Abstract
It has been documented previously that defects in the generation of C18-ceramide, a product of ceramide synthase 1 (CerS1), also known as longevity assurance gene 1 (hLASS1), play important roles in the pathogenesis and/or progression of HNSCC. However, whether altered levels of ceramide generation in HNSCC tumors have any clinical relevance remains unknown. In this study, the levels of endogenous ceramides were measured in tumor tissues of 45 HNSCC patients as compared to their normal tissues using high-pressure liquid chromatography/mass spectrometry (LC/MS), and then possible link between ceramide levels and the clinical parameters of HNSCC were examined. The data showed that the levels of C16-, C24-, C24:1-ceramide were significantly elevated in the majority of tumor tissues compared to their normal tissues, while the levels of only C18-ceramide were significantly decreased in HNSCC tumors, especially in tumor tissues of male patients. Importantly, it was also shown here that decreased C18-ceramide levels in HNSCC tumor tissues were significantly associated with the higher incidences of lymphovascular invasion, and pathologic nodal metastasis. Importantly, attenuation of C18-ceramide was also positively linked to the higher overall stages of the primary HNSCC tumors. Therefore, these data suggest, for the first time, that the defects in the generation/accumulation of C18-ceramide might have important clinical roles in HNSCC, especially in lymphovascular invasion and nodal disease.
Keywords: Ceramide, Ceramide synthase, Longevity assurance gene (LASS), Head and neck cancer, Lymphovascular spread, Nodal metastasis
1. Introduction
Squamous cell carcinomas of the head and neck (HNSCC) are among the most aggressive group of cancers. Despite emerging new surgical techniques and chemoradiation protocols, HNSCC remains among the five leading causes of solid tumor-related deaths in the United States [1]. Five-year survival rates of patients with advanced stages of HNSCC remain around 50%, with little improvement for several decades. Although several tissue biomarkers such as p16, p53, cyclin D1, cyclo-oxygenase 2 (COX-2), epidermal growth factor receptor (EGFR), vascular endothelial growth factor (VEGF), and matrix metalloproteinases have been associated with HNSCC, there is still a need for new diagnostic and/or prognostic markers [2].
The biologically active sphingolipid ceramide has been a source of interest in the regulation of cancer cell growth and response to therapy. In addition to its important role as a key molecule in sphingolipid metabolism, ceramide also has important effector functions, which involve the regulation of apoptosis, cell cycle arrest, or senescence [3].
Previous studies have demonstrated a role for sphingolipids in the regulation of HNSCC growth and progression. For example, ceramide and sphingosine have been shown to inhibit EGF receptor kinase in epidermoid cell A431 carcinoma cells [4,5]. In addition, altering the levels of membrane glycolipids of human A431 cells by an inhibitor of glucosylceramide synthase resulted in a rapid loss of epithelial cell morphology, a reduced rate of cell growth, and inhibition of cell-substrate interaction [6]. In another study, treatment of squamous cell carcinoma cell line, DJM-1, with an exogenous ceramide promoted differentiation, and inhibited proliferation, suggesting a regulatory role of ceramide in growth and differentiation of keratinocytes [7]. Moreover, there have been additional studies which confirmed the active regulatory role of ceramide in HNSCC cells regarding the induction of apoptosis [8], EGF receptor modulation [9,10], reversing drug and radiation resistance [11,12], inhibition of neo-vascularization [13], and enhancing the anti-cancer actions of various chemotherapy agents [14,15], or photodynamic therapy [16].
Recently, treatment of human UM-SCC-22A (SCC of the hypopharynx) cells with a novel exogenous ceramide analogue, L-threo-C6-pyridinium-ceramide (L-t-C6-Pyr-Cer) resulted in a significant inhibition of telomerase activity, and prevented the growth of HNSCC xenografts in SCID mice in vivo [17]. It is well recognized that telomerase is found to be active in 80-90% of HNSCC and is proposed to be an essential step for cancer cell immortalization. Telomerase is also associated with poor prognosis of patients with HNSCC [18,19].
In another line of investigation, the functions of specific subspecies of endogenous ceramides with different fatty acid chain length in HNSCC growth and/or progression has been examined. Analysis of the levels of endogenous ceramides between tumor and normal mucosa tissues of the same patients with HNSCC showed that only C18-ceramide levels were decreased in the majority of tumor tissues, whereas the levels of other ceramides, such as C16-, and C24-ceramides were increased in HNSCC tumor tissues when compared to their normal counterparts [20]. Further experiments showed that overexpression of the mammalian homologue of the yeast longevity assurance gene 1 (LASS1), also referred to as ceramide synthase1 (CerS1), which is known to selectively generate C18-ceramide, resulted in the inhibition of HNSCC cell growth, and enhanced chemotherapy-induced apoptosis in UMSCC22A cells in situ, and in HNSCC xenografts in vivo [20, 21].
Although, these data demonstrated an important role for ceramide signaling in the regulation of HNSCC pathogenesis and/or progression both in situ and in vivo, the clinical relevance of endogenous ceramide levels in HNSCC is still unknown. Therefore, in this study, we examined the association between changes in the levels of endogenous ceramide and clinical parameters of HNSCC. The data demonstrated that alterations of the C18-ceramide levels in HNSCC tumors are significantly correlated with the presence of lymphovascular invasion and nodal metastatic disease, suggesting that attenuation of C18-ceramide is highly associated with the advanced HNSCC in the clinic.
2. Materials and methods
2.1. Clinical samples
With the permission of the Institutional Review Board, randomized tissue samples of 33 male (73%) and 12 female (27%) HNSCC patients were obtained from the tumor bank of the Hollings Cancer Center at Medical University of South Carolina. For each patient, paired tissue samples, obtained from the tumor or from the pathologically negative healthy mucosa near tumor site, were studied. Therefore, randomization was performed among the patients whose paired tissue specimens included both the pathologically documented tumor and healthy mucosa in the tumor bank. The demographics and clinicopathologic findings of the patients are shown in Table 1. The mean follow up time for the group was 15.1 months (ranging from 1 to 58 months).
Table 1.
Clinicopathologic parameters of 45 HNSCC patients
| Parameter | Number of patients | % of patients |
|---|---|---|
| Sex | ||
| Male | 33 | 73.3 |
| Female | 12 | 26.7 |
| Site | ||
| Oral cavity and Hypopharynx | 28 | 62.2 |
| Larynx | 10 | 22.2 |
| Sinonasal cavity | 5 | 11.1 |
| Unknown primary | 2 | 4.5 |
| T stage* | ||
| 1 | 3 | 6.7 |
| 2 | 7 | 15.5 |
| 3 | 6 | 13.3 |
| 4 | 13 | 28.9 |
| Recurrent | 14 | 31.1 |
| Unknown | 2 | 4.5 |
| N stage* | ||
| 0 | 21 | 46.6 |
| 1 | 6 | 13.3 |
| 2 | 16 | 35.6 |
| Undetermined | 2 | 4.5 |
| Overall stage* | ||
| 1,2 | 7 | 15.5 |
| 3,4 | 24 | 53.4 |
| Recurrent | 14 | 31.1 |
| Lymphovascular invasion | ||
| Absent | 26 | 57.8 |
| Present | 14 | 31.1 |
| Undetermined | 5 | 11.1 |
| Neural spread | ||
| Absent | 19 | 42.3 |
| Present | 20 | 44.4 |
| Undetermined | 6 | 13.3 |
According to the guidelines of American Joint Committee of Cancer [21]
2.2. Measurement of endogenous ceramides and sphingosine-1-phosphate (S1P)
Measurements of the ceramide subspecies and S1P in HNSCC tumor versus their normal tissues were performed by LC/MS as previously described [22]. The ceramide levels were normalized to total protein levels. The mRNA levels of human longevity assurance gene 1 and 6 (LASS1/CerS1 and LASS6/CerS6) in tumor and normal tissues of randomly selected 12 patients were measured using real-time PCR.
2.3. Analysis of the association between ceramide levels and clinical parameters
Electronic chart reviews of the patients were performed with data collection encompassing demographics, site and stage of the tumor, pathologic findings including nodal metastatic disease, perineuronal spread and lymphovascular invasion. The date of the surgery in which the tissues were sampled, is accepted as the reference day for data collection regardless of the previous or consecutive treatment or surgery results.
2.4. Statistical analysis
The statistical analysis was performed using SPSS 14.0 (SPSS, Chicago, IL) and SAS version 9.1 (SAS Inc., Cary, NC). Comparison of ceramide levels between tumor and normal tissues of the patients were analyzed using the paired T-Test. Correlation between age or stages of the tumors with ceramide levels were analyzed using standard and multiple linear regression methods. Comparison of the risk of nodal metastasis in patients with higher or lower tumor levels of C18-ceramide as compared to their normal counterparts were performed using Fisher’s exact-test. In these studies P value of .05 was considered significant.
3. Results
3.1. The levels of endogenous ceramides in HNSCC tumor tissues as compared to normal tissues
To examine the clinical relevance of ceramide, first the levels of endogenous ceramides were measured in 45 pairs of tissues (normal as compared to tumor tissues) obtained from patients with HNSCC using LC/MS, and results are presented in Table 2. Consistent with the previously published data [20], which were obtained from a smaller cohort (n=14 pairs), the current data in this study using a larger cohort of patients (n=45 pairs) showed that endogenous C16-, C24-, C24:1-ceramides were significantly increased in tumor tissues whereas the levels of only C18-ceramide (both C18:0- and C18:1-ceramides) were significantly decreased in HNSCC compared to their normal tissues (Table 2). The differences of other minor ceramides, such as C14- and C20-ceramides in tumor and normal tissue levels were not significant (Table 2). Thus, these data suggest that attenuation of C18-ceramide, which is known to mediate anti-proliferative functions [20,21], in HNSCC tissues may play a role in the pathogenesis and/or progression of this disease.
Table 2.
Ceramide levels in tumor and counterpart normal tissues (pmol/1mg protein)
| Ceramide subspecie | Normal Tissue level * | Tumor level* | P** |
|---|---|---|---|
| Total Ceramide | 275.43±229.94 | 391.61±311.06 | < .01 |
| C14-ceramide | 13.12±22.0 | 20.05±18.21 | .07 |
| C16-ceramide | 97.55±92.02 | 175.61±150.49 | <.01 |
| C18-ceramide | 25.42±18.22 | 14.08±15.85 | <.01 |
| C18:1-ceramide | 8.87±9.81 | 6.91±6.98 | .04 |
| C20-ceramide | 10.59±7.81 | 9.86±7.83 | .63 |
| C24-ceramide | 49.38±64.42 | 69.39±76.17 | <.01 |
| C24:1-ceramide | 71.44±70.57 | 97.24±94.19 | .01 |
Mean + SD
Paired T test
Then, possible correlation between ceramide levels and clinical parameters, such as gender, lymphovascular invasion, nodal metastasis, and overall stage of the tumors were analyzed.
3.2. The analysis of relationship between ceramide levels and gender
The study group consisted of 12 female and 33 male patients. Interestingly, the levels of ceramide, in general, in the normal and tumor tissues of female patients were higher than that of males. Importantly, the data showed that C18-ceramide was significantly lower in HNSCC tumor tissues, compared to their normal counterparts in the majority of the male patients (P = .02), whereas the levels of other ceramide species did not show any significant changes based on the gender of these patients (Table 3).
Table 3.
Ceramide levels* in female and male genders
| Subspecies | Female (n=12) | Male (n=33) | P** |
|---|---|---|---|
| Total Ceramide | |||
| Normal | 374±299 | 240±192 | .08 |
| Tumor | 525±330 | 343±294 | .08 |
| C16-ceramide | |||
| Normal | 141±125 | 81.9±73.0 | .06 |
| Tumor | 231±177 | 155±137 | .14 |
| C18-ceramide | |||
| Normal | 31.2±25.1 | 23.3±15.0 | .21 |
| Tumor | 23.3±26.6 | 10.7±7.61 | .02 |
| C18:1-ceramide | |||
| Normal | 11.6±11.2 | 7.90±9.27 | .27 |
| Tumor | 10.2±8.47 | 5.71±6.08 | .06 |
| C24-ceramide | |||
| Normal | 50.3±50.6 | 47.7±68.9 | .91 |
| Tumor | 92.4±81.7 | 60.8±73.5 | .22 |
| C24:1-ceramide | |||
| Normal | 104±103 | 59.7±51.4 | .06 |
| Tumor | 130±98.4 | 85.5±91.3 | .17 |
(pmol/1mg protein), mean+SD
Unpaired T Test
3.3. Decrease in C18-ceramide in HNSCC tumor tissues significantly correlates with lymphovascular invasion and nodal metastasis
Among 45 patients with HNSCC included in this study, 14 patients were presented with lymphovascular invasion in their pathologic specimens, while 26 patients showed no lymphovascular invasion, and reports of 5 patients did not specify the presence or absence of this feature (Table 1). As shown in Table 4, the tumor levels of C18-ceramide in patients who presented with lymphovascular invasion were significantly lower than the patients who did not (P = .05). Eight patients had higher C18-ceramide levels in their tumor tissues compared to normal tissues, and impressively, none of these 8 patients were diagnosed with lymphovascular invasion. On the other hand, 14 patients of 32 (44%), whose tumor C18-ceramide levels were lower than normal tissue levels presented with lymphovascular spread. The difference between two groups was also significant (Fisher’s exact test, P = .02). The other ceramide subspecies in these tumors presented no significant changes regarding lymphovascular spread.
Table 4.
Ceramide levels * of patients with or without lymphovascular invasion
| Subspecies | Without
Lymphovascular Invasion (n=26) |
With
Lymphovascular Invasion (n=14) |
P** |
|---|---|---|---|
| Total Ceramide | |||
| Normal | 258±193 | 328±317 | .39 |
| Tumor | 380±299 | 382±321 | .98 |
| C16-ceramide | |||
| Normal | 101±91.4 | 99.9±106 | .98 |
| Tumor | 183±140 | 144±112 | .38 |
| C18-ceramide | |||
| Normal | 25.0±18.1 | 26.5±19.7 | .81 |
| Tumor | 14.6±10.7 | 8.49±5.94 | .05 |
| C18:1-ceramide | |||
| Normal | 8.95±10.7 | 8.87±9.61 | .98 |
| Tumor | 7.33±7.59 | 6.13±6.02 | .61 |
| C24-ceramide | |||
| Normal | 42.3±67.3 | 60.7±64.9 | .41 |
| Tumor | 58.0±74.1 | 87.3±87.0 | .27 |
| C24:1-ceramide | |||
| Normal | 60.0±43.9 | 99.2±108 | .11 |
| Tumor | 87.2±90.3 | 112±111 | .45 |
pmol/1mg protein, mean+SD
Unpaired T Test
Retrospective reviews of the pathologic specimens revealed that 22 patients exhibited pathological presence of nodal metastasis at the time of surgery, and among these, 6 and 16 patients presented N1 and N2 neck disease, respectively (Table 1). Interestingly, all the ceramide levels were higher in tissue samples obtained from patients without nodal metastasis than in tissues of patients with nodal disease. More importantly, C18-ceramide levels in tumors with nodal disease were significantly lower (P = .02) when compared to its levels in tumor tissues of patients without nodal metastasis (Table 5).
Table 5.
Ceramide levels* of patients with or without nodal metastasis disease
| Subspecies | Without nodal
metastasis (n=21) |
With nodal
metastasis (n=22) |
P** |
|---|---|---|---|
| Total Ceramide | |||
| Normal | 317±263 | 238±204 | .27 |
| Tumor | 392±265 | 362±325 | .74 |
| C16-ceramide | |||
| Normal | 114±110 | 77.8±72.5 | .21 |
| Tumor | 174±114 | 154±140 | .62 |
| C18-ceramide | |||
| Normal | 29.6±22.3 | 22.2±13.1 | .19 |
| Tumor | 15.8±11.2 | 9.12±6.25 | .02 |
| C18:1-ceramide | |||
| Normal | 11.7±12.1 | 6.55±6.94 | .09 |
| Tumor | 8.64±8.34 | 4.76±4.50 | .06 |
| C24-ceramide | |||
| Normal | 44.7±45 | 54.6±80.8 | .62 |
| Tumor | 69±70.6 | 71.7±85.4 | .91 |
| C24:1-ceramide | |||
| Normal | 87.7±87.5 | 57.9±51.5 | .18 |
| Tumor | 97.7±87 | 94.4±105 | .91 |
pmol/1mg protein, mean+SD
Unpaired T Test
Importantly, regression analysis of the correlation between ceramide levels and the overall stages of the patients (presented in Table 1) showed that the difference in the levels of C18-ceramide between normal and tumor tissues (normal – tumor) is positively associated with higher overall stages of HNSCC (P = .004), and these data are shown in Table 6.
Table 6.
Regression model* for the C18-ceramide difference between normal and tumor tissues
| Variable | Parameter Estimate | Standard Error | t-statistic | P |
|---|---|---|---|---|
| Intercept | -63.84101 | 24.49990 | -2.61 | 0.0150 |
| Age | 0.04165 | 0.32219 | 0.13 | .90 |
| Stage | 13.27076 | 4.27539 | 3.10 | < .01 |
Only the effects for the variables alone were considered in this model; no interactions between the independent variables were considered.
Taken together, these data show for the first time that attenuation of C18-ceramide in HNSCC tumors significantly associate with lymphovascular invasion and nodal metastasis, which are among the known parameters of advanced clinical disease. However, there were not enough clinical data to assess whether attenuation of C18-ceramide is linked to overall survival and/or disease-free survival of these patients with HNSCC, which needs to be explored further.
3.4. The expression levels of human LASS1 and LASS6 in HNSCC
In order to obtain a mechanistic insight into the perturbations of ceramide species, the mRNA levels of human homologue of the yeast longevity assurance gene 1 (LASS1)/CerS1, which is known to be involved in the generation of C18-ceramide, were also examined in 12 pairs of HNSCC tumor and normal tissues using real-time PCR. The results showed that decreased tumor levels of C18-ceramide, which were detected in 9 of 12 patients, were also associated with decreased mRNA levels of LASS1 in 5 of these 9 patients. However, lower levels of C18-ceramide in the remaining 4 patients did not correlate with LASS1/CerS1 mRNA levels (Figure 1). Interestingly, higher levels of C16-ceramide in HNSCC tumors were highly linked to the increased mRNA levels of human LASS6/CerS6, which is implicated in the generation of C16-ceramide. It was observed C16-ceramide was increased in the HNSCC tumor tissues of 11 out of 12 patients, and induced LASS6/CerS6 mRNA levels correlated with increased C16-ceramide levels in 8 of those 11 patients. However, there was no increase in LASS6 mRNA expression in 3 patients who had increased levels of C16-ceramide in their tumor tissues (Figure 2). Therefore, these data demonstrate that in addition to transcriptional regulation of LASS1/CerS1 and LASS6/CerS6, there might be a post-transcriptional or -translational regulation of these proteins, or modulation of their (dihydro)ceramide synthase activities in some patients, which are involved in the down- or up-regulation of C18- or C16-ceramides, respectively.
Fig. 1.
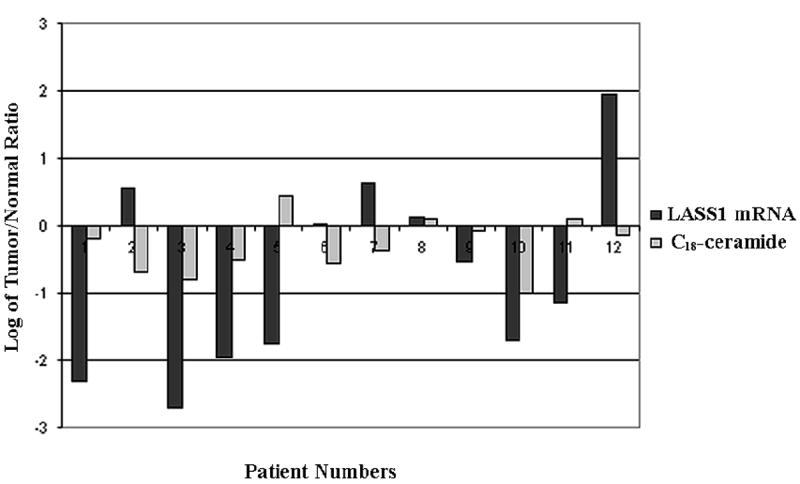
The alterations of LASS1/CerS1 mRNA and C18-ceramide levels in HNSCC. The correlation between the expression levels of human LASS1/CerS1 mRNA, measured by real-time PCR, and C18-ceramide levels, measured by LC/MS, in 12 HNSCC tumors as compared to their normal counterparts, was analyzed, and the fold-changes in their levels were presented in logarithmic scale. Negative numbers represent decreases in the levels of LASS1/CerS1 mRNA and ceramide levels. The measurements were performed in duplicates, and standard errors were within 0.5-1% of the presented values.
Fig. 2.
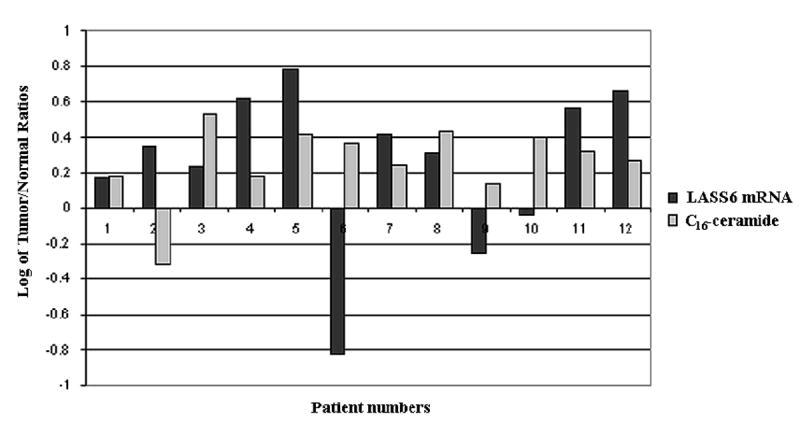
The alterations of LASS6/CerS6 mRNA and C16-ceramide levels in HNSCC. The correlation between the expression levels of human LASS6/CerS6 mRNA, measured by real-time PCR, and C16-ceramide levels, measured by LC/MS, in 12 HNSCC tumors as compared to their normal counterparts, was analyzed and presented in logarithmic scale. Negative numbers represent decreases in the levels of LASS6/CerS6 mRNA and ceramide levels. The measurements were performed in duplicates, and standard errors were within 0.5-1% of the presented values.
4. Discussion
In this report, the clinical relevance of endogenous ceramides in 45 pairs of HNSCC tumor and non-cancerous (normal) tissues was examined. Consistent with the previous data [20], results presented here demonstrated that the levels of only C18-ceramide was significantly lower in the tumor tissues of patients with HNSCC, whereas the levels of other major ceramides, especially C16-, and C24-ceramides, were higher in these tumors, when compared to their normal counterparts. Interestingly, levels of C18-ceramide in HNSCC tumors appeared to be significantly altered in male patients, and, in general, female patients contained higher levels of ceramides in their tissues (both normal and tumor tissues) as compared to males. More impressively, the data revealed for the first time that the attenuation of C18-ceramide in HNSCC tumor as compared to normal tissues significantly correlated with lymphovascular spread, and nodal metastatic disease, which are known to be independent markers of poor prognosis and advanced disease for HNSCC patients in the clinic.
Previously, an important study revealed that total ceramide levels, without any information about fatty acid chain length specificity, were inversely correlated with malignant progression of human astrocytomas, and associated with poor patient prognosis [23]. In an independent study, the data showed that defects in the generation of C18-ceramide in the majority of HNSCC tumors might play a role in the pathogenesis of this disease, and that reconstitution of the levels of C18-ceramide via hLASS1/CerS1 expression resulted in apoptosis, and enhanced chemotherapy-induced cell death in HNSCC both in situ and in vivo [20, 21]. The data presented here also showed similar results, confirming that decreased levels of C18-ceramide in HNSCC tumor tissues compared to their normal counterparts using larger cohort of patients might play important roles in the pathogenesis of HNSCC. More importantly, results presented here showed also the clinical relevance of ceramide in HNSCC, revealing that the lower levels of C18-ceramide in tumor tissues associate with lymphovascular invasion and nodal metastatic disease in patients with HNSCC, which links the altered C18-ceramide generation and/or accumulation with the advanced clinical disease.
The different incidence rates of HNSCC, especially for larynx cancer, in men and women have been a subject of research, and it has been reported that there is a high ratio of male to female rates of the laryngeal cancer incidences, especially in the glottic tumors [24,25]. Even though there exists a controversy regarding the existence of sex hormone receptors in larynx [26-28], the voice changes after hormone replacement therapies support of the presence of a relationship between sex hormones and the larynx [29,30]. In parallel with these, our data showed a significant difference in ceramide levels between female and male patients. Specifically, ceramide levels in the tissues of female patients were higher than males, and more importantly, C18-ceramide levels were significantly lower only in the HNSCC tumor tissues of the male patients, which is also consistent with the higher incidence rates of these cancers in males. The relationship between ceramide metabolism, HNSCC and gender has not been reported previously, and thus these data are novel and interesting. Although, the reason for increased levels of ceramide in females, and decreased C18-ceramides in males are not clear, these findings of the presented study warrant further analysis.
Lymphovascular invasion of the tumor has been found to be positively associated with lymph node metastasis of HNSCC [31-34]. The negative effect of lymphovascular invasion on disease specific survival has been shown for HNSCC previously [35]. Moreover, existence of perivascular invasion reduces the time interval between surgery and development of recurrence [34]. Finally, recent studies suggest that in addition to advanced nodal stage and perineuronal invasion, lymphovascular spread is an independent prognostic variable for hypopharynx carcinomas, and a predictor of poor survival [36]. In the current study, tumors tissues of patients who were presented with lymphovascular invasion contained significantly lower levels of C18-ceramide when compared to their normal tissues (P = .05). Remarkably, none of the eight (0/8) HNSCC patients whose tumor C18-ceramide levels were higher than that of their normal tissues exhibited any detectable lymphovascular spread, again supporting the role of C18-ceramide in its regulation.
Nodal metastatic disease is a well recognized independent factor for poor survival in different sites of HNSCC, including tongue, floor of mouth, oropharynx, hypopharynx and larynx [37-40]. The survival is hampered by the presence of extracapsular spread feature of the tumor [41,42]. Several clinicopathologic parameters have been associated with nodal disease, such as tumor depth [43], lymphovascular invasion [31-34], histological grade [44], tumor differentiation [45], tumor size [31], and double DNA aneuploidy [45]. In the presented study, patients who had a nodal disease exhibited significantly lower levels of C18-ceramide in their tumors, compared to their normal tissues (P = .02). The patients whose tumor C18-ceramide levels were higher than their normal tissues presented a lower risk of nodal disease (33.3%) as compared to patients whose tumor C18-ceramide levels were lower than that of their normal tissues (56%).
However, mechanisms that regulate C18-ceramide generation and/or accumulation in HNSCC, leading to its altered levels, are still unknown and need to be determined. The analysis of the mRNA levels of hLASS1/CerS1, which is known to generate mainly C18-ceramide [46,47], by real-time PCR showed that decreased tumor levels of C18-ceramide, which were detected in 9 of 12 patients, were associated with decreased mRNA levels of LASS1/CerS1 in only about 50% of these patients (5 out of 9 patients). Therefore, these data demonstrate that in addition to transcriptional regulation of LASS1/CerS1, there might be a post-transcriptional and/or -translational regulation of LASS1/CerS1, or modulation of its (dihydro)ceramide synthase activity in some HNSCC patients, which might be involved in the down- regulation of C18-ceramide.
Similarly, the biochemical mechanisms involved in the ceramide-mediated regulation of lymphovascular invasion, and the consequences of altered levels of C18-ceramide, leading to this feature in HNSCC, however, are still unknown, and will require further examination. Recent studies have elucidated a role for protein phosphatase 2A (PP2A) in the regulation of vascular invasion, and since ceramide is a known activator of PP2A [3], its attenuation in HNSCC tumors might lead to negative modulation of PP2A activity [13]. Although this might be a plausible possibility, whether C18-ceramide specifically plays a role in the activation of PP2A, and whether further metabolism of C18-ceramide into other complex lipids, might inhibit PP2A function, are still unknown. For example, it is possible that further metabolism of C18-ceramide to generate pro-survival lipids [48], such as sphingosine-1-phosphate (S1P) and/or ceramide-1-phosphate (C1P), might be important in the increased incidences of lymphovascular invasion and nodal metastasis. It is well documented that increased generation of S1P by sphingosine kinase 1 (SK1) plays important roles in malignant transformation, alteration of ceramide-induced apoptosis, altered autophagy, and drug resistance [49-51]. However, our analysis of S1P levels in 18 pairs of HNSCC tumor tissues used in this study as compared to their normal counterparts did not reveal any statistically significant differences. The average values of S1P in HNSCC tumor and normal tissues were 3.50 and 3.23 pmol/mg of protein, respectively (n=18 pairs, and P >.05). Nevertheless, the levels of S1P in larger cohort of patients, especially in the serum of HNSCC patients as compared to that of healthy individuals should be determined to examine its possible clinical roles in the pathogenesis of HNSCC, since it might be readily secreted from squamous tissues into the blood, which is a known characteristic of S1P in some cell/tissue types [52]. Indeed, targeting of extracellular S1P has been shown to exert anti-proliferative functions against various cancers [53].
Similarly, it has been recently reported that generation of C1P by ceramide kinase (CK) plays a role in the induction of growth in various cell types [54,55]. Therefore, increased levels of C1P, especially C18-ceramide-1-phosphate, in HNSCC tissues and/or serum of patients with the disease might be very important to evaluate in future studies.
These data also demonstrated that the levels of C16-ceramide were highly up-regulated in the majority of HNSCC tumor tissues. Although there were no significant correlation between increased C16-ceramide and any of the clinical parameters tested, its modulation might still play very important roles in the pro-survival of HNSCC cells. Taken together, these data also suggest that endogenous ceramides with different fatty acid chain lengths, such as C18- and C16-ceramides, might play distinct roles in the regulation of cell growth and/or survival. Indeed, our recent data demonstrated that while the C18-ceramide generated by hLASS1/CerS1 inhibits telomerase, which is one of the best studied negative prognostic markers of most cancers, including HNSCC, generation of C16-ceramide via LASS6/CerS6 induced the activity of the promoter of the human telomerase reverse transcriptase (L.G. Wooten-Blanks, P. Song, C.E. Senkal, and B. Ogretmen, unpublished data). Nevertheless, specific roles of hLASS/CerS-generated C18- and C16-ceramides in the regulation of PP2A function, if any, are still unknown, and need to be determined. Therefore, differential sub-cellular localization/topology of these endogenous ceramides, and the regulation of their distinct down-stream targets might be important for their distinct biological functions in human cancers, which should be further defined.
In conclusion, this study demonstrates the clinical relevance of ceramide signaling in the pathogenesis of HNSCC, and reveal for the first time that lower levels of C18-ceramide in HNSCC tumors as compared to normal tissues is significantly associated with lymphovascular invasion and nodal disease. Although there were not enough clinical data to evaluate the possible link between C18-ceramide levels and the overall survival, it is believed that this possibility should be determined in an independent trial with a prospective study design. Nevertheless, these data reveal that lower C18-ceramide levels, which is known to be generated by the function of hLASS1/CerS1 [46,47], in HNSCC tumors is significantly correlated with the parameters of advanced clinical disease, and support the view that C18-ceramide might play important roles in the regulation of HNSCC growth and/or pathogenesis in the clinic.
Acknowledgments
We thank the members of Ogretmen, Hannun, and Norris laboratories for their helpful discussions. This study was supported by research grants from the National Institutes of Health DE01657 (B.O.), and CA097132 (Y.A.H.), Department of Defense, phase VII program project grant through Hollings Cancer Center (B.O.), and the National Science Foundation/EPSCoR, EPS-0132573 (B.O.). The animal facility used in this study was supported by the National Institutes of Health, Grant Number C06 RR015455 (MUSC) from the Extramural Research Facilities Program of the National Center for Research Resources. The Lipidomics Core Facility was supported by grants from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Thomas GR, Nadiminti H, Regalado J. Molecular predictors of clinical outcome in patients with head and neck squamous cell carcinoma. Int J Exp Pathol. 2005;86:347–363. doi: 10.1111/j.0959-9673.2005.00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 4.Igarashi Y, Kitamura K, Toyokuni T, Dean B, Fenderson B, Ogawass T, Hokomori S. A specific enhancing effect of N,N-dimethylsphingosine on epidermal growth factor receptor autophosphorylation. Demonstration of its endogenous occurrence (and the virtual absence of unsubstituted sphingosine) in human epidermoid carcinoma A431 cells. J Biol Chem. 1990;265:5385–5389. [PubMed] [Google Scholar]
- 5.Goldkorn T, Dressler KA, Muindi J, Radin NS, Mendelsohn J, Menaldino D, Liotta D, Kolesnick RN. Ceramide stimulates epidermal growth factor receptor phosphorylation in A431 human epidermoid carcinoma cells. Evidence that ceramide may mediate sphingosine action. J Biol Chem. 1991;266:16092–16097. [PubMed] [Google Scholar]
- 6.Barbour S, Edidin M, Felding-Habermann B, Taylor-Norton J, Radin NS, Fenderson BA. Glycolipid depletion using a ceramide analogue (PDMP) alters growth, adhesion, and membrane lipid organization in human A431 cells. J Cell Physiol. 1992;150:610–619. doi: 10.1002/jcp.1041500322. [DOI] [PubMed] [Google Scholar]
- 7.Wakita H, Tokura Y, Yagi H, Nishimura K, Furukowa F, Takigawa M. Keratinocyte differentiation is induced by cell-permeant ceramides and its proliferation is promoted by sphingosine. Arch Dermatol Res. 1994;286:350–354. doi: 10.1007/BF00402228. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Lafrasse C, Alphonse G, Broquet P, Aloy MT, Louisot P, Rousson R. Temporal relationships between ceramide production, caspase activation and mitochondrial dysfunction in cell lines with varying sensitivity to anti-Fas-induced apoptosis. Biochem J. 2001;357:407–416. doi: 10.1042/0264-6021:3570407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meuillet EJ, Mania-Farnell B, George D, Inokuchi JI, Bremer EG. Modulation of EGF receptor activity by changes in the GM3 content in a human epidermoid carcinoma cell line, A431. Exp Cell Res. 2000;256:74–82. doi: 10.1006/excr.1999.4509. [DOI] [PubMed] [Google Scholar]
- 10.Hudson PL, Pedersen WA, Saltsman WS, Liscowitch M, MacLaughlin DT, Donahoe PK, Blutsztajin JK. Modulation by sphingolipids of calcium signals evoked by epidermal growth factor. J Biol Chem. 1994;269:21885–21890. [PubMed] [Google Scholar]
- 11.Alphonse G, Bionda C, Aloy MT, Ardail D, Rousson R, Rodriquez-Lafrasse C. Overcoming resistance to gamma-rays in squamous carcinoma cells by poly-drug elevation of ceramide levels. Oncogene. 2004;23:2703–2715. doi: 10.1038/sj.onc.1207357. [DOI] [PubMed] [Google Scholar]
- 12.Chmura SJ, Mauceri HJ, Advani S, Heimann R, Beckett MA, Nodzsenki E, Quintas J, Kufe DW, Weichselbaum RR. Decreasing the apoptotic threshold of tumor cells through protein kinase C inhibition and sphingomyelinase activation increases tumor killing by ionizing radiation. Cancer Res. 1997;57:4340–4347. [PubMed] [Google Scholar]
- 13.Meisinger J, Patel S, Vellody K, Bergstrom R, Benefield J, Lozano Y, Young MR. Protein phosphatase-2A association with microtubules and its role in restricting the invasiveness of human head and neck squamous cell carcinoma cells. Cancer Lett. 1997;111:87–95. doi: 10.1016/s0304-3835(96)04517-x. [DOI] [PubMed] [Google Scholar]
- 14.Mehta S, Blackinton D, Omar I, Kouttab N, Myrick D, Klostergaard J, Wanebo H. Combined cytotoxic action of paclitaxel and ceramide against the human Tu138 head and neck squamous carcinoma cell line. Cancer Chemother Pharmacol. 2000;46:85–92. doi: 10.1007/s002800000140. [DOI] [PubMed] [Google Scholar]
- 15.Senkal CE, Ponnusamy S, Rossi MJ, Sundararaj K, Szulc Z, Bielawski J, Bielawska A, Meyer M, Cobanoglu B, Koybasi S, Sinha D, Day TA, Obeid LM, Hannun YA, Ogretmen B. Potent antitumor activity of a novel cationic pyridinium-ceramide alone or in combination with gemcitabine against human head and neck squamous cell carcinomas in vitro and in vivo. J Pharmacol Exp Ther. 2006;317:1188–1199. doi: 10.1124/jpet.106.101949. [DOI] [PubMed] [Google Scholar]
- 16.Nagy B, Chiu SM, Separovic D. Fumonisin B1 does not prevent apoptosis in A431 human epidermoid carcinoma cells after photosensitization with a silicon phthalocyanine. J Photochem Photobiol B. 2000;57:132–141. doi: 10.1016/s1011-1344(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 17.Rossi MJ, Sundararaj K, Koybasi S, Phillips MS, Szulc ZM, Belawska A, Day TA, Obeid LM, Hannun YA, Ogretmen B. Inhibition of growth and telomerase activity by novel cationic ceramide analogs with high solubility in human head and neck squamous cell carcinoma cells. Otolaryngol Head Neck Surg. 2005;132:55–62. doi: 10.1016/j.otohns.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Liao CT, Tung-Chieh Chang J, Wang HM, Chen IH, Lin CY, Chen TM, Hsieh LL, Cheng AJ. Telomerase as an independent prognostic factor in head and neck squamous cell carcinoma. Head Neck. 2004;26:504–512. doi: 10.1002/hed.20007. [DOI] [PubMed] [Google Scholar]
- 19.Patel MM, Parekh LJ, Jha FP, Sainger RN, Patel JB, Shah PM, Patel PS. Clinical usefulness of telomerase activation and telomere length in head and neck cancer. Head Neck. 2002;24:1060–1067. doi: 10.1002/hed.10169. [DOI] [PubMed] [Google Scholar]
- 20.Koybasi S, Senkal CE, Sundararaj K, Spassieva S, Bielawski J, Osta W, Day TA, Jiang JC, Jazwinski SM, Hannun YA, Obeid LM, Ogretmen B. Defects in cell growth regulation by C18:0-ceramide and longevity assurance gene 1 in human head and neck squamous cell carcinomas. J Biol Chem. 2004;279:44311–44319. doi: 10.1074/jbc.M406920200. [DOI] [PubMed] [Google Scholar]
- 21.Senkal CE, Ponnusamy S, Rossi MJ, Bielawski J, Sinha D, Jiang JC, Jazwinski SM, Hannun YA, Ogretmen B. Role of human longevity assurance gene 1 and C18-ceramide in chemotherapy-induced cell death in human head and neck squamous cell carcinomas. Mol Cancer Ther. 2007;6:712–722. doi: 10.1158/1535-7163.MCT-06-0558. [DOI] [PubMed] [Google Scholar]
- 22.Kraveka JM, Li L, Bielawski J, Obeid LM, Ogretmen B. Involvement of endogenous ceramide in the inhibition of telomerase activity and induction of morphologic differentiation in response to all-trans-retinoic acid in human neuroblastoma cells. Arch Biochem Biophys. 2003;419:110–119. doi: 10.1016/j.abb.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 23.Riboni L, Campanella R, Bassi R, Villani R, Gaini SM, Martinelli-Boneschi F, Vianni P, Tettamanti G. Ceramide levels are inversely associated with malignant progression of human glial tumors. Glia. 2002;39:105–113. doi: 10.1002/glia.10087. [DOI] [PubMed] [Google Scholar]
- 24.Yang PC, Thomas DB, Daling JR, Davis S. Differences in the sex ratio of laryngeal cancer incidence rates by anatomic subsite. J Clin Epidemiol. 1989;42:755–758. doi: 10.1016/0895-4356(89)90072-3. [DOI] [PubMed] [Google Scholar]
- 25.Stephenson WT, Barnes DE, Holmes FF, Norris CW. Gender influences subsite of origin of laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1991;117:774–778. doi: 10.1001/archotol.1991.01870190086018. [DOI] [PubMed] [Google Scholar]
- 26.Newman SR, Butler J, Hammond EH, Gray SD. Preliminary report on hormone receptors in the human vocal fold. J Voice. 2000;14:72–81. doi: 10.1016/s0892-1997(00)80096-x. [DOI] [PubMed] [Google Scholar]
- 27.Ferguson BJ, Hudson WR, Jr, McCarty KS. Sex steroid receptor distribution in the human larynx and laryngeal carcinoma. Arch Otolaryngol Head Neck Surg. 1987;113:1311–1315. doi: 10.1001/archotol.1987.01860120057008. [DOI] [PubMed] [Google Scholar]
- 28.Schneider B, Cohen E, Stani J, Kolbus A, Rudas M, Horvat R, vanTrotsenburg M. Towards the Expression of Sex Hormone Receptors in the Human Vocal Fold. J Voice. 2006 Mar 24; doi: 10.1016/j.jvoice.2006.01.002. in press. [DOI] [PubMed] [Google Scholar]
- 29.Gerritsma EJ, Brocaar MP, Hakkesteegt MM, Birkenhager JC. Virilization of the voice in post-menopausal women due to the anabolic steroid nandrolone decanoate (Decadurabolin). The effects of medication for one year. Clin Otolaryngol Allied Sci. 1994;19:79–84. doi: 10.1111/j.1365-2273.1994.tb01153.x. [DOI] [PubMed] [Google Scholar]
- 30.Akcam T, Bolu E, Merati AL, Durmus C, Gerek M, Ozkaptan Y. Voice changes after androgen therapy for hypogonadotrophic hypogonadism. Laryngoscope. 2004;114:1587–1591. doi: 10.1097/00005537-200409000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Kurokawa H, Yamashita Y, Takeda S, Zhang M, Fukuyama H, Takahashi T. Risk factors for late cervical lymph node metastases in patients with stage I or II carcinoma of the tongue. Head Neck. 2002;24:731–736. doi: 10.1002/hed.10130. [DOI] [PubMed] [Google Scholar]
- 32.Pimenta Amaral TM, Da Silva Freire AR, Carvalho AL, Pinto CA, Kowalski LP. Predictive factors of occult metastasis and prognosis of clinical stages I and II squamous cell carcinoma of the tongue and floor of the mouth. Oral Oncol. 2004;40:780–786. doi: 10.1016/j.oraloncology.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 33.Moore BA, Weber RS, Prieto V, El-Nagaar A, Holsinger FC, Zhou X, Lee JJ, Lippman S, Clayman GL. Lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115:1561–1567. doi: 10.1097/01.mlg.0000173202.56739.9f. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz T, Hosal AS, Gedikoglu G, Onerci M, Gursel B. Prognostic significance of vascular and perineural invasion in cancer of the larynx. Am J Otolaryngol. 1998;19:83–88. doi: 10.1016/s0196-0709(98)90100-4. [DOI] [PubMed] [Google Scholar]
- 35.Clark JR, de Almeida J, Gilbert R, Irish J, Brown D, Neligan P, Gullane PJ. Primary and salvage (hypo)pharyngectomy: Analysis and outcome. Head Neck. 2006;28:671–677. doi: 10.1002/hed.20428. [DOI] [PubMed] [Google Scholar]
- 36.Bova R, Goh R, Poulson M, Coman WB. Total pharyngolaryngectomy for squamous cell carcinoma of the hypopharynx: a review. Laryngoscope. 2005;115:864–869. doi: 10.1097/01.MLG.0000158348.38763.5D. [DOI] [PubMed] [Google Scholar]
- 37.Layland MK, Sessions DG, Lenox J. The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+ Laryngoscope. 2005;115:629–639. doi: 10.1097/01.mlg.0000161338.54515.b1. [DOI] [PubMed] [Google Scholar]
- 38.Sessions DG, Spector GJ, Lenox J, Parriott S, Haughey B, Chao C, Marks J, Perez C. Analysis of treatment results for floor-of-mouth cancer. Laryngoscope. 2000;110:1764–1772. doi: 10.1097/00005537-200010000-00038. [DOI] [PubMed] [Google Scholar]
- 39.Sessions DG, Lenox J, Spector GJ, Chao C, Chaudry OA. Analysis of treatment results for base of tongue cancer. Laryngoscope. 2003;113:1252–1261. doi: 10.1097/00005537-200307000-00026. [DOI] [PubMed] [Google Scholar]
- 40.Hahn SS, Spaulding CA, Kim JA, Constable WC. The prognostic significance of lymph node involvement in pyriform sinus and supraglottic cancers. Int J Radiat Oncol Biol Phys. 1987;13:1143–1147. doi: 10.1016/0360-3016(87)90186-6. [DOI] [PubMed] [Google Scholar]
- 41.Brasilino de Carvalho M. Quantitative analysis of the extent of extracapsular invasion and its prognostic significance: a prospective study of 170 cases of carcinoma of the larynx and hypopharynx. Head Neck. 1998;20:16–21. doi: 10.1002/(sici)1097-0347(199801)20:1<16::aid-hed3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Shingaki S, Takada M, Sasai K, Bibi R, Kobayashi T, Nomura T, Saito C. Impact of lymph node metastasis on the pattern of failure and survival in oral carcinomas. Am J Surg. 2003;185:278–284. doi: 10.1016/s0002-9610(02)01378-8. [DOI] [PubMed] [Google Scholar]
- 43.Pentenero M, Gandolfo S, Carrozzo M. Importance of tumor thickness and depth of invasion in nodal involvement and prognosis of oral squamous cell carcinoma: a review of the literature. Head Neck. 2005;27:1080–1091. doi: 10.1002/hed.20275. [DOI] [PubMed] [Google Scholar]
- 44.Umeda M, Yokoo S, Take Y, Omori A, Nakanishi K, Shimada K. Lymph node metastasis in squamous cell carcinoma of the oral cavity: correlation between histologic features and the prevalence of metastasis. Head Neck. 1992;14:263–272. doi: 10.1002/hed.2880140402. [DOI] [PubMed] [Google Scholar]
- 45.Byers RM, El-Naggar AK, Lee YY, Rao B, Fornage B, Terry NH, Sample D, Hankins P, Smith TL, Wolf PJ. Can we detect or predict the presence of occult nodal metastases in patients with squamous carcinoma of the oral tongue? Head Neck. 1998;20:138–144. doi: 10.1002/(sici)1097-0347(199803)20:2<138::aid-hed7>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 46.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281:25001–25005. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 47.Venkataraman K, Riebeling C, Bodennec J, Riezman H, Allegood JC, Sullards MC, Merrill AH, Jr, Futerman AH. Upstream of growth and differentiation factor 1 (uog1), a mammalian homolog of the yeast longevity assurance gene 1 (LAG1), regulates N-steraroyl-sphinganine (C18-(dihydro)ceramide) synthesis in a fumonisin B1-independent manner in mammalian cells. J Biol Chem. 2002;277:35642–35649. doi: 10.1074/jbc.M205211200. [DOI] [PubMed] [Google Scholar]
- 48.Ogretmen B. Sphingolipids in cancer: regulation of pathogenesis and therapy. FEBS Lett. 2006;580:5467–5476. doi: 10.1016/j.febslet.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 49.Segui B, Andrieu-Abadie N, Jaffrezou JP, Benoist H, Levade T. Sphingolipids as modulators of cancer cell death: potential therapeutic targets. Biochim Biophys Acta. 2006;1758:2104–2120. doi: 10.1016/j.bbamem.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 50.Spiegel S, Milstien S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem Soc Trans. 2003;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- 51.Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282:10922–10934. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- 52.Bassi R, Anelli V, Giussani P, Tettamanti G, Viani P, Riboni L. Sphingosine-1-phosphate is released by cerebral astrocytes in response to bFGF and induces astrocyte proliferation through Gi-protein-coupled receptors. Glia. 2006;53:621–630. doi: 10.1002/glia.20324. [DOI] [PubMed] [Google Scholar]
- 53.Sabbadini RA. Targeting sphingosine-1-phosphate for cancer therapy. Br J Cancer. 2006;95:1131–1135. doi: 10.1038/sj.bjc.6603400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gomez-Munoz A, Kong JY, Parhar K, Wang SW, Gangoiti P, Gonzales M, Eivemark S, Salh B, Duronio V, Steinbrecher UP. Ceramide-1-phosphate promotes cell survival through activation of the phosphatidylinositol 3-kinase/protein B pathway. FEBS Lett. 2005;579:3744–3750. doi: 10.1016/j.febslet.2005.05.067. [DOI] [PubMed] [Google Scholar]
- 55.Mitra P, Maceyka M, Payne SG, Lamour N, Milstien S, Chalfant CE, Spiegel S. Ceramide kinase regulates growth and survival of A549 human lung adenocarcinoma cells. FEBS Lett. 2007;581:735–740. doi: 10.1016/j.febslet.2007.01.041. [DOI] [PubMed] [Google Scholar]


