Abstract
We previously reported that intravenous delivery of the human tissue kallikrein (HK) gene reduced blood pressure and plasma insulin levels in fructose-induced hypertensive rats with insulin resistance. In the current study, we evaluated the potential of a recombinant adeno-associated viral vector expressing the HK cDNA (rAAV·HK) as a sole, long term therapy to correct insulin resistance and prevent renal damage in streptozotocin-induced type-2 diabetic rats. Administration of streptozotocin in conjunction with a high fat diet induced systemic hypertension, diabetes and renal damage in rats. Delivery of rAAV·HK resulted in a long-term reduction in blood pressure, and fasting plasma insulin was significantly lower in the rAAV·HK group than in the control group. The expression of PI3-kinase p110 catalytic subunit, and the levels of phosphorylation at residue Thr-308 of Akt, insulin receptor B and AMP-activated protein kinases (AMPK) were significantly decreased in organs from diabetic animals. These changes were significantly attenuated following rAAV-mediated HK gene therapy. Moreover, rAAV·HK significantly decreased urinary microalbumin excretion, improved creatinine clearance and increased urinary osmolarity. HK gene therapy also attenuated diabetic renal damage as assessed by histology. Together, these findings demonstrate that rAAV·HK delivery can efficiently attenuate hypertension, insulin resistance and diabetic nephropathy in streptozotocin-induced diabetic rats.
Keywords: recombinant adeno-associated virus, human tissue kallikrein, diabetic nephropathy, type 2 diabetes, insulin resistance, gene therapy
INTRODUCTION
Type 2 diabetes is a worldwide epidemic associated with significant morbidity and mortality. People with diabetes have higher all-cause mortality rates than those without diabetes, mainly attributable to cardiovascular causes (1, 2). The pathogenesis of type 2 diabetes includes reductions in insulin sensitivity and Beta-cell function. Insulin resistance may play a major role in type 2 diabetes, especially when glucose tolerance is still normal (3).
Insulin resistance in animal and humans is characterized by decreased rates of insulin-mediated glucose uptake and glycogen synthesis in peripheral tissues including skeletal muscle, liver and fat. It has been previously demonstrated that bradykinin increases insulin sensitivity and stimulates glucose uptake both in vivo and in vitro (4). Isami et al reported that bradykinin increased insulin-induced glucose uptake, stimulated insulin-induced translocation of GLUT4, and potentiated insulin-induced phosphorylation of the insulin receptor and insulin receptor substrate-1 (IRS-1) in isolated dog adipocytes (5). Tissue kallikrein is a serine protease that converts kininogen to the peptide hormone bradykinin. A reduction of renal kallikrein has been found in non-insulin-treated diabetic individuals, suggesting that an impaired kallikrein-kinin system (KKS) may be involved in the development of diabetes (6).
Our previous study demonstrated that intravenous delivery of the pcDNA3.1 vector carrying the human tissue kallikrein (HK) cDNA reduced blood pressure and plasma insulin levels in fructose-induced hypertensive rats with diabetes (7). Recently, Montanari et al., (8) used an adenovirus vector to deliver the HK gene to diabetic rats to further support our findings (7). Together, these data suggest the tissue kallikrein may be an effective gene for the treatment of insulin-resistance in type 2 diabetes. Plasmid or adenovirus vectors mediate only transient gene expression and produce short-term therapeutic effects (∼3 to 4 weeks), and the later is toxic and immunogenic. Because of these complications, neither is suitable for gene therapy of a chronic disease such as insulin-resistant diabetes. Comparatively, recombinant adeno-associated virus (rAAV) vectors possess numerous advantages, including stable and long-term transgene expression, the ability to infect both dividing and non-dividing cells (such as muscle and liver), non-pathogenecity and low vector toxicity. Recently, the establishment of adenovirus-independent package and replication systems and the improvement of carrying capacity (9-11) make rAAV the most attractive vector for long-term gene therapy.
The objectives in the current study were: 1) to confirm that tissue kallikrein gene delivery attenuates insulin resistance in streptozotocin-induced diabetes; 2) to determine if HK gene delivery attenuates the kidney complications associated with diabetes; and 3) to determine the impact of rAAV·HK gene therapy on the signaling mechanisms involved in diabetic pathology. To address these issues, we have evaluated the effectiveness of HK gene-expressing rAAV as a sole therapy to correct insulin resistance and to prevent renal complications in type 2 diabetic rats.
METHODS
Materials
Horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG were obtained from Jackson Immuno Research Laboratories (Soham, Cambridgeshire, UK). Enhanced chemiluminescence reagent was purchased from Pierce Chemical (Rockford, IL), prestained molecular weight standards from Bio-Rad (Hercules, CA), and PVDF membranes from Schleicher and Schuell (Dassel, Germany). Akt (p-Thr-308) phosphorylation specific polyclonal antibody was purchased from AnaSpec, Inc (San Jose, CA). Polyclonal antibodies against (PI3-kinase), Akt, p42/p44 MAPK and phosphorylated MAPK (p-MAPK), and monoclonal antibodies against Bax and Bcl-2 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). AMP-activated protein kinases (AMPK) and phospharylatedd AMPK (p-AMPK), insulin receptor beta (IR-β) and p-IR-β were from Cell Signaling Technology Inc. (Danvers, MA). All other chemicals and reagents were from Sigma-Aldrich (St. Louis, MO) unless otherwise specified.
Construction and preparation of rAAV
The rAAV vector plasmid pXXUF1 and rAAV plasmid containing the reporter gene, pdxII-lacZ, were from Dr. Xiao Xiao. The pXXUF1 contains 2 inverted terminal repeats, a cytomegalovirus (CMV) promoter and a poly-A tail. The rAAV packaging plasmids were constructed based on plasmid pAAV/Ad as we reported previously (9, 12). Packaging plasmid pXX2 (for serotype 2 of adeno-associated virus) was constructed from pACG2 by inserting a promoter p5 element downstream of the capsid gene. The adenovirus helper plasmid pXX6 was constructed by inserting the large ClaI/SalI fragment of pXX5 into plasmid pBluescript KS. A 860-base pair human tissue kallikrein (HK) cDNA fragment (Not I/Not I) containing the open reading frame (NM 002257) was subcloned into pXXUF1 downstream of the CMV promoter to create the plasmid pUF1·HK. rAAVs containing either LacZ or HK genes were prepared and purified as described previously (13, 14) and the rAAV titers were determined by dot blot hybridization. The resultant rAAV vectors were designated rAAV·LacZ and rAAV·HK, respectively.
Animal treatment
Animals treatment has been described in detail elsewhere (15). Briefly, two month old male Wistar rats weighing 180−200 g were supplied from the Experimental Animal Center in Shanghai, China. Experimental protocols complied with National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Academy of Sciences of China. All animals were housed (four per cage) in a temperature (25±3°C) and humidity (50%±20%)-controlled room with 12-hour light/dark cycles and were allowed free access to normal rat chow (fat 9%, protein 20%, starch 53%, fiber 5%) plus water for 1 week to obtain baseline blood pressure and blood samples. The animals were then divided randomly into two groups. Normal rats (n=16) were fed normal rat chow and remained untreated for the duration of the study. Experimental rats (n=32) were fed a diet enriched in fat (25% fat, 15% protein, 51% starch and 5% fiber) as previously described (16). After 1 month on their respective diets, experimental rats were injected intravenously with streptozotocin 25 mg/kg body weight and normal rats were injected with vehicle (0.05 mol/L citric acid, pH 4.5). Both the low dose of STZ and the high fat diet are essential elements of the model designed to induce type 2 diabetes with insulin resistance. After 1 month, rats were fasted overnight and given 20% glucose 3g/kg body weight. Blood samples were taken from the tail vein to measure glucose and insulin levels at 2 hrs and 12 hrs after glucose administration. Only rats with increased postprandial glucose (>200mg/dl) were considered diabetic and were randomly divided into 2 groups: rAAV·HK treatment (HK group) and rAAV·lacZ treatment (LacZ group) (n=16 rats in each group).
Gene delivery
Diabetic animals were first anesthetized with pentobarbital at a dose of 50 mg/kg body weight intraperitoneally. A single intravenous injection of rAAV·HK or rAAV·LacZ (1×1011 viron particles in 1 ml of saline solution) was then given into the via tail vein of diabetic rats. This dose has previously been shown to result in persistent HK gene expression for up to 19 weeks (13). After the injection, the rats were kept warm under an infrared lamp until they returned to consciousness. All animals were sacrificed 12 weeks after rAAV gene delivery under pentobarbital anesthesia (50 mg/kg body wt) and heart, kidney, skeletal muscle, liver, lung and fat were collected, frozen on liquid nitrogen and stored at −80°C until further analyses were done.
Systolic blood pressure measurements
After rAAV injections, systolic blood pressure was measured every 2 weeks in conscious rats with a manometer-tachometer (Rat Tail NIBP system, ADI Instruments) by means of the tail-cuff method (17). The rats were minimally warmed (usually 15 minutes) before blood pressure determination and after a brief period of restraint in a plastic cage. For each animal, systolic blood pressure was taken as the mean of five daily recordings. All measurements were made between 9:00AM and noon.
Plasma and urine analysis
Blood samples were taken after an overnight fast from the tail vein immediately before injection of rAAV and at the end of the experiment. Plasma was prepared and stored at −80°C. Every two weeks, 24-hour urine samples were collected in metabolic cages into brown bottles containing 500μl toluene to prevent decay. Plasma glucose, cholesterol, triglyceride, creatinine and urine creatinine were measured in duplicate on an AEROSET Clinical Chemistry System (Abbott Laboratories). Plasma insulin was measured with the use of a magnetic solid phase enzyme immunoassay kit from BioChem ImmunoSystem. Insulin resistance was calculated by means of the hemostasis model assessment (HOMA IR) method (18), which has been used previously in rodents (19-21). Urine osmolarity was measured on a model 3D3 osmometer (Advanced Instruments, Inc). Urinary microalbumin was measured to estimate albumin excretion rate (AER) by ELISA method with a kit from Shanghai Debo Bbiotechnology Inc. Creatinine clearance (Ccr) was calculated according to the method of Cockcroft and Gault (22).
ELISA specific for human tissue kallikrein
ELISA reagents specific for human tissue kallikrein are a gift from Dr. Lee Chao (Medical University of South Carolina). ELISA for HK in animal urine samples was performed according to previously described methods (23, 24). The plates were read at 405 nm on Elx 800 ELISA reader (Bio-tek instruments, INC).
RT-PCR analysis of human tissue kallikrein mRNA
Total RNA was extracted from frozen rat lung, liver, heart, kidney, skeletal muscle and fat using TRIZOL reagent (Invitrogen Life Technologies). RT-PCR analysis specific for HK mRNA utilized the following primers: 5' primer, 5'-CATTTCAGCACTTTCCA-3' ; 3' primer, 5'-GCCACAAGGGACGTAGC-3'); RT-PCR analysis for β-actin housekeeping gene use the following primers: 5` primer, 5'-GGAGAAGATGACCCAGATC-3', 3' primer, 5'-GATCTTCATGAGGTAGTCAG-3'). The RT-PCR method was performed according to manufacturer's instructions using an RT-PCR kit from TAKARA Biotechnology Co. Ltd. After incubation with Moloney murine leukemia virus reverse transcriptase, amplification was performed with reagents from Applied Biosystems under the following conditions: denaturing phase of 1 min at 94°C, annealing phase of 40s at 65°C, and extension phase of 1 min at 72°C for 20 cycles. PCR products were electrophoresed on 1.5% agarose gels. The quantity of specific HK transcripts was normalized to the expression of β-actin to control for RNA quality and amount.
Analysis of renal morphology and collagen deposition
A portion of the kidney from each animal was preserved in 4% PBS-buffered formaldehyde solution and embedded in paraffin. Four 3-μm paraffin sections were randomly chosen from one kidney from each animal and stained with periodic acid Schiff reagent (PAS) or Masson's trichrome to evaluate morphologic changes, and with Sirius red using the method described previously to evaluate collagen deposition (25). In each section, six fields of kidney cortex were randomly selected at a magnification of 40X using an Olympus BH-2 light microscope (Olympus, Tokyo, Japan). Extracellular matrix (ECM) production was quantified as the ratio of collagen to non-collagen area (percentage of ECM) with the use of HAIPS pathologic image analysis system. All sections were evaluated by investigators who were blinded to treatment group assignment.
Apoptosis assays
Apoptotic cells were detected using a TUNEL assay on paraffin-embedded sections using a commercially available kit for detecting end-labeled DNA (RD Systems, Inc., Minneapolis, MN) as described previously (26). All experiments were repeated at least three times for each animal kidney. Double-stranded DNA in nuclei was counterstained after TUNEL staining with methyl green. TUNEL-positive cells per high-power field were counted in 10 random high power fields (HPFs) from each of 5 sections, and the counts were averaged as the apoptotic index (AI).
Western blotting
Proteins for western blotting were obtained from skeletal muscle and liver using TRIZOL reagent. Protein concentrations were estimated by the Bradford method (27). Equal amounts of protein sample were separated by electrophoresis on 10% SDS/PAGE gels. The resolved proteins were electrophoretically transferred to PVDF membranes using a transfer buffer containing 192 mmol/L glycine, 20% (vol/vol) methanol and 0.02% SDS. The membranes were incubated in 10mmol/L Tris-Cl (pH 7.5), 100mmol/L NaCl, and 0.1% Tween-20 (TBS-T) with 5% nonfat dried milk for 2 hr at room temperature, and then incubated overnight at 4°C with the indicated primary antibodies. Immunoreactive proteins were visualized by enhanced chemiluminescence using horseradish peroxidase–labeled anti-rabbit or anti-mouse IgG (Amersham Life Science) and quantified by densitometric analysis using Rio image system (Syngene, a Division of SYNOPTIC, Ltd).
Statistical Analysis
Results are expressed as mean±SEM. Data were analyzed by either the unpaired Student's t test or ANOVA, with the use of SYSTAT software (SYSTAT Inc). Values were considered significantly different if P<0.05.
RESULTS
Streptozotocin and high fat diet induce diabetes in rats
Table 1 shows the biochemical and physiological parameters in streptozotocin treated rats on a high fat diet and normal rats. There were no significant differences in systolic blood pressure, body weight, glucose, insulin, triglycerides, cholesterol and the insulin resistance index assessed by homeostasis model assessment (HOMA IR) between the two groups at baseline. After streptozotocin injection and high fat feeding, experimental rats displayed lower body weight (P < 0.05), higher systolic blood pressure (P < 0.01), higher serum post-prandial glucose (P < 0.01), higher fasting insulin levels (P < 0.01), higher triglycerides, and higher cholesterol compared to nondiabetic control rats. HOMA IR was significantly increased in experimental rats compared to nondiabetic controls. These data validate the use of streptozotocin and high fat diet to induce the metabolic abnormalities characteristic of type 2 diabetes in rats.
Table 1.
Physiological parameters in normal and diabetic rats following induction of Type 2 diabetes but preceding HK gene delivery
| Nondiabetic Normal Rats(n=16) | Diabetic Rats (n=32) | |||
|---|---|---|---|---|
| Baseline | Week 8 | Baseline | Week 8 | |
| Body weight (g) | 216.1±14.8 | 258.7±20.1 | 214.9±15.7 | 233.6±17.9* |
| Systolic pressure (mmHg) | 94.5±4.4 | 94.5±3.5 | 95±5.1 | 124.1±3.9* |
| Fasting glucose (mmol/L) | 5.43±0.68 | 4.61±0.82 | 5.49±0.74 | 3.94±0.56 |
| 2h glucose (mmol/L) | 6.77±1.02 | 15.66±2.41* | ||
| Fasting insulin (mlU/L) | 2.92±0.72 | 3.44±0.88 | 2.81±0.82 | 10.12±2.47* |
| Triglycerides (mmol/L) | 1.04±0.21 | 1.18±0.52 | 1.08±0.30 | 2.11±0.48* |
| Total cholesterol (mmol/L) | 1.36±0.17 | 1.27±0.15 | 1.40±0.16 | 2.61±0.44* |
| HOMA IR | 0.71±0.02 | 0.71±0.03 | 0.68±0.03 | 1.77±0.06* |
Values are mean±SEM. Normal rats were fed with normal rat chow (Normal). Diabetic rats received streptozotocin injection and high fat diet (Diabetes). HOMA IR indicates homeostasis model assessment of insulin resistance.
P<0.05 vs. normal.
rAAV·HK induces in vivo expression of human tissue kallikrein
We analyzed HK mRNA levels in rats after rAAV gene delivery by RT-PCR. Total RNA was prepared from lung, liver, heart, kidney, skeletal muscle and fat 16 weeks after intravenous injection of rAAV·HK. HK mRNA was detected in lung, liver, heart, kidney and skeletal muscle in rAAV-HK-treated rats, but not in rAAV·lacZ-treated diabetic rats (Figure 1A). Equivalent levels of β-actin mRNA were detected in tissues of HK and lacZ groups. We also observed abundant HK protein expression in rAAV·HK treated rats compared to rAAV·LacZ treated rats, indicating that the increase in HK mRNA levels is accompanied by abundant HK protein expression (Figure 1B). Immunoreactivity observed in rAAV·LacZ lanes is likely cross-reactivity of our antibodies with endogenous rat tissue kallikrein. Together, these results indicate that HK is stably expressed in multiple tissues related to cardiovascular and renal function after rAAV·HK gene transfer in rats.
Figure 1.
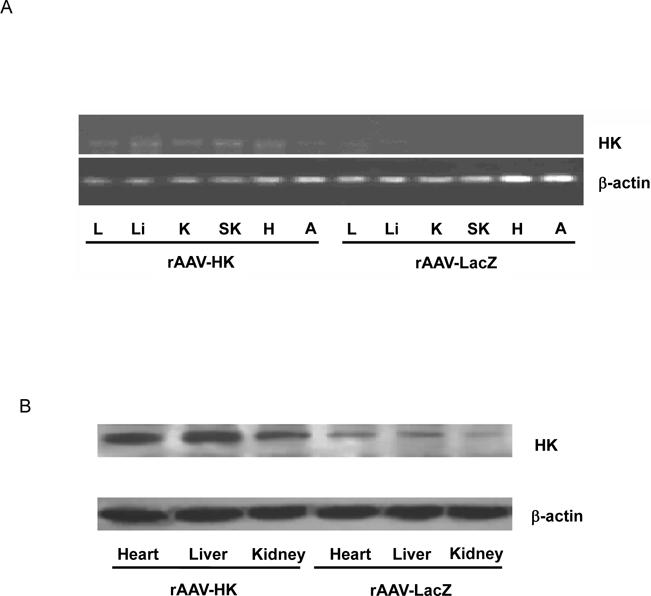
Expression of kallikrein. Panel A: RT-PCR analysis in diabetic rats treated with rAAV·HK (HK) or rAAV·LacZ (LacZ). Total RNA was prepared from lung (L), liver (Li), kidney (K), skeletal muscle (SK), heart (H), and adipose tissue (A). Panel B: Representative immunoblotting results showing increased HK expression in heart, kidney and liver of rAAV·HK treated diabetic rats compared to rAAV·LacZ-treated diabetic rats. Experiments were repeated 3 or 4 times.
We also measured kallikrein protein levels in urine by ELISA. Low levels of immunoreactive kallikrein were detected in the urine of saline treated nondiabetic normal control rats and rAAV·lacZ treated diabetic animals. In contrast, in rAAV·HK-treated diabetic animals, the urinary level of immunoreactive kallikrein was 1.91±0.73 ng/ml 2 weeks after injection and was maintained at high levels (1.92± 0.49 ng/ml) throughout the duration of the experiment, which significantly higher than those in nondiabetic normal control and rAAV·lacZ treated diabetic rats (Figure 2). These results demonstrate that rAAV-mediated gene transfer achieves HK gene expression in vivo for an extended period of time.
Figure 2.
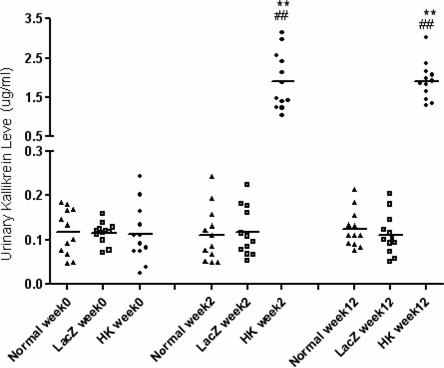
Urinary expression of kallikrein by ELISA in non-diabetic rats (Normal), diabetic rats treated with rAAV·LacZ (LacZ) and diabetic rats treated with rAAV·HK (HK) at various time points after treatment. Values are mean ± SEM (n=6 for each group); **P<0.01 vs. Normal; ## P<0.01 vs. LacZ.
rAAV·HK reduces blood pressure in diabetic rats
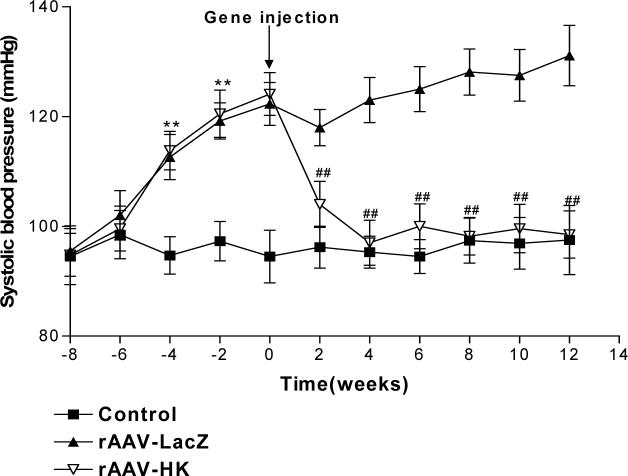
Blood pressure was measured at baseline, after treatment with streptozotocin and high fat diet, and after injection of saline, rAAV·LacZ and rAAV·HK. The results shown in Figure 3 indicate that systolic blood pressure was significantly higher in diabetic rats than in control rats. Moreover, rAAV·HK treatment significantly reduced systolic blood pressure within 2 weeks of injection and normalized systolic blood pressure from the 4th week post-injection until the end of the experiment (16 weeks). In contrast, the rAAV·LacZ-treated animals remained significantly hypertensive throughout the study period (Figure 3). These results indicate that rAAV·HK gene delivery induce a sustained reduction in blood pressure in diabetic rats.
Figure 3.
Systolic blood pressure in non-diabetic rats injected with saline (Normal), diabetic rats injected with rAAV·LacZ (LacZ) and diabetic rats injected with rAAV·HK (HK). Values are mean ± SEM (n=16 per group); **P<0.01 vs. Normal, ##P<0.01 vs. LacZ.
rAAV·HK attenuates diabetic nephropathy
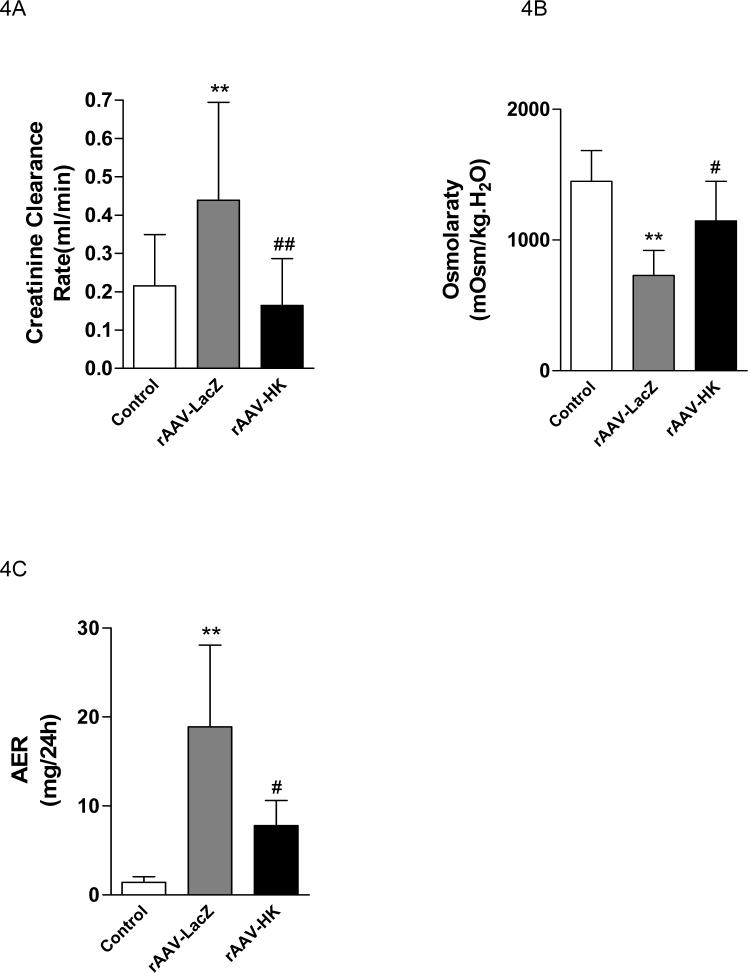
Biochemical tests indicated that creatinine clearance (Ccr) was increased in the rAAV·LacZ group compared to normal consistent with enhanced glomerular filtration with streptozotocin treatment. Treatment with rAAV·HK significantly prevented the increase in Ccr (Figure 4A). In addition, rAAV·LacZ injected streptozotocin-treated rats had reduced urine osmolarity, suggesting renal tubular damage. In contrast, urine osmolarity was not significantly different from normal in rAAV·HK treated rats (Figure 4B). Moreover, urinary microalbumin levels were significantly decreased in the rAAV·HK group compared with the rAAV·LacZ group (Figure 4C). Together, these results suggest that HK gene delivery significantly preserves renal glomerular and tubular functions after induction of diabetes in rats.
Figure 4.
Creatinine clearance (Panel A), urine osmolarity (Panel B) and urinary microalbumin (Panel C) in non-diabetic rats injected with saline (Normal), diabetic rats injected with rAAV·LacZ (LacZ) and diabetic rats injected with rAAV·HK (HK). Values are mean ± SEM (n= 6 for each group); #P<0.05 vs. LacZ; ##P<0.01 vs. LacZ; ** P<0.01 vs. Normal.
Effect of rAAV·HK on renal histology
Histological analysis of kidneys revealed moderate mesangial cell proliferation and extracellular matrix (ECM) accumulation in rats with streptozotocin-induced diabetes (Figure 5B, 5J). Severe interstitial lymphocyte infiltration, renal tubular vacuolation, protein casts in renal tubules, and hyaline caps were observed in rats with diabetes (Figure 5D, 5G). Moderate collagen deposition and blood capillary thrombosis was also observed in the diabetic rats. In contrast, rats treated with rAAV·HK displayed only mild mesangial cell proliferation, a small degree of interstitial lymphocyte infiltration, and nearly normal renal tubular morphology (Figure 5C, 5E, 5H, 5K). To quantify collagen accumulation in kidneys, red-staining ECM was expressed as a percentage of the total tissue area and was used as an index of fibrosis. There was a significant difference in ECM accumulation between the rAAV·LacZ group (0.277 ± 0.069) and the HK group (0.209 ± 0.032) (p<0.01). Together, these results suggest that HK gene delivery attenuates the renal injury associated with diabetes.
Figure 5.
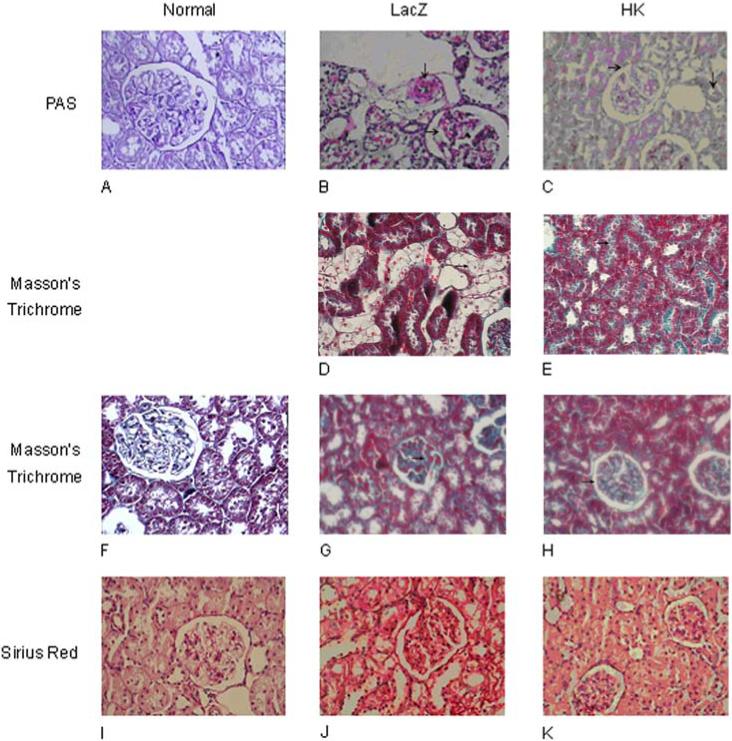
Histological assessment of rat kidneys. Paraffin sections of kidney cortex from non-diabetic rats (Panels A, F, I) or from diabetic rats treated with rAAV·HK (Panels C, E, H, K) or rAAV·LacZ (Panels B, D, G, J) stained with periodic acid Schiff (PAS) (Panels A, B, C), Masson' s trichrome (Panels D, E, F, G, H) or Sirius red reagent (Panels I, J, K). Magnification was 400X (Panels A-C, F-K) or 200X (Panels D, E). PAS staining shows that rAAV·LacZ-treated rats had moderate mesangial cell proliferation (→) with severe blood vessel narrowing (↓) (Panel B), and rAAV·HK-treated rats had minimal glomerulosclerosis (→) (Panel C), much milder than rAAV·LacZ-treated diabetic rats (B) and nearly normal blood vessels (A). Masson's trichrome staining shows renal tubule vacuolation (→) in rAAV·LacZ treated rats (Panel D) but almost normal renal tubules (→) in rAAV·HK treated rats (Panel E). Masson's trichrome staining also showed significant capillary thrombosis (→) in rAAV·LacZ treated diabetic rats (Panel G), but minimal capillary thrombosis (→) in rAAV·HK treated diabetic rats (Panel H). Sirius Red staining shows rAAV·LacZ treated diabetic kidneys with moderate collagen deposition (Panel J), but only mild collagen deposition in kidneys from rAAV·HK treated diabetic rats (Panel K). One section from each experimental animal was prepared and representative slices are shown.
Kallikrein decreases hyperinsulinemia and insulin resistance
Fasting glucose and insulin levels were measured prior to rAAV·LacZ and rAAV·HK injections and at the end of the study. Fasting glucose was significantly higher in both the rAAV·HK group and the rAAV·LacZ group compared with the control group at the end of study, indicating that kallikrein expression did not alter this parameter (Table 2). However, fasting insulin levels were significantly lower in the rAAV·HK group than the rAAV·LacZ group despite lack of an observed change in fasting glucose (Table 2). These findings suggest that less insulin is required to maintain the same glucose level in the HK group than the LacZ group. Consistent with this data, insulin resistance (as measured via HOMA IR) was significantly decreased in the rAAV·HK group compared with the rAAV·LacZ group (Table 2). We also found that HK gene delivery lowers circulating levels of triglycerides and cholesterol, important indicators of cardiovascular risk. Together, these data indicate that HK decreases hyperinsulinemia and insulin resistance, and improves lipid profiles in streptozotocin-induced diabetic rats.
Table 2.
Physiological parameters in nondiabetic normal, diabetic-LacZ and diabetic-HK rats 12 weeks after introduction of rAAV-HK and rAAV-LacZ.
| nondiabetic normal | diabetic-HK | diabetic-LacZ | |
|---|---|---|---|
| Weight (g) | 285.7±11.2 | 253.2±9.7 | 242.5±8.1 |
| Systolic pressure (mmHg) | 97.5±3.8 | 98.5±5.5## | 131.1±6.3** |
| Glucose (mmol/L) | 5.51±0.79 | 13.09±3.01** | 13.58±2.88** |
| Insulin (mIU/L) | 3.56±0.69 | 8.19±2.45## | 13.85±3.76** |
| HOMA IR | 0.87±0.02 | 4.76±0.33## | 8.36±0.48** |
| Triglyceride (mmol/L) | 1.10±0.47 | 1.54±0.66## | 2.38±0.89** |
| Total cholesterol (mmol/L) | 1.36±0.55 | 1.48±0.62## | 2.55±1.01** |
Values are mean±SEM for 16 rats per group. Plasma glucose, insulin, triglyceride and total cholesterol were measured after an overnight fast at the end of experiment. Nondiabetic normal rats were injected with saline, diabetic rats were injected with rAAV·LacZ (LacZ) or rAAV·HK (HK). HOMA IR indicates homeostasis model assessment of insulin resistance.
P<0.01 vs. LacZ.
P<0.01 vs. Normal.
Effects of kallikrein on the PI3-kinase/Akt and AMPK signaling pathways
PI3-kinase is recruited to insulin-receptor-substrate (IRS) signaling complexes through binding of SH2 domains in its 85-kDa regulatory subunit to specific phosphotyrosine residues in IRS (28). This process leads to activation of the PI3-kinase p110 catalytic subunit. To begin to investigate the signaling mechanisms through which HK attenuates insulin resistance, we evaluated the expression of PI3-kinase and Akt associated with the insulin signaling cascade in kidney, liver and skeletal muscle. PI3-kinase p110 catalytic subunit expression was determined by immunoblotting with p110-specific antibodies. Results showed that PI3-kinase p110 catalytic subunit expression was significantly decreased in kidneys from rAAV·LacZ treated rats compared with nondiabetic controls. In contrast, rAAV·HK treatment increased p110 expression to normal levels (Figure 6A, 6B). Similarly, rAAV·HK treatment restored partially PI3 kinase levels in liver and skeletal muscle compared with rAAV·LacZ-treated diabetic rats (Figure 6C, 6D).
Figure 6.
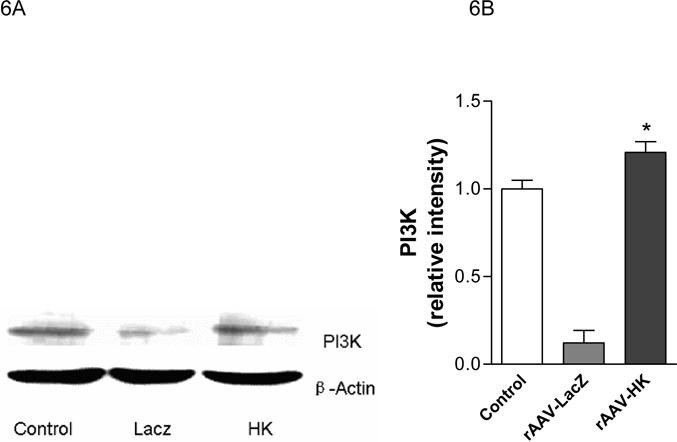
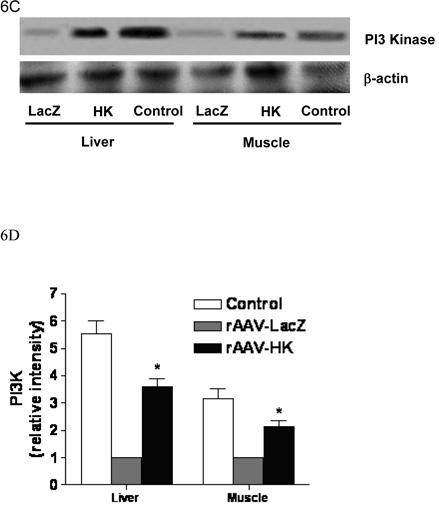
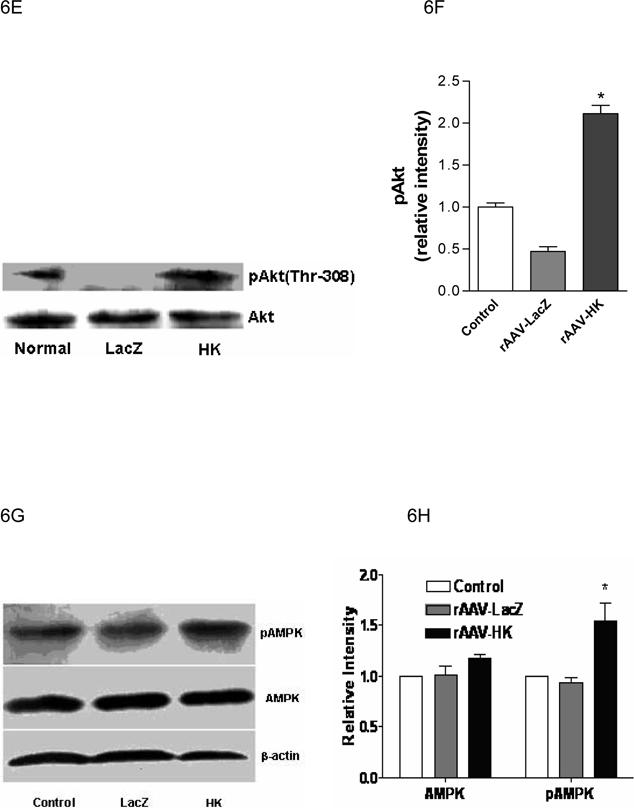
Representative western blots and densitometric quantification of at least three repeats for each experiment showing the effects of rAAV-mediated HK gene therapy on PI3 kinase/Akt and pAMPK signaling pathways. Effects of HK gene therapy on the expression of PI3-kinase p110 catalytic subunit in kidney (Panels A and B), liver and skeletal muscle (Panels C and D). Effects of HK gene therapy on total and p-Akt at Thr-308 in kidney (Panels E and F). Effects of HK gene therapy on total and p-AMPK in kidney (Panels G and H). Blots were scanned, and relative expression levels were normalized to total β-actin. Values shown are the means ± S.E. of three independent experiments. * p < 0.05 versus rAAV-LacZ.
The expression of Akt, a serine/threonine kinase that lies downstream of PI3-kinase, was also assessed in kidneys of the experimental animals. Because phosphorylation of Akt is required for its activation (29), levels of pAkt at position Thr-308 were determined by immunoblotting with a phosphospecific anti-Akt antibody. A significant decrease of pAkt was noted in kidneys of diabetic rats treated with rAAV·LacZ compared with normal control animals, but rAAV·HK treatment restored pAkt to control levels (Figure 6E, 6F). There were no changes in total Akt levels between the groups. Together, these data indicate that reduced insulin resistance in rAAV·HK treated rats was associated with partial activation of the PI3-kinase/Akt signaling pathway.
We also probed the phosphorylation state of AMP-activated protein kinase (AMPK). We found that AMPK phosphorylation was not downregulated in our model of type 2 diabetes. However, rAAV-HK treatment enhanced phosphorylation of AMPK while total level of AMPK was not changed by rAAV-HK treatment (Figure 6G, 6H).
Effects of kallikrein on activity of p42/44 MAPK and IR-β
The p42/44 mitogen activated protein kinases (MAPK) are important signaling molecules activated during injury in multiple cells and tissues. We found that in rAAV·LacZ treated diabetic rats, phosphorylated p42/p44 MAPK (p-MAPK) levels were markedly reduced in kidney relative to control rats. Importantly, rAAV·HK therapy returned p-MAPK to normal levels (Figure 7A,7B). We also investigated insulin receptor-β (IR-β) expression and activity in kidneys of normal, rAAV·LacZ, and rAAV·HK treated rats. Figures 7C and 7D show that IR-β expression was comparable in all groups; however, the extent of IR-β phosphorylation was reduced in rAAV·LacZ treated diabetic rats. In contrast, rAAV·HK treatment restored IR-β phosphorylation to normal levels. Together, these results suggest that reversal of diabetic pathology in rAAV·HK treated rats is associated with activation of both p42/p44 MAPK and IR-β.
Figure 7.
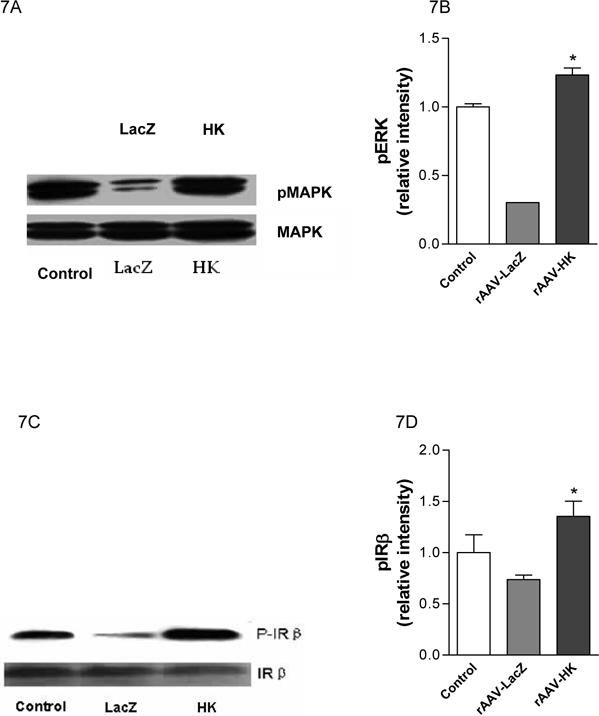
Representative western blots and densitometric quantification of at least three experiments showing effects of rAAV-mediated HK gene therapy on phosphorylation of p42/44 MAPK and IR-β. Results show decreased phosphorylation of MAPK in diabetic kidney is reversed by HK overexpression (Panels A and B) and that decreased phosphorylation of IR-β in diabetic treated rats is also reversed by rAAV·HK treatment (Panels C and D). Results are representative of 3 independent experiments. Blots were scanned and relative p-ERK (B) and p-IR-β levels (D) were normalized to total ERK and total IR-β, respectively. Values shown are the means ± S.E. of three independent experiments. *, p < 0.05 versus rAAV-LacZ.
Kallikrein decreases renal apoptosis in diabetic rats
Tubular and interstitial cell apoptosis is known to occur in streptozotocin-induced diabetic rats (30, 31). Hence, we examined the effects of rAAV·HK treatment on renal apoptosis and expression of apoptosis-related genes in diabetic rats. Consistent with prior literature, we found that the number of apoptotic cells (as assessed by Tunnel staining) in kidneys from rAAV·LacZ treated diabetic rats was significantly increased compared to control rats (Figure 8A, 8B). Apoptotic cells were observed in the renal tubules but not in the glomerulus in diabetic rats. Interestingly, rAAV·HK treatment significantly attenuated this effect (Figure 8A, 8B).
Figure 8.
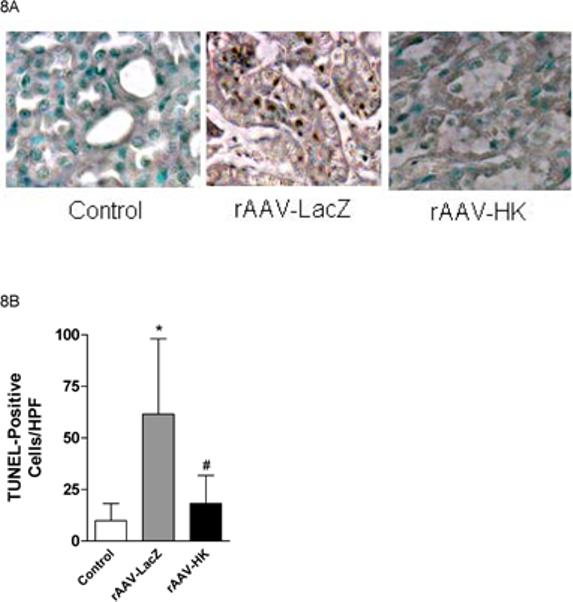
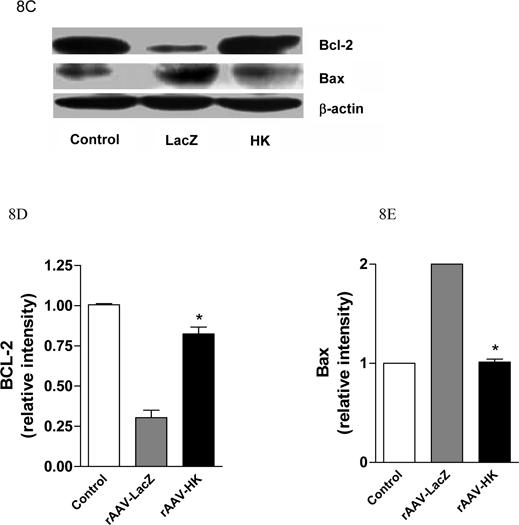
Effects of kallikrein on renal apoptosis. TUNEL staining of kidney showing significantly increased TUNEL-positive cells in diabetic rat renal tubules from rAAV-LacZ rats compared with non-diabetic controls and rAAV-HK-treated rats (Panel A). The number of TUNEL staining apoptotic cells/HPF is quantified, *p<0.01 vs. Normal Control; #p<0.01 vs. rAAV-LacZ (Panel B). Representative western blots showing expression of anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bax in kidney (Panel C). Blots were scanned and Bcl-2 and Bax levels (D and E, respectively) were normalized to β-actin. Values shown are the means ± S.E. of three independent experiments. *, p < 0.05 versus rAAV-LacZ.
Apoptosis-related protein levels were assessed using Western blotting. Levels of the anti-apoptotic protein Bcl-2 was decreased while levels of the pro-apoptotic protein Bax was increased in rAAV·LacZ treated diabetic rats (Figure 8C, 8D, 8E). Interestingly, rAAV·HK treatment reversed these changes (Figure 8C). Together, these results indicate renal tubular apoptosis is an important component of diabetic nephropathy and that this is attenuated by HK treatment.
DISCUSSION
In our previous studies, intravenous delivery of a plasmid carrying the human kallikrein cDNA was found to reduce blood pressure and reverse hyperinsulinemia in fructose-induced hypertensive rats. However, these effects persisted for only 3−4 weeks, likely due to the transient nature of the pcDNA3.1-HK expression system. In the current study, we delivered the HK cDNA in a rAAV-based vector to type 2 diabetic rats induced by streptozotocin and high fat diet. A single injection of rAAV·HK resulted in persistent and stable expression of HK, and caused significant reductions in blood pressure and insulin resistance in diabetic rats for up to 12 weeks. In contrast, injection of the control rAAV·LacZ did not affect blood pressure or reduce circulating insulin levels in diabetic rats. We also found that HK gene therapy may elicit these changes by altering a number of signal transduction pathways associated with diabetes. Indeed, mechanistic studies revealed an enhancement in the expression of PI3-kinase and increased phosphorylation of Akt suggesting activation of the PI3-kinase/protein kinase B (Akt) pathway in kidney, liver and skeletal muscle. Moreover, we observed activation of MAPK and enhanced phosphorylation of IR-β in rAAV·HK treated rats.
There is considerable controversy as to the relative roles of insulin resistance and insulin secretion in the pathogenesis of type 2 diabetes mellitus. Recent evidence suggests that insulin resistance can be observed in non-diabetic first-degree relatives of patients with type 2 diabetes (32). Normal glucose tolerance is maintained in these subjects because the pancreas is able to secrete sufficient insulin to overcome the insulin resistance, and frank hyperglycemia does not occur until this compensatory response fails. Previous studies have shown that fructose-fed or high fat-fed rodents become hyperinsulinemic and hypertensive but are still able to maintain normal glucose levels (33, 34), a condition that is similar to the aforementioned “pre-diabetic state” in humans. Administration of relatively low-doses of streptozotocin to such animals leads to significant hyperglycemia, whereas the same dose does not decrease the insulin secretory capacity enough to cause hyperglycemia in the rodents that were fed with conventional chow. In this study, we used high fat feed and low dose streptozotocin injection to induce a diabetic state characterized with hyperglycemia, hyperinsulinemia, and hypertension – a state that closely resembles the metabolic abnormalities of patients with type 2 diabetes.
The majority of individuals with essential hypertension also display insulin resistance and hyperinsulinemia (35). Angiotensin-converting enzyme (ACE) inhibitors are frequently used for the treatment of high blood pressure. ACE inhibitors not only reduce blood pressure but also improve insulin action and peripheral glucose utilization, as shown in both animal studies (36) and clinical investigations (37). ACE inhibitors have at least two effects at the tissue level: 1) inhibition of the conversion of ANG I to ANG II; and 2) enhancement of bradykinin levels through inhibition of kininase II-mediated degradation of this peptide (4). There is evidence to suggest that bradykinin itself may have an effect on the enhanced insulin action and insulin signaling at the skeletal muscle level (38). Tissue kallikrein is a serine protease that converts kininogen to the peptide hormone bradykinin. Considering these data, we hypothesized that HK gene therapy would reduce blood pressure and improve insulin resistance in diabetic rats.
In the present study, we used a rAAV vector which can drive efficient and prolonged transgene expression via persistent episomal concatamers (39, 40) or by genomic integration (41, 42). High levels of HK protein in urine was detected by ELISA at 2 weeks after intravenous injection of rAAV·HK, and this level of HK protein was maintained for up to 12 weeks. Moreover, RT-PCR analysis revealed HK mRNA was abundant in heart, lung, liver, skeletal muscle, fat and kidney from rAAV·HK treated diabetic rats at 12 weeks post-injection. This suggests that rAAV mediates stable and long-term expression of the HK gene in numerous target organs in diabetic rats.
The blood pressure in rAAV·HK-injected rats was decreased within 2 weeks after initiating therapy. This is consistent with the increased urinary concentrations of HK at 2 weeks post-injection and suggests that the reduction of blood pressure was caused by the increased HK expression, as we have previously shown (13). In diabetic subjects, hypertension and hyperinsulinemia may be caused by related physiological processes, with insulin resistance being a common feature. In the present study, insulin resistance was attenuated in rats treated with rAAV·HK, although the fasting glucose levels were not significantly reduced compared with control rats.
A previous study (43) demonstrated that bradykinin administration significantly reduced fasting insulin levels by 20%, whereas fasting glucose was not significantly altered. In that study, the glucose areas under the curve (AUC) was reduced by 21% during an oral glucose tolerance test, which suggests that glucose metabolism was significantly improved. The model used by Henriksen et al. utilized chronic administration of bradykinin (twice daily for 14 consecutive days at a dose of 40 ug/kg body weight). Not even a very high level of HK gene expression can achieve high kinin levels in vivo. For example, Emanueli et al (44) used a mouse model of adenovirus mediated HK delivery (1.8×109 plaque forming units/rat) to induce high level expression of HK (350 ng/day in urine) and only observed a modest increase in urine kinin levels of 80−100 ng/day. More recently, Montanari et al. (45) reported adenovirus-mediated HK delivery that resulted in a very high expression level of HK, but only a moderate reduction in post-prandial blood glucose (575.8±28.3 to 450.8±29.9 mg/dl). Furthermore, Montanari et al. used a type 1 diabetes model which produces very high post-prandial blood glucose levels, whereas our manuscript used a type 2 diabetes model with insulin resistance that produces much lower glucose levels (13−15 mmol/l = 234−270 mg/dl). Comparatively, our rAAV mediated long-term HK gene delivery system resulted in levels of ∼2 ng/ml in urine and ∼1.2 ng/ml in serum. These differences may explain why glucose levels were not reduced by rAAV-HK therapy.
Published data consistent with our findings suggest that kallikrein and bradykinin additively increase adipocyte hexose transport under conditions of maximal intrinsic insulin stimulation and this action occurs at post-insulin binding sites (46). Miyata, et al. also reported that bradykinin infusion could increase glucose uptake in peripheral tissues (47). This effect could be explained by the ability of bradykinin to upregulate insulin receptor tyrosine kinase activity, which stimulates phosphorylation of insulin receptor substrate-1 (IRS-1), and increases glucose transporter 4 (GLUT4) translocation. However, this effect was only found when insulin was present, and not in the absence of insulin. This could also partially explain why the fasting glucose was not different between rAAV·HK and rAAV·LacZ groups.
A growing body of evidence indicates that defects in the early and intermediate steps of the insulin signaling pathway, including the IRS-1 and PI3-kinase/Akt pathways, contribute to insulin resistance in skeletal muscle of diabetic rodents and humans (38, 48). Data indicate that activation of PI3-kinase is a necessary, albeit not sufficient, step for insulin-induced glucose transport. The present study aimed to determine whether the impairment in PI3-kinase activation in diabetes could be reversed by HK gene therapy. We found that the levels of the PI3-kinase p110 catalytic subunit were significantly decreased in skeletal muscle and liver of diabetic rats and rAAV·HK treatment corrected this defect. Recent evidence indicates that the serine/threonine protein kinase B (PKB/Akt) mediates many of the downstream events controlled by PI3-kinase. Following the activation of PI3-kinase, Akt isoforms are recruited from the cytosol to the plasma membrane through interactions with PtdIns(3,4,5)P3 and/or PtdIns(3,4)P2, where they are thought to undergo a conformational change and become activated by phosphorylation at two residues. Akt isoforms are phosphorylated at the T-loop (Thr-308 in PKB α) by 3-phosphoinositide dependent protein kinase 1 (PDK1) and this phosphorylation appears to be crucial for activation of Akt (49). Buren has demonstrated that high glucose levels combined with high insulin produce impaired sensitivity to insulin stimulation of Akt activity (50). In this regard, we evaluated Akt phosphorylation on Thr-308 in skeletal muscle and liver of diabetic rats. We found that Akt phosphorylation on Thr-308 was decreased in diabetic rats, but this was enhanced by HK gene therapy. These data suggest that the improvement of insulin resistance following HK gene therapy could be caused, at least in part, by increased activation of PI3-kinase and Akt phosphorylation in skeletal muscle and liver. We found that HK gene treatment also enhanced AMPK phosphorylation, which has been shown to ameliorate insulin resistance (51).
Diabetic nephropathy occurs in ∼30% of all patients with diabetes and has become the leading cause of end-stage renal disease in the Western societies; it is also becoming more prevalent in China (52-54). Diabetic nephropathy is characterized by persistent albuminuria, elevated blood pressure, glomerular hyperfiltration at early stages and a relentless decline in the glomerular filtration rate (GFR) at later stages, as well as a high risk for cardiovascular morbidity and mortality (55). Diabetic nephropathy involves progressive injury to both the glomerulus and the tubulointerstitium that is accompanied by increased protein excretion and ultimately a decline in renal function (56). In the present study, diabetic rats developed features similar to human diabetic nephropathy, including severe glomerulosclerosis, tubulointerstitial abnormalities and albuminuria. We also found significant apoptosis in the renal tubules of diabetic rats. HK gene delivery significantly decreased urinary albumin excretion, increased urinary osmolarity and attenuated renal tubule apoptosis, possibly by modifying expression of apoptosis-related proteins Bcl-2 and Bax. Morphological studies showed that both glomerular sclerotic lesions and tubular damage were reversed by HK gene transfer. These results demonstrate that persistent expression of HK gene protects against nephropathy in diabetic rats.
The mechanisms by which kallikrein prevents renal complications in diabetic rats are poorly understood. Previous studies have shown that bradykinin is able to reduce blood pressure, attenuate endothelial cell apoptosis, increase tPA and increase MMP (57). Together, these effects may contribute to the protection of the kidney from injury induced by the diabetic state. MAPK and AMPK activity is essential for cell survival. Our study demonstrated that HK gene delivery significantly enhanced phosphorylation of renal p42/p44 MAPK and AMPK supporting the notion of a protective role of kallikrein in the kidney(58). The role of bradykinin and related products of the plasma KKS in diabetic renal damage are currently undefined. Other researchers reported that renal tissue kallikrein and kinin increased in type 1 diabetic patients at risk for developing nephropathy (59). Treatment of diabetic rats with aprotinin, a kallikrein inhibitor, reduced glomerular filtration rate and renal plasma flow, indicating that tissue kallikrein and kinins help to mediate renal hyperfiltration in diabetes (60, 61). Some published data indicated that bradykinin can attenuate vascular disease associated with diabetes when there is an intact endothelium, but could aggravate vascular disease when the endothelium is damaged (59, 62, 63). In the present study, HK gene delivery during the early period of diabetes appears to exert several protective effects in the kidney.
In summary, our studies demonstrate that rAAV-mediated HK gene delivery can efficiently attenuate insulin resistance, prevent diabetic nephropathy and reduce blood pressure in streptozotocin-induced diabetic rats. HK gene therapy may mediate these effects by restoring activation of the insulin receptor, PI3 kinase/Akt, MAPK and AMPK signaling pathways. These results suggest that rAAV-mediated HK gene administration may be a promising tool for the therapy of insulin resistance.
Acknowledgements
This work was supported by grants from National 863 Plan project (No. 2001AA217121), 973 project (No. G2000056901), National Nature Science Foundation Committee grant (No. 30470824), Wuhan City International Collaboration project (No. 997005135G) and funds from the Intramural Research Program of the National Institutes of Health, National Institutes of Environenmental Health Sciences.
REFERENCES
- 1.Gu K, Cowie C, Harris M. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971−1993. Diabetes Care. 1998;21:1138–1145. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 2.Yu YSL, Yu H, Wang C, Tang H. Insulin resistance and endothelial dysfunction in type 2 diabetes patients with or without microalbuminuria. Diabetes Res Clin Pract. 2004;65:95–104. doi: 10.1016/j.diabres.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Martin BCWJ, Krolewski AS, Bergman RN, Soeldner JS, Kahn CR. Role of glucose and insulin resistance in development of type 2 diabetes mellitus: results of a 25-year follow-up study. Lancet. 1992;17:925–929. doi: 10.1016/0140-6736(92)92814-v. [DOI] [PubMed] [Google Scholar]
- 4.Uehara MKH, Isami S, Kisanuki K, Ohkubo Y, Miyamura N, Miyata T, Yano T, Shichiri M. Effect on insulin sensitivity of angiotensin converting enzyme inhibitors with or without a sulphydryl group: bradykinin may improve insulin resistance in dogs and humans. Diabetologia. 1994;37:300–307. doi: 10.1007/BF00398058. [DOI] [PubMed] [Google Scholar]
- 5.Isami SKH, Araki E, Uehara M, Kaneko K, Shirotani T, Todaka M, Ura S, Motoyoshi S, Matsumoto K, Miyamura N, Shichiri M. Bradykinin enhances GLUT4 translocation through the increase of insulin receptor tyrosine kinase in primary adipocytes: evidence that bradykinin stimulates the insulin signalling pathway. Diabetologia. 1996;39:412–420. doi: 10.1007/BF00400672. [DOI] [PubMed] [Google Scholar]
- 6.Tschope C, Reinecke A, Seidl U, Yu M, Gavriluk V, Riester U, Gohlke P, Graf K, Bader M, Hilgenfeldt U, Pesquero JB, Ritz E, Unger T. Functional, biochemical, and molecular investigations of renal kallikrein-kinin system in diabetic rats. Am J Physiol Heart Circ Physiol. 1999;277:H2333–2340. doi: 10.1152/ajpheart.1999.277.6.H2333. [DOI] [PubMed] [Google Scholar]
- 7.Zhao C, Wang P, Xiao X, Chao J, Chao L, Wang DW, Zeldin DC. Gene Therapy With Human Tissue Kallikrein Reduces Hypertension and Hyperinsulinemia in Fructose-Induced Hypertensive Rats. Hypertension. 2003;42:1026–1033. doi: 10.1161/01.HYP.0000097603.55404.35. [DOI] [PubMed] [Google Scholar]
- 8.David Montanari HY, Dobrzynski Eric, Agata Jun, Yoshida Hideaki, Chao Julie, Chao Lee. Kallikrein Gene Delivery Improves Serum Glucose and Lipid Profiles and Cardiac Function in Streptozotocin-Induced Diabetic Rats. Diabetes. 2005;54:1573–1580. doi: 10.2337/diabetes.54.5.1573. [DOI] [PubMed] [Google Scholar]
- 9.Xiao X, Li J, Samulski RJ. Production of High-Titer Recombinant Adeno-Associated Virus Vectors in the Absence of Helper Adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monahan PESR. AAV vectors: is clinical success on the horizon? Gene Ther. 2000;7:24–30. doi: 10.1038/sj.gt.3301109. [DOI] [PubMed] [Google Scholar]
- 11.Duan DYY, Yan Z, Engelhardt JF. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat Med. 2000;6:595–598. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]
- 12.Xiao X, Li J, Samulski R. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang TLH, Zhao C, Chen C, Li J, Chao J, Chao L, Xiao X, Wang DW. Recombinant adeno-associated virus-mediated kallikrein gene therapy reduces hypertension and attenuates its cardiovascular injuries. Gene Ther. 2004;3:1038. doi: 10.1038/sj.gt.3302294. [DOI] [PubMed] [Google Scholar]
- 14.Auricchio A, Hildinger M, O'Connor E, Gao G-P, Wilson JM. Isolation of Highly Infectious and Pure Adeno-Associated Virus Type 2 Vectors with a Single-Step Gravity-Flow Column. Human Gene Therapy. 2001;12:71–76. doi: 10.1089/104303401450988. [DOI] [PubMed] [Google Scholar]
- 15.Giorgino F, Logoluso F, Davalli A, Napoli R, Laviola L, Hirshman M, Horton E, Weir G, Smith R. Islet transplantation restores normal levels of insulin receptor and substrate tyrosine phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle and myocardium of streptozocin-induced diabetic rats. Diabetes. 1999;48:801–812. doi: 10.2337/diabetes.48.4.801. [DOI] [PubMed] [Google Scholar]
- 16.Jian LJQ, Joyce T, Christina KH, Cynthia S, Richard H, Gerald MR. Nongenetic mouse modles of non-insulin dependent diabetes mellirus. Metabolism. 1998;47:663–668. doi: 10.1016/s0026-0495(98)90027-0. [DOI] [PubMed] [Google Scholar]
- 17.Wang JXW, Yang Z, Davis T, Dewey MJ, Chao J, Chao L. Human tissue kallikrein induces hypotension in transgenic mice. Hypertension. 1994;23:236–243. doi: 10.1161/01.hyp.23.2.236. [DOI] [PubMed] [Google Scholar]
- 18.Sathanur R, Srinivasan MGFaGSB. Longitudinal changes in risk variables of insulin resistance syndrome from childhood to young adulthood in offspring of parents with type 2 diabetes: The Bogalusa Heart Study. Metabolism. 2003;52:443–450. doi: 10.1053/meta.2003.50065. [DOI] [PubMed] [Google Scholar]
- 19.Thule PM, Campbell AG, Kleinhenz DJ, Olson DE, Boutwell JJ, Sutliff RL, Hart CM. Hepatic insulin gene therapy prevents deterioration of vascular function and improves adipocytokine profile in STZ-diabetic rats. Am J Physiol Endocrinol Metab. 2006;290:E114–E122. doi: 10.1152/ajpendo.00134.2005. [DOI] [PubMed] [Google Scholar]
- 20.Tomie Furuya D, Binsack R, Onishi ME, Monteiro Seraphim P, Fabres Machado U. Low ethanol consumption induces enhancement of insulin sensitivity in liver of normal rats. Life Sci. 2005;77:1813–1824. doi: 10.1016/j.lfs.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 21.Lee HJ, Choi SS, Park MK, An YJ, Seo SY, Kim MC, Hong SH, Hwang TH, Kang DY, Garber AJ, Kim DK. Fenofibrate lowers abdominal and skeletal adiposity and improves insulin sensitivity in OLETF rats. Biochem Biophys Res Commun. 2002;296:293–299. doi: 10.1016/s0006-291x(02)00822-7. [DOI] [PubMed] [Google Scholar]
- 22.Walter J, Mortasawi A, Arnrich B, Albert A, Frerichs I, Rosendahl U, Ennker J. Creatinine clearance versus serum creatinine as a risk factor in cardiac surgery. BMC Surgery. 2003;3:4. doi: 10.1186/1471-2482-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong W, Chao J, Chao L. Muscle Delivery of Human Kallikrein Gene Reduces Blood Pressure in Hypertensive Rats. Hypertension. 1995;25:715–719. doi: 10.1161/01.hyp.25.4.715. [DOI] [PubMed] [Google Scholar]
- 24.Guesdon JLTT, Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979;27:1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Dobrzynski E, Chao J, Chao L. Adrenomedullin gene delivery attenuates renal damage and cardiac hypertrophy in Goldblatt hypertensive rats. Am J Physiol Renal Physiol. 2001;280:F964–971. doi: 10.1152/ajprenal.2001.280.6.F964. [DOI] [PubMed] [Google Scholar]
- 26.Yasuhiro Maejima SA, Hiroshi Ito, Nobori Kiyoshi, Tamamori-Adachi Mimi, Isobe Mitsuaki. Nitric oxide inhibits ischemia/reperfusion-induced myocardial apoptosis by modulating cyclin A-associated kinase activity. Cardiovascular Research. 2003;59:308–320. doi: 10.1016/s0008-6363(03)00425-5. [DOI] [PubMed] [Google Scholar]
- 27.MM B. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 28.Virkamaki A, Ueki K, Kahn CR. Protein–protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J Clin Invest. 1999;103:931–943. doi: 10.1172/JCI6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alessi DRCP. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 30.Kumar DZJ, Robertson S, Burns KD. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron Exp Nephrol. 2004;96:e77–88. doi: 10.1159/000076749. [DOI] [PubMed] [Google Scholar]
- 31.Susztak KCE, McCue P, Sharma K, Bottinger EP. Multiple Metabolic Hits Converge on CD36 as Novel Mediator of Tubular Epithelial Apoptosis in Diabetic Nephropathy. PLoS Med. 2005;2(2):e45. doi: 10.1371/journal.pmed.0020045. Epub 2005 Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson S, Bagstaff S, Lynn S, Yeaman S, Turnbull D, Walker M. Decreased insulin responsiveness of glucose uptake in cultured human skeletal muscle cells from insulin-resistant nondiabetic relatives of type 2 diabetic families. Diabetes. 2000;49:1169–1177. doi: 10.2337/diabetes.49.7.1169. [DOI] [PubMed] [Google Scholar]
- 33.Storlien LHPD, Kriketos AD, Baur LA. High fat diet-induced insulin resistance. Lessons and implications from animal studies. Ann N Y Acad Sci. 1993;683:82–90. doi: 10.1111/j.1749-6632.1993.tb35694.x. [DOI] [PubMed] [Google Scholar]
- 34.Soter Dai JHM. Fructose-induced hypertension in rats is concentration- and duration-dependent. J Pharmacol Toxicol Methods. 1995;33:101–107. doi: 10.1016/1056-8719(94)00063-a. [DOI] [PubMed] [Google Scholar]
- 35.P T. Insulin sensitivity and blood lipids during antihypertensive treatment with special reference to ACE inhibition. J Diabet Complications. 1990;4:75–78. doi: 10.1016/0891-6632(90)90039-8. [DOI] [PubMed] [Google Scholar]
- 36.Jacob SHE, Fogt DL, Dietze GJ. Effects of trandolapril and verapamil on glucose transport in insulin-resistant rat skeletal muscle. Metabolism. 1996;45:535–541. doi: 10.1016/s0026-0495(96)90021-9. [DOI] [PubMed] [Google Scholar]
- 37.Vuorinen-Markkola HY-JH. Antihypertensive therapy with enalapril improves glucose storage and insulin sensitivity in hypertensive patients with non-insulin-dependent diabetes mellitus. Metabolism. 1995;44:85–89. doi: 10.1016/0026-0495(95)90293-7. [DOI] [PubMed] [Google Scholar]
- 38.Kim Y-B, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. J Clin Invest. 1999;104:733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yan ZZY, Duan D, Engelhardt JF. From the Cover: Trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. PNAS. 2000;97:6716–6721. doi: 10.1073/pnas.97.12.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang JZW, Zhang Y, Zidon T, Ritchie T, Engelhardt JF. Concatamerization of Adeno-Associated Virus Circular Genomes Occurs through Intermolecular Recombination. J Virol. 1999;73:9468–9477. doi: 10.1128/jvi.73.11.9468-9477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samulski RSM, Muzyczka N. Adeno-associated viral vectors. In: F T, editor. Development of Human Gene Therapy. 1999. pp. 131–172. [Google Scholar]
- 42.Wang ZMH, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen EJJS, Fogt DL, Dietze GJ. Effect of chronic bradykinin administration on insulin action in an animal model of insulin resistance. Am J Physiol. 1998;275:R40–45. doi: 10.1152/ajpregu.1998.275.1.R40. [DOI] [PubMed] [Google Scholar]
- 44.Emanueli C, Salis MB, Chao J, Chao L, Agata J, Lin KF, Munao A, Straino S, Minasi A, Capogrossi MC, Madeddu P. Adenovirus-mediated human tissue kallikrein gene delivery inhibits neointima formation induced by interruption of blood flow in mice. Arterioscler Thromb Vasc Biol. 2000;20:1459–1466. doi: 10.1161/01.atv.20.6.1459. [DOI] [PubMed] [Google Scholar]
- 45.Montanari D, Yin H, Dobrzynski E, Agata J, Yoshida H, Chao J, Chao L. Kallikrein gene delivery improves serum glucose and lipid profiles and cardiac function in streptozotocin-induced diabetic rats. Diabetes. 2005;54:1573–1580. doi: 10.2337/diabetes.54.5.1573. [DOI] [PubMed] [Google Scholar]
- 46.Goldman JPD, Vukmirovich R. Potentiation of insulin stimulation of hexose transport by kallikrein and bradykinin in isolated rat adipocytes. Mol Cell Endocrinol. 1987;53:183–191. doi: 10.1016/0303-7207(87)90016-5. [DOI] [PubMed] [Google Scholar]
- 47.Miyata TTT, Uehara M, Isami S, Kishikawa H, Kaneko K, Araki E, Shichiri M. Bradykinin potentiates insulin-stimulated glucose uptake and enhances insulin signal through the bradykinin B2 receptor in dog skeletal muscle and rat L6 myoblasts. Eur J Endocrinol. 1998;138:344–352. doi: 10.1530/eje.0.1380344. [DOI] [PubMed] [Google Scholar]
- 48.Bjornholm M, Kawano Y, Lehtihet M, Zierath J. Insulin receptor substrate-1 phosphorylation and phosphatidylinositol 3-kinase activity in skeletal muscle from NIDDM subjects after in vivo insulin stimulation. Diabetes. 1997;46:524–527. doi: 10.2337/diab.46.3.524. [DOI] [PubMed] [Google Scholar]
- 49.Bevan P. Insulin signalling. J Cell Sci. 2001;114:1429–1430. doi: 10.1242/jcs.114.8.1429. [DOI] [PubMed] [Google Scholar]
- 50.Buren JLH, Lauritz J, Eriksson JW. High glucose and insulin in combination cause insulin receptor substrate-1 and −2 depletion and protein kinase B desensitisation in primary cultured rat adipocytes: possible implications for insulin resistance in type 2 diabetes. Eur J Endocrinol. 2003;148:157–167. doi: 10.1530/eje.0.1480157. [DOI] [PubMed] [Google Scholar]
- 51.Ido YCD, Ruderman N. Hyperglycemia-Induced Apoptosis in Human Umbilical Vein Endothelial Cells. Inhibition by the AMP-Activated Protein Kinase Activation. Diabetes. 2002;51:159–167. doi: 10.2337/diabetes.51.1.159. [DOI] [PubMed] [Google Scholar]
- 52.Tseng C-H. Mortality and Causes of Death in a National Sample of Diabetic Patients in Taiwan. Diabetes Care. 2004;27:1605–1609. doi: 10.2337/diacare.27.7.1605. [DOI] [PubMed] [Google Scholar]
- 53.Mauer M, Drummond K. The Early Natural History of Nephropathy in Type 1 Diabetes: I. Study Design and Baseline Characteristics of the Study Participants. Diabetes. 2002;51:1572–1579. doi: 10.2337/diabetes.51.5.1572. [DOI] [PubMed] [Google Scholar]
- 54.Drummond K, Mauer M. The Early Natural History of Nephropathy in Type 1 Diabetes: II. Early Renal Structural Changes in Type 1 Diabetes. Diabetes. 2002;51:1580–1587. doi: 10.2337/diabetes.51.5.1580. [DOI] [PubMed] [Google Scholar]
- 55.Thomson SC, Deng A, Bao D, Satriano J, Blantz RC, Vallon V. Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes. J Clin Invest. 2001;107:217–224. doi: 10.1172/JCI10963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida H, Zhang JJ, Chao L, Chao J. Kallikrein Gene Delivery Attenuates Myocardial Infarction and Apoptosis After Myocardial Ischemia and Reperfusion. Hypertension. 2000;35:25–31. doi: 10.1161/01.hyp.35.1.25. [DOI] [PubMed] [Google Scholar]
- 58.Olsen GSHB. AMP kinase activation ameliorates insulin resistance induced by free fatty acids in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2002;283:E965–E970. doi: 10.1152/ajpendo.00118.2002. [DOI] [PubMed] [Google Scholar]
- 59.Jaffa AA, Durazo-Arvizu R, Zheng D, Lackland DT, Srikanth S, Garvey WT, Schmaier AH. Plasma Prekallikrein: A Risk Marker for Hypertension and Nephropathy in Type 1 Diabetes. Diabetes. 2003;52:1215–1221. doi: 10.2337/diabetes.52.5.1215. [DOI] [PubMed] [Google Scholar]
- 60.Jaffa AARP, Mayfield RK. Kinin, a mediator of diabetes-induced glomerular hyperfiltration. Diabetes. 1995;44:156–160. doi: 10.2337/diab.44.2.156. [DOI] [PubMed] [Google Scholar]
- 61.Harvey JNJA, Margolius HS, Mayfield RK. Renal kallikrein and hemodynamic abnormalities of diabetic kidney. Diabetes. 1990;39:299–304. doi: 10.2337/diab.39.3.299. [DOI] [PubMed] [Google Scholar]
- 62.Douillet CD, Velarde V, Christopher JT, Mayfield RK, Trojanowska ME, Jaffa AA. Mechanisms by which bradykinin promotes fibrosis in vascular smooth muscle cells: role of TGF-beta and MAPK. Am J Physiol Heart Circ Physiol. 2000;279:H2829–2837. doi: 10.1152/ajpheart.2000.279.6.H2829. [DOI] [PubMed] [Google Scholar]
- 63.Greene EL, Velarde V, Jaffa AA. Role of Reactive Oxygen Species in Bradykinin-Induced Mitogen-Activated Protein Kinase and c-fos Induction in Vascular Cells. Hypertension. 2000;35:942–947. doi: 10.1161/01.hyp.35.4.942. [DOI] [PubMed] [Google Scholar]