Abstract
The role of dihydroceramide desaturase as a key enzyme in the de-novo pathway of ceramide generation was investigated in human neuroblastoma cells (SMS-KCNR). A novel assay using water soluble analogs of dihydroceramide, dihydroceramidoids (D-e-dhCCPS analogs) was used to measure desaturase activity in-situ. Conversion of D-e-C12-dhCCPS (C12-dhCCPS) to its 4,5-desaturated counterpart: D-e-C12-CCPS (C12-CCPS) was determined by LC/MS analysis. The validity of the assay was confirmed by C8-cyclopropenylceramide, a competitive inhibitor of dihydroceramide desaturase. A human homologue (DEGS-1) of the Drosophila melanogaster degenerative-spermatocyte-gene-1 (des-1) was recently identified, and reported to have desaturase activity. Transfection of SMS-KCNR cells with siRNA to DEGS-1 significantly blocked the conversion of C12-dhCCPS to C12-CCPS. The associated accumulation of endogenous dihydroceramides confirmed DEGS-1 as the main active dihydroceramide desaturase in these cells. The partial loss of DEGS-1 inhibited cell growth with cell cycle arrest at G0/G1. This was accompanied by significant decrease in the amount of phosphorylated retinoblastoma protein (pRb). This hypophosphorylation was inhibited by tautomycin and not by okadaic acid, suggesting the involvement of protein phosphatase 1. Additionally, we found that treatment of SMS-KCNR cells with fenretinide inhibited desaturase activity in a dose dependent manner. Increase of dihydroceramides, but not ceramides, paralled this process as measured by LC/MS. There were no effects on the mRNA or protein levels of DEGS-1, suggesting that fenretinide acts at the post-translational level as an inhibitor of this enzyme. Tautomycin was also able to block the hypophosphorylation of Rb observed with fenretinide treatment. These findings suggest a novel biologic function for dihydroceramides.
Sphingolipids, in addition to their roles as structural components of cell membranes, play important roles as regulators of signal transduction in cell differentiation, cell proliferation, and apoptosis. One of the most studied sphingolipids is ceramide (1–5). Ceramide (Cer) is the central building block for sphingolipids. It serves as a precursor for the synthesis of more complex sphingolipids; and is generated in cells by multiple pathways. Ceramide can be produced de-novo from serine and palmitoyl-CoA via dihydroceramide (dhCer), followed by its desaturation to Cer by dihydroceramide desaturase. While there has been a great body of investigation on many of the enzymes of the de-novo pathway and Cer, the enzyme dihydroceramide desaturase and its substrate dhCer have not been well characterized.
Dihydroceramide desaturase is responsible for inserting the 4,5-trans-double bond to the sphingoid backbone of dhCer. The enzyme was previously characterized and an in-vitro assay was developed to determine its activity (6–9). A crude enzyme preparation was isolated from rat liver microsomes. In an independent study, a family of sphingolipid Δ4-desaturases (homologues of the Drosophila melanogaster degenerative spermatocyte gene-1 (des-1) was identified via a bioinformatics approach (10). These proteins contain three His-containing consensus motifs that are characteristic of a group of membrane fatty acid desaturases. The human homologue of des-1 is now referred to as DEGS-1 (DES1), was first cloned in 1997 and named as Membrane Lipid Desaturase (MLD) (11). It was identified using a yeast two-hybrid screen. It was reported that over-expression of MLD inhibited biosynthesis of the epidermal growth factor receptor (EGFR) in 293 EBNA human embryonic kidney cells.
Dihydroceramides are believed to be biologically inactive molecules. Treatment of cells with exogenous short chain dhCers has failed to inhibit cell growth and induce apoptosis (12–14). In the present study on dihydroceramide desaturase in SMS-KCNR cells, we used a novel in-situ assay to measure the activity of this enzyme using cell-permeable dihydroceramidoids (dhCCPS analogs) (15). DhCCPS analogs represent a novel class of water soluble long chain dhCers, which can be delivered to cells in culture.
The obtained data demonstrate that DEGS-1 is the main dihydroceramide desaturase active in SMS-KCNR human neuroblastoma cells, and that inhibition of DEGS-1 with siRNA leads to the accumulation of endogenous dhCers, with subsequent effects on cell growth with cell cycle arrest and hypophosphorylation of the retinoblastoma protein (Rb). Activation of ceramide activated protein phosphatases appears to be involved in this process. Furthermore, we have confirmed that dihydroceramide desaturase is a target for the synthetic retinoid, fenretinide (4-HPR).
EXPERIMENTAL PROCEDURES
Reagents
D-erythro-C12-ceramide
(D-e-C12-CCPS) D-erythro-2-N-[12′-(1″-pyridinium)-dodecanoyl]-sphingosine bromide (C12-CCPS) and D-erythro-C12-dihydroceramide: (D-e-C12-dhCCPS) D-erythro-2-N-[12′-(1″-Pyridinium)-dodecanoyl]-4,5-dihydrosphingosine bromide (C12-dhCCPS) were synthesized by the Lipidomics Core Facility at the Medical University of South Carolina (MUSC) (15). Fenretinide (4-HPR), All-trans-retinoic acid (ATRA), and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma (St Louis, MO). C8-Cyclopropenylceramide (C8-CPPC) was obtained from Matreya LLC (Pleasant Gap, PA). Okadaic Acid (OA) and Tautomycin (TMY) were obtained from Biomol (Plymouth Meeting, PA).
Cell lines and culture conditions
The SMS-KCNR cell line was obtained from Dr. C. Pat Reynolds (Children’s Hospital of Los Angeles) The MCF-7 cell line was obtained from Dr. Chiara Luberto (MUSC). Cells were maintained in growth medium (RPMI-1640) containing 10% fetal calf serum (FCS) (Invitrogen Carlsbad, California) at 37 °C in 5% CO2. C8-CPPC was dissolved in methanol at a stock concentration of 10 mM, 4-HPR in 95% ethanol at a stock concentration of 100 mM, and ATRA in DMSO at a stock concentration of 100 mM. Stock solutions were diluted to required concentrations (0.01–2.5 μM for C8-CPPC, 0.5–10 μM for 4-HPR, and 10 μM for ATRA) just prior to use, then directly added to the cells in growth medium. OA and TMY were dissolved in ethanol at a stock concentration of 10 μM. OA and TMY were directly added to the cells in growth medium to obtain a final concentration of 10 nM, 18 hours prior to siRNA transfection or 4-HPR treatment. The final volume of methanol, ethanol or DMSO in the medium was <0.02%, which had no effect on cell growth or survival.
In-situ dihydroceramide desaturase assay
C12-dhCCPS was used as the substrate for this enzyme. C12-dhCCPS was dissolved in 100% ethanol at concentrations of 100 mM and this stock solution was diluted just prior to use and directly added to the cells in medium containing 10% fetal calf serum to obtain a final concentration of 0.5 μM. Cells were incubated with C12-dhCCPS for 15 minutes, 2 hours, 6 hours and 24 hours. The cells were collected at these time points and levels of C12-dhCCPS and its product C12-CCPS were detected by LC/MS (15,16) The percentage of the conversion C12-CCPS was depicted in the figures.
LC-MS analysis of endogenous Cers and cellular level of CCPS and dhCCPS analogs
Advanced analyses were performed by the Lipidomics Core Facility at MUSC (http://hcc.musc.edu/research/shared_resources/lipidomics.cfm) on a ThermoFinningan TSQ 7000, triple-stage quadrupole mass spectrometer operating in a Multiple Reaction Monitoring (MRM) positive ionization mode as described (15,16).
RNA Interference Transfection
Transient transfections were performed using HiPerfect (Qiagen, Valencia, CA) following the manufacturer’s recommendations. siRNA to human DEGS-1 (NM_144780, NM_003676) and a nonspecific (Silencer® Negative Control) siRNA were purchased from Ambion (Austin, TX). Cells were treated with siRNA at least 24–48 hours prior to the addition of dhCCPS analogs. Knockdown of target gene expression was confirmed by RT-PCR and Western blotting.
RNA isolation, RT-PCR
One μg of total RNA, isolated using an RNA isolation kit (Qiagen), was used in reverse transcription reactions with AMV-reverse transcriptase and random primers (Promega, Madison, WI) as described by the manufacturer. The resulting total cDNA was then used in PCR to measure the mRNA levels of DEGS-1. The mRNA levels of 28s rRNA were used as internal controls. Linear amplification cycles were determined separately for each gene. The following primers were used for PCR amplification:
DEGS-1 (forward)
5′-TTCTTCTGTACCGCTTTCAG-3′
DEGS-1 (reverse)
5′-TTACTCCAGCACCATCTCT-3′
rRNA (forward)
5′-TTACCAAAAGTGGCCCACTA-3′
rRNA (reverse)
5′-GAAAGATGGTGAACTATGCC-3′
The reactions were performed at 95°C for 1 min, 55°C for 2 min and 72°C for 2 min for 30 cycles. The concentrations of rRNA primers were used at a 1:6 ratio compared with other primers to achieve linear amplification conditions in the same reactions. The RT-PCR products were quantitated by densitometry using Quantity One 1-D Analysis Software (BioRad, Hercules, CA). The mRNA levels of DEGS-1 were normalized to the rRNA levels used as internal controls. The amplified fragments were separated on 2% agarose gels and visualized by ethidium bromide staining.
Immunoblotting
The protein levels of DEGS-1, pRb, Rb, and β-Actin were detected by Western blot analysis. In short, total proteins (30 μg/lane) were separated by 10% SDS-PAGE, blotted onto an Immobilon™ membranes (Millipore Billerica, MA), and the proteins were detected using primary: anti-DEGS-1 (1:2000), anti-pRb (1:1000), anti-Rb (1:1000) or anti-β-actin (1:1000) antibodies for 1 hour, followed by peroxidase-conjugated secondary antibodies: anti-rabbit or anti-mouse (1:10,000) for 1 hour. The proteins were visualized by chemiluminescence using the Amersham ECL™ protein detection kit (GE Healthcare Piscataway, NJ) as described by the manufacturer. Anti-DEGS-1 (MLD 3906) was kindly provided by Dr. Gordon N. Gill (University of California, San Diego). Anti-β-actin was purchased from Sigma. Anti-Phospho-Rb (Ser795) was obtained from Cell Signaling Technology, Inc (Danvers, MA). Anti-Rb was obtained from BD Pharmingen (San Jose, CA) Secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). The protein levels were quantitated by densitometry using Quantity One 1-D Analysis Software (BioRad, Hercules, CA).
Cell growth assays
Trypan Blue Assay
The effects of DEGS-1 siRNA cell growth were detected using a trypan blue exclusion method as described previously (17). SMS-KCNR cells were transfected with 10 nM siRNA to DEGS-1 (DEGS-1) or a nonspecific siRNA (SCR). These cells along with untransfected cells were re-plated 24 hours after transfection in 6 well plates at a density of ~5000 cells/well. At 2, 4 and, 6 days after plating, the cells were trypsinized and diluted in phosphate-buffered saline. The floating (dead cells) in the medium and cells that remained attached to the plates were then counted using a hematocytometer in the presence of trypan blue solution at a 1:1 ratio (v/v) (Sigma) as described by the manufacturer. Triplicate wells were used for each treatment. MTT Assay: The effects of increasing concentrations of 4-HPR on cell growth were detected using an MTT cell survival assay (18). In brief, cells (~2000) were plated in 24 well dishes in the absence or presence of increasing concentrations of 4-HPR. On days 0, 2, 4, and 6, the cells were incubated with MTT reagent at 37°C for 4 hours and then lysed in lysis buffer at room temperature overnight. MTT uptake was measured at 570 nm in a microplate reader. Untreated cells were used as controls. 4-HPR was added directly to the media every 48 hours. Triplicate wells were used for each treatment.
Cell cycle analysis
The effects of inhibition of DEGS-1 by siRNA on the cell cycle profiles of SMS-KCNR cells at 48 hours were analyzed in the presence of DNase-free RNase and propidium iodide by flow cytometry in the Flow Cytometry Facility at MUSC as described previously (19). Untreated and nonspecific siRNA (SCR) transfected cells were used as controls.
RESULTS
Conversion of 4,5-dhCers to the Corresponding Cers
Positively-charged cell permeable analogs of D-erythro-C12-ceramide and D-erythro-C12-dihydroceramide: C12-CCPS and C12-dhCCPS were used as molecular probes to study dihydroceramide desaturase activity by monitoring conversion of C12-dhCPPS to C12-CCPS in intact cells by an LC-MS approach (16). This conversion is depicted in Figure 1A.
Figure 1. Conversion of C12-dhCCPS to C12-CCPS in cells.

(A) The scheme depicts the intracellular oxidation of dihydro-ceramidoid C12-dhCCPS to the corresponding ceramidoid C12-CCPS by the enzyme dihydroceramide desaturase (B) SMS-KCNR and MCF-7 cells were treated with 0.5 μM C12-dhCCPS for 15 min, 2 h, 6 h and 24 h. Cells were harvested at these time points, and pyridinium conjugated ceramidoids (Pyr-Cers) were measured by LC/MS as described in the “Experimental Procedures.” The percentage of the total detected Pyr-Cers that was converted to C12-dhCCPS are depicted here. The data presented are representative of the mean of at least three independent experiments ± S.D. The error bars represent the standard deviations, and when not seen, they are smaller than the thickness of the lines on the graphs.
SMS-KCNR human neuroblastoma cells and MCF-7 human breast carcinoma cells were incubated with 0.5 μM C12-dhCCPS for 15 minutes, 2 hours, 6 hours and 24 hours (Fig. 1B). The cells were collected at these time points, and levels of C12-dhCCPS and C12-CCPS were measured by LC/MS as described in “Experimental Procedures.” The percentage of the conversion is shown in Figure 1B. Desaturation to C12-CCPS was detected as early as 15 minutes (8 and 11% in SMS-KCNR and MCF-7 cells, respectively), and near total conversion was achieved by 24 hours, with greater than 80% conversion at 6 hours. This assay was also performed with other human cancer cell lines, including lung adenocarcinoma (A549), squamous cell carcinoma (UM-SCC-1), and other neuroblastomas (SK-N-SH, IMR-32, and SK-N-RA), and similar results were obtained (data not shown), These studies demonstrate that the desaturase is an active enzyme in these cells, proving this approach as a powerful method to study the activity of this enzyme in intact cells.
Since there was over 80% conversion of C12-dhCCPS to C12-CCPS at 6 hours, this time point was selected to study potential inhibitors and activators of the desaturase enzyme. Prior to testing the action of various compounds on enzyme activity, the cytotoxicity of C12-dhCCPS. was examined by MTT assay. There was no significant effect on cell viability with increasing concentrations up to 10 μM at 6 hours in SMS-KCNR and MCF-7 cells (data not shown). Measurement of endogenous ceramide and dihydroceramide levels was also performed and no changes in these sphingolipid levels were observed at 6 hours (data not shown).
Effects of the Desaturase Inhibitor, C8-Cyclopropenylceramide, on Conversion of C12-dhCCPS to C12-CCPS
C8-Cyclopropenylceramide (C8-CPPC) is a known in vitro competitive inhibitor of dihydroceramide desaturase (20). At low concentrations, C8-CPPC inhibits dihydroceramide desaturase while at high concentrations it also inhibits sphingosine-1-phosphate lyase and serine palmitoyl transferase (21). SMS-KCNR cells were pre-treated with increasing (0, 0.01, 0.1, 0.5, 1, and 2.5 μM) concentrations of C8-CPPC for 30 minutes, and then 0.5 μM C12-dhCCPS was added for 6 hours (Fig. 2A). Cells were collected after 6 hours, and the conversion to C12-CCPS was determined. Inhibition of DEGS-1 activity was observed in a dose dependent manner, starting at 0.1 μM, decreasing conversion levels to ~40% C12-CCPS as compared to ~66% conversion in the untreated cells. The percent of the conversion to C12-CCPS in cells treated with 0.5, 1, and 2.5 μM C8-CPPC was ~27%, ~22%, and ~5%, respectively (Fig. 2A). Measurement of total endogenous Cer, dhCer, and sphingosine levels was also performed (Fig 2B-E) by LC/MS. Approximately 2.4, 8.4, and 4.8 fold increases in total endogenous dhCers were observed in cells treated with 0.5, 1, and 2.5 μM C8-CPPC, respectively, correlating with the inhibition of DEGS-1 (Fig. 2B). There was also a modest concentration dependent decrease in total Cer levels with ~ 8%, 22%, and 27% (Fig. 2B). There were no significant changes in dihydrosphingosine, dihydrosphingosine-1-phosphate, sphingosine, nor sphinogosine-1-phosphate levels (Fig. 2C). The largest decrease in Cer species occurred in C24, which was the predominant Cer species in this cell line (Fig. 2D) All dhCer species were elevated, the largest increases occurred for those species that were predominant in this cell line, mainly C16, C24, C24:1, C26, and C26:1. (Fig. 2E). These results verify that C8-CPPC is a potent and total (not showing specificity to dhCer species) inhibitor of dihydroceramide desaturase and that inhibition of this enzyme with C8-CPPC leads to the accumulation of endogenous dhCers.
Figure 2. Effects of C8-CPPC on desaturase activity.

(A) SMS-KCNR cells were pre-treated with increasing concentrations of the dihydroceramide desaturase inhibitor C8-CPPC for 30 minutes and then 0.5 μM C12-dhCCPS was added for 6 h. Cells were collected after 6 h and the conversion to C12-CCPS was measured by LC/MS as described in “Experimental Procedures.” (B) Total endogenous levels of ceramides (Cer), dihydroceramides (dhCer), (C) dihydrosphingosine (dhSph), dihydrosphingosine-1- phosphate (dhSph-1P), sphingosine (Sph), sphinogosine-1-phosphate (Sph-1P), (D) Cer species, (E) and dhCer species were measured by LC/MS as described under “Experimental Procedures.” The sphingolipid levels were normalized to total lipid phosphate. The data presented are representative of the mean of 2 independent experiments, performed in duplicate ± S.D. The error bars represent the standard deviations, and when not seen, they are smaller than the thickness of the lines on the graphs.
Role of DEGS-1 in Mediating the Conversion of C12-dhCCPS to C12-CCPS
Although the studies above revealed the action of a desaturase, it became important to determine if this was mediated specifically by the primary dihydroceramide desaturase, DEGS-1. Therefore, short interfering RNA against the human desaturase enzyme, DEGS-1 was used to attenuate its expression and consequently inhibit its activity. SMS-KCNR cells were transfected with 10 nM DEGS-1 siRNA (DEGS-1) or a nonspecific siRNA (SCR), and the conversion of C12-dhCCPS was evaluated after 48 hours with the in-situ desaturase assay. Knockdown of DEGS-1 mRNA levels was confirmed by RT-PCR (Fig. 3A left panel), with greater than 75% knockdown of DEGS-1 mRNA achieved in SMS-KCNR cells. Decreased expression of the DEGS-1 protein in DEGS-1 siRNA-treated SMS-KCNR cells was also confirmed by Western blotting (Fig. 3A right panel).
Figure 3. Effects of inhibition of human DEGS-1 with siRNA on desaturase activity and sphingolipid levels.
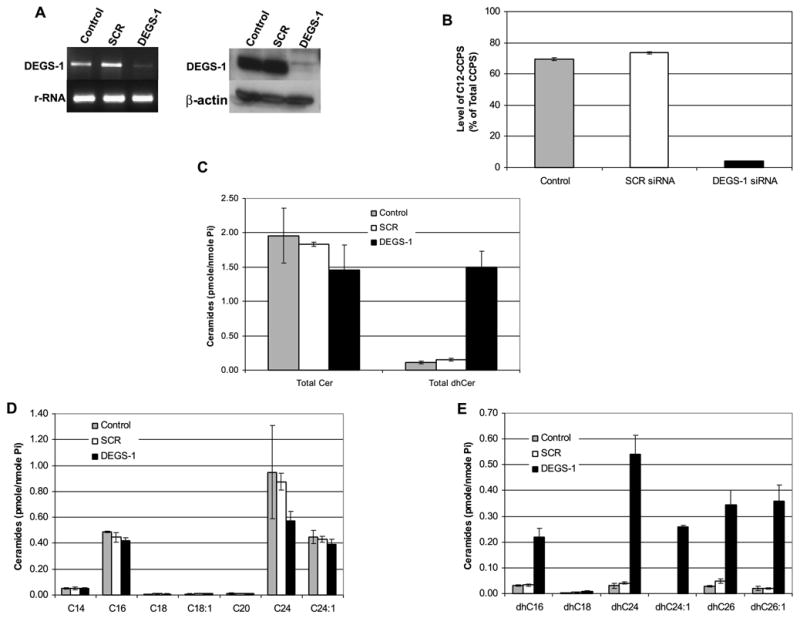
SMS-KCNR cells were transfected with 10 nM DEGS-1 or a nonspecific siRNA (SCR) and collected after 48 h. Control refers to untreated cells. Knockdown of DEGS-1 mRNA levels was confirmed by semi-quantitative RT-PCR (A left panel). The mRNA levels of 28S rRNA were used as internal controls. Expression of the DEGS-1 protein was also detected by Western blotting (A right panel). Total cell lysates were prepared 48 h after siRNA transfection. Equal amounts of proteins (30 μg) were run on 10% SDS-PAGE and blotted onto an Immobilon membrane as described in “Experimental Procedures.” β-actin was probed to verify equal loading of proteins per lane. The figures presented are representative of at least three independent experiments. (B) Inhibition of the DEGS-1 activity was confirmed using our in-situ assay for dihydroceramide desaturase activity. In siRNA transfected (DEGS-1 and SCR) and untransfected (control) cells, 0.5 μM C12-dhCCPS was added to the media 48 h post-transfection for 6 h. The conversion to C12-CCPS was measured by LC/MS as described in “Experimental Procedures.” (C) Total endogenous levels of ceramides (Cer), dihydroceramides (dhCer), (D) Cer species, (E) and dhCer species were measured by LC/MS as described under “Experimental Procedures.” The sphingolipid levels were normalized to total lipid phosphate. The data presented are representative of the mean of 3 independent experiments ± S.D. The error bars represent the standard deviations, and when not seen, they are smaller than the thickness of the lines on the graphs.
Next, the effects of the loss of DEGS-1 on the conversion of dihydroceramide were evaluated (Fig 3B). SMS-KCNR cells were transfected with siRNA (DEGS-1 or SCR) for 48 hours and, then along with untransfected (control) cells, were incubated with 0.5 μM C12-dhCCPS for 6 hours. In the untreated (control) and SCR siRNA-transfected cells, ~70% of the substrate C12-dhCCPS was converted to C12-CCPS. However, when expression of the enzyme was inhibited with siRNA, the conversion was significantly reduced, with only ~4% conversion observed. Similar results were also obtained with MCF-7 cells (data not shown). These results demonstrate that DEGS-1 is the major dihydroceramide desaturase in these cells.
We next examined what effects inhibition of DEGS-1 would exert on endogenous sphingolipids (Fig. 3C-E). For these studies, cells were treated with 10 nM DEGS-1 siRNA or SCR siRNA for 48 hours, and key sphingolipids were quantitated using LC/MS. As expected, there was an increase in endogenous dhCers ~13-fold when the enzyme was inhibited whereas levels of endogenous Cer were decreased approximately 25% and 20% when compared to untreated and SCR siRNA transfected cells (Fig. 3C,3D). As seen with C8-CPPC, all dhCer species were elevated, with the largest increases occurring in the ceramide species predominant in these cell lines (C16, C24, C24:1, C26, and C26:1) (Fig. 3E). There were no significant changes seen in endogenous sphingosine, dihydrosphingosine, or sphinogosine-1-phosphate levels (data not shown).
A time course study using siRNA against DEGS-1 (Fig. 4A) showed that the DEGS-1 protein was reduced about 50% by as early as 24 hours (data not shown), and persisted even 6 days after siRNA transfection. LC/MS measurement of endogenous Cers and dhCers (Fig. 4B-D) was performed 2, 4, and 6 days after siRNA transfection and demonstrated the persistent elevation of endogenous dhCers after silencing of the DEGS-1 protein. Endogenous dhCers were elevated ~18 fold, ~22 fold, and ~8 fold when compared to untreated cells on days 2, 4, and 6 after siRNA transfection. Taken together, these results demonstrate that DEGS-1 is the major dihydroceramide desaturase active in these cells, and that inhibition of this enzyme with siRNA leads to the accumulation of endogenous dhCers.
Figure 4. Effects of DEGS-1 inhibition at 2,4, and 6 days after siRna on sphingolipid levels.

(A) SMS-KCNR cells were transfected with 10 nM DEGS-1 or a nonspecific siRNA (SCR) and total cell lysates collected 2, 4, and 6 days after transfection. Control refers to untreated cells. Knockdown of the DEGS-1 protein was detected by Western blotting. Equal amounts of proteins (30 μg) were run on 10% SDS-PAGE and blotted onto an Immobilon membrane as described in “Experimental Procedures.” β-actin was probed to verify equal loading of proteins per lane. The figures presented are representative of at least two independent experiments. (B-D) Total endogenous levels of ceramides (Cer) and dihydroceramides (dhCer) were measured by LC/MS on days 2 (B), 4 (C) and 6 (D) after siRNA transfecton as described under “Experimental Procedures.” The sphingolipid levels were normalized to total lipid phosphate. The data presented are representative of the mean of 2 independent experiments performed in duplicate ± S.D. The error bars represent the standard deviations, and when not seen, they are smaller than the thickness of the lines on the graphs.
Effects of Loss of DEGS-1 on Cell Growth
In most cell studies, exogenous dhCers behave as “biological inactive compounds” compared to exogenous ceramides (12–14). In order to evaluate the biological roles of loss of DEGS-1 (which results in elevation of endogenous dhCers), we examined its effects on cell growth using a trypan blue exclusion method. SMS-KCNR cells were transfected with 10 nM DEGS-1 siRNA or a non-targeting siRNA (SCR). As shown in Figure 5A, treatment of these cells with DEGS-1 siRNA resulted in a significant inhibition of cell growth (about 65%) at days 4 and 6.
Figure 5. Effects of DEGS-1 inhibition on Cell Growth, Cell Cycle and pRb.
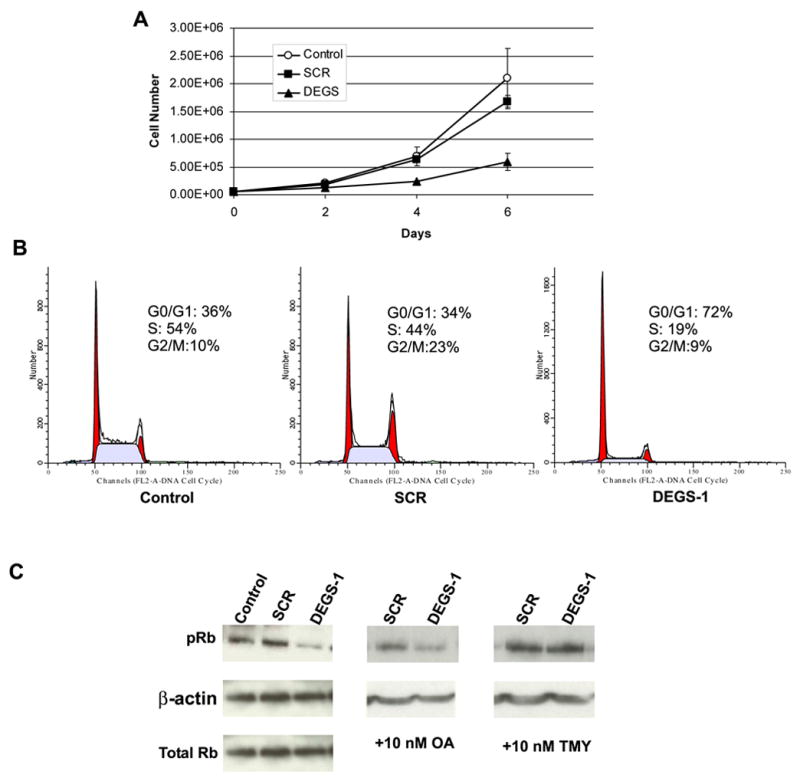
(A) The effects of loss of DEGS-1 on cell growth were determined by the trypan blue exclusion method as described under “Experimental Procedures.” SMS-KCNR cells were transfected with 10 nM DEGS-1 or a nonspecific siRNA (SCR) for 24 h and then split and plated, along with untransfected cells (control) in 6-well dishes (~ 5000 cells/well) in triplicate. Cells were counted on days 2, 4, and 6, and then the inhibition of growth in response to siRNA was determined from the cell survival plots. (B) The effects of siRNA to DEGS-1 on cell cycle profiles were determined and compared to that of untreated and non-specific siRNA (SCR) treated cells after 48 h using flow cytometry as described in “Experimental Procedures.” The figures presented are representative of at least two independent experiments. (C) Protein levels of pRb, total Rb, and β-actin were detected by Western blotting. Cells were pretreated for ~18 h with either 10 nM OA (C, center panel) or 10 nM TMY (C, right panel) prior to siRNA transfection. Total cell lysates were prepared 48 h after siRNA transfection. Equal amounts of proteins (30 μg) were run on 10% SDS-PAGE and blotted onto an Immobilon membrane as described in “Experimental Procedures.” β-actin was probed with to verify equal loading of proteins per lane. The figures presented are representative of at least three independent experiments.
We next determined the cell cycle profile of these cells after the reduction of DEGS-1 expression at 48 hours (Fig. 5B). Loss of DEGS-1 resulted in cell cycle arrest at the G0/G1 phase. DEGS-1 siRNA treated cells showed 72% of the cell population in the G0/G1 phase and 19% in the S phase whereas in the SCR siRNA-treated and in untreated cells, 34% and 36% of the cell population was in the G1 phase with 44% and 54% in the S phase, respectively.
Since loss of DEGS-1 resulted in G1 arrest, the phosphorylation status of the retinoblastoma protein (Rb) was examined next (Fig. 5C). pRb is critical for cell cycle progression; it regulates the G1/S phase restriction point, thereby controlling entry into S phase (21). Western blotting was performed and over 50 % decrease in amount of pRb was seen in cells treated with siRNA to DEGS-1. There was no change in the level of total Rb protein. (Fig 5C, left panel)
Ceramide has been shown to induce the dephosphorylation of Rb (22–25) and activate the serine/threonine protein phosphatases, protein phosphatase 1 (PP1) and 2A (PP2A) (26,27). PP1 in particular has been associated with ceramide mediated Rb dephosphorylation (28). In order to determine if a ceramide activated protein phosphatase was involved in this process, we pre-treated cells with either Okadiac Acid (OA) (Fig 5C, center panel), a specific PP2A inhibitor or Tautomycin (TMY) (Fig 5C, right panel), a specific PP1 inhibitor prior to siRNA transfection. As seen in Figure 5C, TMY was able to inhibit the hypophosphorylation of Rb. OA had minimal effects.
These data suggest that DEGS-1 activity may be required for normal progression through the cell cycle, and that like ceramide, endogenous dihydroceramides may activate serine/threonine protein phosphatases, specifically PP-1.
Effects of Fenretinide on Desaturase Activity
Fenretinide, N-(4-hydroxyphenyl)retinamide (4-HPR) is a synthetic analog of all-trans-retinoic acid (ATRA). This compound has been reported to increase Cer levels via de-novo synthesis within 6 hours of treatment (29). We therefore initially aimed at testing if the inhibition of DEGS-1 by siRNA would block the 4-HPR induced Cer generation and the anti-tumor effects of 4-HPR. First, the levels of endogenous ceramide in response to increasing concentrations (0.5, 1, 2.5, 5, and 10 μM) of 4-HPR for 6 hours were measured (Fig. 6A-C). Interestingly, it was found that dhCers and not Cers increased. Indeed, endogenous dhCers increased substantially in a dose dependent manner, with approximately 4, 14, 28, 45, and 67 fold increases over untreated cells with 0.5, 1, 2.5, 5, and 10 μM 4-HPR respectively. Endogenous Cer levels decreased to a lesser extent, but also in a dose dependent manner: ~24%, ~43% and ~45% decrease with 1, 2.5 and 5 μM 4-HPR. An ~15% increase in Cer levels was observed with 0.5 μM 4-HPR. There were no significant changes seen in endogenous sphingosine, dihydrosphingosine, or sphinogosine-1- phosphate levels (data not shown).
Figure 6. Effects of Fenretinide on Endogenous Sphingolipids and Desaturase Activity.
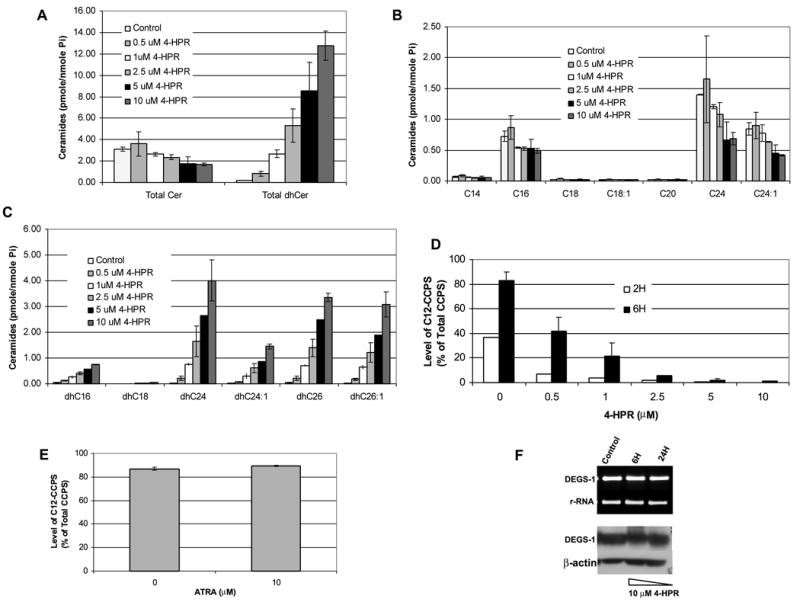
(A) SMS-KCNR cells were treated with increasing concentrations of 4-HPR for 6 h. Total endogenous levels of ceramides (Cer), dihydroceramides (dhCer), (B) Cer species, (C) and dhCer species were measured by LC/MS as described under “Experimental Procedures.” The sphingolipid levels were normalized to total lipid phosphate. The data presented are representative of the mean of 3 independent experiments ± S.D. (D,E) Desaturase activity was measured using our in-situ assay. Cells were treated with increasing concentrations of 4-HPR for 2 and 6 h (D) or 10 μM ATRA for 6 h (E). C12-dhCCPS was added at the same time as 4-HPR or ATRA. Cells were collected at these time points, and the conversion to C12-CCPS was determined by LC/MS. (F) The mRNA levels of 28s rRNA and DEGS-1 in response to 10 μM 4-HPR treatment for 6 and 24 h were measured by semi-quantitative RT-PCR. (F, upper panel) Expression of the DEGS-1 protein was detected by Western blotting (F, lower panel) Total cell lysates were prepared 6 and 24 h after 4-HPR treatment and, run on 10% SDS-PAGE. Equal amounts of proteins (30 μg) were blotted onto an Immobilon membrane as described in “Experimental Procedures.” β-actin was probed to verify equal loading of proteins per lane. The figures presented are representative of at least two independent experiments. The error bars represent the standard deviations, and when not seen, they are smaller than the thickness of the lines on the graphs.
While this study was in progress, a publication by Schulz et al. (30), also reported accumulation of dhCers in response to 4-HPR treatments. These findings raised the possibility that 4-HPR may result in inhibition of the desaturase. Given these findings, we then tested the ability of 4-HPR to inhibit desaturase activity using the in-situ assay for C12-dhCCPS (Fig. 6D). SMS-KCNR cells were treated with increasing concentrations (0.5, 1, 2.5, 5, and 10 μM) of 4-HPR for 2 and 6 hours. The substrate C12-dhCCPS (0.5 μM) was added at the same time as 4-HPR, and the conversion to C12-CCPS was measured by LC/MS. 4-HPR inhibited desaturase activity in a dose dependent manner. Inhibition was observed even at the lowest dose at both 2 and 6 hours. In untreated (control) cells, ~36% of measured Pyr-Cers were converted to C12-CCPS at 2 hours. The conversion was decreased to ~7%, 4%, ~2%, and < 1% in cells treated with 0.5, 1, 2.5, 5, and 10 μM 4-HPR. At 6 hours, there was ~83% conversion to C12-CCPS in untreated (control) cells. This conversion was decreased to ~42%, ~21%, ~6%, ~2%, and ~1% in cells treated with 0.5, 1, 2.5, 5, and 10 μM 4-HPR respectively.
We have previously reported that treatment of neuroblastoma cells with ATRA resulted in generation of long chain ceramides (C24:0 and C24: 1) (17). Therefore, the ability of other retinoids, such as ATRA to inhibit desaturase activity was tested with the in-situ assay. SMS-KCNR cells were treated with 10 μM ATRA for 6 hours; no inhibition of desaturase activity was detected (Fig. 6E). These results demonstrate specificity of inhibition of the desaturase by 4-HPR.
In order to determine the mechanism of action of 4-HPR on desaturase activity, semi-quantitative RT-PCR and Western blotting were then performed to study if the inhibition of the desaturase by 4-HPR was due to decreased transcription or translation of DEGS-1 (Fig. 6F). There were no changes seen in DEGS-1 mRNA levels (Fig. 6F upper panel) or protein levels (Fig 6F. lower panel.). This suggests that 4-HPR may be a direct an/or post translational inhibitor of this enzyme.
High concentrations of 4-HPR (>5 μM) have been shown to induce apoptosis and necrosis (29), while lower concentrations (3 μM) of 4-HPR have been reported to induce G1-S arrest and hyophosphorylation of Rb (31,32). Since loss of DEGS-1 resulted in cell cycle arrest, we examined what effects lower concentrations of 4-HPR (1 and 2.5 μM) would have on cell growth in an MTT assay (Fig. 7A). SMS-KNCR cells were plated as described in the “Experimental Procedures.” Cells were collected on days 2, 4, and 6. As shown in figure 7A, low dose 4-HPR inhibited cell growth in similar fashion to cells transfected with DEGS-1 siRNA. There was an ~ 41% and ~47% decrease in growth on days 4 and 8 with 1 μM 4-HPR. In cells treated with 2.5 μM 4-HPR there was an ~ 70% decrease in cell growth on both days. When cells were treated with 5 and 10 μM 4-HPR marked cell death was observed within 48–72 hours (data not shown). We next examined the effects of low dose 4-HPR (2.5 μM) on the phosphorylation of Rb. (Fig. 7B) The results obtained were similar to those of siRNA transfected cells (Fig. 5C). After 48 hours of 4-HPR treatment, a decrease in amount of pRb was observed. The hyophosphorylation of Rb was also inhibited by TMY. DEGS-1 protein levels were unchanged.
Figure 7. Effects of Fenretinide on Cell Growth and pRB.
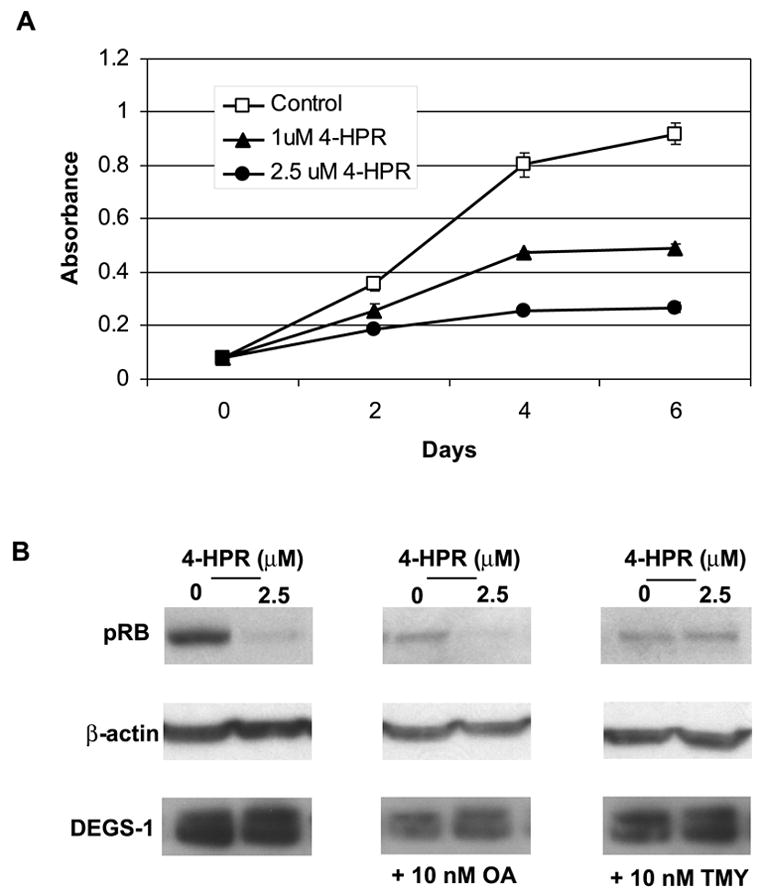
(A) The effects of low dose 4-HPR treatment (1 and 2.5 μM) on cell growth were determined via MTT assay as described in “Experimental Procedures.” The figures presented are representative of at least two independent experiments performed in triplicate. The error bars represent the standard deviations, and when not seen, they are smaller than the thickness of the lines on the graphs. (B) Protein levels of pRb, β-actin, and DEGS-1 were detected by Western blotting. Cells were pretreated for ~18 h with either 10 nM OA (B, center panel) or 10 nM TMY (C, right panel) prior to 2.5 μM 4-HPR treatment. Total cell lysates were prepared 48 h after 4-HPR treatment. Equal amounts of proteins (30 μg) were run on 10% SDS-PAGE and blotted onto an Immobilon membrane as described in “Experimental Procedures.” β-actin was probed with to verify equal loading of proteins per lane. The figures presented are representative of at least three independent experiments.
Taken together these results show that 4-HPR is a potent and rapid inhibitor of dihydroceramide desaturase, which induces dihydroceramide (and not ceramide) generation in a dose dependent manner. It does not have any effects on the mRNA or protein levels of DEGS-1. The hypophosphorylation of Rb involves the activation of PP1.
DISCUSSION
In this study, we used a novel assay to measure dihydroceramide desaturase activity in intact cells employing cationic dihydroceramidoids (dhCCPS). This is the first study to employ siRNA to attenuate dihydroceramide desaturase function. The data presented demonstrate that human desaturase, DEGS-1, is the major desaturase active in SMS-KCNR cells, and that inhibition of its function with siRNA, along with the subsequent accumulation of endogenous dhCers leads to cell cycle arrest with de-phosphorylation of the retinoblastoma protein. It is the first study to implicate dihydroceramides in serine/threonine protein phosphatase activation.
Because of limitations in the solubility and bioavailability of conventional exogenous dhCers, novel cationic dhCers (dhCCPS analogs) with high solubility, cell membrane permeability, and cellular uptake were designed and synthesized (15). Measuring the conversion of the dhCCPS to the corresponding CCPS counterparts by LC/MS methodology was very accurate and highly reproducible. Since enzyme activity was measured within cells, there was no need for the addition of cofactors.
The in-situ assay was able to confirm the utility of the cyclopropene-containing ceramide analog, C8-CPPC as an inhibitor of the desaturase in intact cells. Interestingly, there was a decrease seen in the generation of dhCers with higher (2.5 μM) concentrations of C8-CPPC. This finding may be due to the effect of C8-CPPC on other sphingolipid enzymes (21). Thus, in addition to a siRNA approach, C8-CPPC treatment may be used as a tool to study the function of the enzyme.
DES family members belong to a desaturase/hydroxylase superfamily which is characterized by three histidine-containing consensus motifs (10). DEGS-1 is the only dihydroceramide desaturase reported to be present in human cells. Its mouse homologue (mDES1) was shown to have desaturase activity (33). hDES2, the human homologue of the mouse DES2 (mDES2) gene, was recently cloned and like mDES2 shown to have dihydroceramide hydroxylase activity (34). While mDES2 has been reported to have both desaturase and hydroxylase activity, no desaturase activity was detected in HEK 293 human embryonic kidney cells overexpressing hDES2 (34). Our data supports that DEGS-1 is the main dihydroceramide desaturase in human cells, since its loss with siRNA blocks its function and increases endogenous dhCers.
The data also show that dihydroceramide desaturase plays an essential role in the regulation of Cer levels, and the function of this enzyme is important in the downstream effects of Cer signaling. Cers have been shown to induce cell cycle arrest and Rb protein dephosphorylation in multiple cell lines (22–25). While both serine/threonine protein phosphatases PP1 and PP2A are involved in Rb dephosphorylation, only PP1 has been demonstrated to directly dephosphorylate Rb and be involved in Cer mediated Rb dephosphorylation (28,35). Previously, there have been no reports of the involvement of dhCers in cell cycle progression. Since the accumulation of dhCers persists even 6 days after siRNA silencing, the cell cycle effects along with the hyophosphorylation of Rb are likely due to the endogenous dhCers themselves.
Prior in-vitro studies with short chain dhCers failed to activate serine/threonine protein phosphatases (28,36). This may have been due to their poor solubility and/or short chain length. In this study tautomycin, a selective PP1 inhibitor, was able to inhibit the hypophosphorylation of Rb associated with dhCer accumulation resulting from siRNA transfection or 4-HPR treatment. Therefore, our results suggest that endogenous dhCers activate PP-1.
4-HPR is currently in clinical trials for neuroblastoma, leukemia, lymphoma, prostate and ovarian cancer. The mechanisms by which 4-HPR mediates anti-proliferative effects are not well understood. It can induce apoptosis independent of retinoid receptor pathways (RAR and RXR) (32,37). Activation of c-Jun N terminal kinase (38), generation of reactive oxygen species (ROS), induction of increased ceramide, gangliosides, and 12-Lipoxygenase have all been implicated (29,39,40).
Our data suggest that 4-HPR’s anti-tumor effects may be related to the accumulation of dhCers and/or more complex dihydrosphingolipids. In our experiments, lower concentrations (<3 μM) of 4-HPR decreased cell growth similar to DEGS-1 siRNA treatment, while higher concentrations (5 and 10 μM), at doses associated with ROS (29) generation caused cell death. Our findings revealed marked accumulation of endogenous dhCers via inhibition of dihydroceramide desaturase activity in SMS-KCNR cells treated with 4-HPR. The role and the mechanisms of ceramide generation in 4-HPR downstream effects has not been clear, and differences have been reported with different neuroblastoma cell lines and the concentration of 4-HPR used (29,39,40). Some of the limitations of these earlier studies were due to the method used for the quantitation of ceramide levels since ceramide was measured by enzymatic or labeling methods where it was difficult to differentiate Cers from dhCers. Given our experimental data, it is likely that dhCers were the sphingolipids that were elevated and not Cers in the previously published studies.
Previous studies on the biological activity of dhCers using their short chain analogs concluded that dhCers were inactive sphingolipids (12–14). This study with dhCCPS analogs suggests a novel biologic function for dhCers. Our findings are in agreement with some recent publications beginning to show biologic functions of dhCers. Jiang et al. (41), reported marked accumulation of dihydroceramide and dihydrosphingosine with -tocopherol treatment, which preceded apoptosis in LNCaP human prostate cancer cells. Tserng and Griffin (42) also reported C16-dhCer accumulation prior to cell death in HL-60 human leukemia cells.
Further investigation of the profile of complex sphingolipids/glycosphingolipids in response to DEGS-1 siRNA or 4-HPR treatment is warranted. Understanding the regulation of this enzyme has potential for future cancer therapy.
Acknowledgments
We would like to thank Barbara Rembiesa and Jason Pierce from the Lipidomics Core Laboratory in the Department of Biochemistry and Molecular Biology at the Medical University of South Carolina. This work is supported by National Institutes of Health Grants: K01-CA100767 (JMK), P01-CA97132 (YAH, BO, LMO), DE-016572 (BO), R01-AG016583 (LMO), C06-RR018823 (Lipidomics Core) and by a MERIT Award to LMO by the Office of Research and Development, Department of Veterans Affairs, Ralph H. Johnson VA Medical Center, Charleston, South Carolina, and by Grant Number P20-RR17677 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
The abbreviations used are
- dhCCPS
dihydroceramidoids
- CCPS
ceramidoids
- C12-dhCCPS
D-erythro-2-N-[12′-(1″-Pyridinium)-dodecanoyl]-4,5-dihydrosphingosine bromide
- C12-CCPS
D-e-C12-dhCCPS D-erythro-2-N-[12′-(1″-pyridinium)-dodecanoyl]-sphingosine bromide
- Cer
ceramide
- dhCer
dihydroceramide
- MUSC
Medical University of South Carolina
- 4-HPR
fenretinide
- ATRA
all-trans-retinoic acid
- OA
Okadiac Acid
- TMY
Tautomycin
- MTT
3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- C8-CPPC
C8-cyclopropenylceramide
- LC/MS
liquid chromatography/mass spectrometry
- siRNA
small interfering RNAs
- RT
reverse transcriptase
- pRb
phosphorylated retinoblastoma protein
- SCR
nonspecific siRna
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
References
- 1.Hannun YA, Obeid LM. J Biol Chem. 2002;277(29):25847–25850. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 2.Hannun YA. Adv Exp Med Biol. 1997;400A:305–312. doi: 10.1007/978-1-4615-5325-0_43. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Luberto C. Trends Cell Biol. 2000;10(2):73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 4.Hannun YA, Obeid LM. Biochem Soc Trans. 1997;25(4):1171–1175. doi: 10.1042/bst0251171. [DOI] [PubMed] [Google Scholar]
- 5.Ogretmen B, Hannun YA. Nat Rev Cancer. 2004;4(8):604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 6.Michel C, van Echten-Deckert G, Rother J, Sandhoff K, Wang E, Merrill AH., Jr J Biol Chem. 1997;272(36):22432–22437. doi: 10.1074/jbc.272.36.22432. [DOI] [PubMed] [Google Scholar]
- 7.Geeraert L, Mannaerts GP, van Veldhoven PP. Biochem J. 1997;327 ( Pt 1):125–132. doi: 10.1042/bj3270125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schulze H, Michel C, van Echten-Deckert G. Methods Enzymol. 2000;311:22–30. doi: 10.1016/s0076-6879(00)11063-8. [DOI] [PubMed] [Google Scholar]
- 9.Causeret C, Geeraert L, Van der Hoeven G, Mannaerts GP, Van Veldhoven PP. Lipids. 2000;35(10):1117–1125. doi: 10.1007/s11745-000-0627-6. [DOI] [PubMed] [Google Scholar]
- 10.Ternes P, Franke S, Zahringer U, Sperling P, Heinz E. J Biol Chem. 2002;277(28):25512–25518. doi: 10.1074/jbc.M202947200. [DOI] [PubMed] [Google Scholar]
- 11.Cadena DL, Kurten RC, Gill GN. Biochemistry. 1997;36(23):6960–6967. doi: 10.1021/bi970091l. [DOI] [PubMed] [Google Scholar]
- 12.Bielawska A, Crane HM, Liotta D, Obeid LM, Hannun YA. J Biol Chem. 1993;268(35):26226–26232. [PubMed] [Google Scholar]
- 13.Sugiki H, Hozumi Y, Maeshima H, Katagata Y, Mitsuhashi Y, Kondo S. Br J Dermatol. 2000;143(6):1154–1163. doi: 10.1046/j.1365-2133.2000.03882.x. [DOI] [PubMed] [Google Scholar]
- 14.Ahn EH, Schroeder JJ. Exp Biol Med (Maywood) 2002;227(5):345–353. doi: 10.1177/153537020222700507. [DOI] [PubMed] [Google Scholar]
- 15.Szulc ZM, Bielawski J, Gracz H, Gustilo M, Mayroo N, Hannun YA, Obeid LM, Bielawska A. Bioorg Med Chem. 2006 doi: 10.1016/j.bmc.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Methods. 2006;39(2):82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Kraveka JM, Li L, Bielawski J, Obeid LM, Ogretmen B. Arch Biochem Biophys. 2003;419(2):110–119. doi: 10.1016/j.abb.2003.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Cancer Res. 1987;47(4):943–946. [PubMed] [Google Scholar]
- 19.Ogretmen B, Schady D, Usta J, Wood R, Kraveka JM, Luberto C, Birbes H, Hannun YA, Obeid LM. J Biol Chem. 2001;276(27):24901–24910. doi: 10.1074/jbc.M100314200. [DOI] [PubMed] [Google Scholar]
- 20.Triola G, Fabrias G, Casas J, Llebaria A. J Org Chem. 2003;68(26):9924–9932. doi: 10.1021/jo030141u. [DOI] [PubMed] [Google Scholar]
- 21.Seville LL, Shah N, Westwell AD, Chan WC. Curr Cancer Drug Targets. 2005;5(3):159–170. doi: 10.2174/1568009053765816. [DOI] [PubMed] [Google Scholar]
- 22.Dbaibo GS, Pushkareva MY, Jayadev S, Schwarz JK, Horowitz JM, Obeid LM, Hannun YA. Proc Natl Acad Sci U S A. 1995;92(5):1347–1351. doi: 10.1073/pnas.92.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayadev S, Liu B, Bielawska AE, Lee JY, Nazaire F, Pushkareva M, Obeid LM, Hannun YA. J Biol Chem. 1995;270(5):2047–2052. doi: 10.1074/jbc.270.5.2047. [DOI] [PubMed] [Google Scholar]
- 24.Lee JY, Bielawska AE, Obeid LM. Exp Cell Res. 2000;261(2):303–311. doi: 10.1006/excr.2000.5028. [DOI] [PubMed] [Google Scholar]
- 25.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM. J Biol Chem. 1995;270(51):30701–30708. doi: 10.1074/jbc.270.51.30701. [DOI] [PubMed] [Google Scholar]
- 26.Chalfant CE, Kishikawa K, Mumby MC, Kamibayashi C, Bielawska A, Hannun YA. J Biol Chem. 1999;274(29):20313–20317. doi: 10.1074/jbc.274.29.20313. [DOI] [PubMed] [Google Scholar]
- 27.Dobrowsky RT, Hannun YA. J Biol Chem. 1992;267(8):5048–5051. [PubMed] [Google Scholar]
- 28.Kishikawa K, Chalfant CE, Perry DK, Bielawska A, Hannun YA. J Biol Chem. 1999;274(30):21335–21341. doi: 10.1074/jbc.274.30.21335. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Maurer BJ, Reynolds CP, Cabot MC. Cancer Res. 2001;61(13):5102–5105. [PubMed] [Google Scholar]
- 30.Schulz A, Mousallem T, Venkataramani M, Persaud-Sawin DA, Zucker A, Luberto C, Bielawska A, Bielawski J, Holthuis JC, Jazwinski SM, Kozhaya L, Dbaibo GS, Boustany RM. J Biol Chem. 2006;281(5):2784–2794. doi: 10.1074/jbc.M509483200. [DOI] [PubMed] [Google Scholar]
- 31.DiPietrantonio AM, Hsieh TC, Olson SC, Wu JM. Int J Cancer. 1998;78(1):53–61. doi: 10.1002/(sici)1097-0215(19980925)78:1<53::aid-ijc10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 32.Wu JM, DiPietrantonio AM, Hsieh TC. Apoptosis. 2001;6(5):377–388. doi: 10.1023/a:1011342220621. [DOI] [PubMed] [Google Scholar]
- 33.Omae F, Miyazaki M, Enomoto A, Suzuki M, Suzuki Y, Suzuki A. Biochem J. 2004;379(Pt 3):687–695. doi: 10.1042/BJ20031425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizutani Y, Kihara A, Igarashi Y. FEBS Lett. 2004;563(1–3):93–97. doi: 10.1016/S0014-5793(04)00274-1. [DOI] [PubMed] [Google Scholar]
- 35.Tamrakar S, Rubin E, Ludlow JW. Front Biosci. 2000;5:D121–137. doi: 10.2741/tamrakar. [DOI] [PubMed] [Google Scholar]
- 36.Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM, Hannun YA. J Biol Chem. 1994;269(30):19605–19609. [PubMed] [Google Scholar]
- 37.Clifford JL, Menter DG, Wang M, Lotan R, Lippman SM. Cancer Res. 1999;59(1):14–18. [PubMed] [Google Scholar]
- 38.Osone S, Hosoi H, Kuwahara Y, Matsumoto Y, Iehara T, Sugimoto T. Int J Cancer. 2004;112(2):219–224. doi: 10.1002/ijc.20412. [DOI] [PubMed] [Google Scholar]
- 39.Lovat PE, Oliverio S, Ranalli M, Corazzari M, Rodolfo C, Bernassola F, Aughton K, Maccarrone M, Hewson QD, Pearson AD, Melino G, Piacentini M, Redfern CP. Cancer Res. 2002;62(18):5158–5167. [PubMed] [Google Scholar]
- 40.Lovat PE, Di Sano F, Corazzari M, Fazi B, Donnorso RP, Pearson AD, Hall AG, Redfern CP, Piacentini M. J Natl Cancer Inst. 2004;96(17):1288–1299. doi: 10.1093/jnci/djh254. [DOI] [PubMed] [Google Scholar]
- 41.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. Proc Natl Acad Sci U S A. 2004;101(51):17825–17830. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tserng KY, Griffin RL. Biochem J. 2004;380(Pt 3):715–722. doi: 10.1042/BJ20031733. [DOI] [PMC free article] [PubMed] [Google Scholar]


