Summary
Following infections, natural killer (NK) cells acquire effector functions presumably by interacting with accessory cells. The cellular and molecular signals required for NK cell priming in vivo remain poorly defined. Using a mouse model for the inducible ablation of dendritic cells (DC), we show that the in vivo priming of NK cell responses to viral and bacterial pathogens depended on the presence of CD11chigh DC. Following peripheral TLR stimulation, priming of NK cells required their recruitment to local lymph nodes and interaction with DC leading to the emergence of effector NK cells in the periphery. NK cell priming depended on the recognition of type I IFN signals by DC and the subsequent production and trans-presentation of IL-15 by DC to resting NK cells. CD11chigh DC-derived IL-15 was necessary and sufficient for the priming of NK cells. Our data define a unique in vivo role of DC for the priming of NK cells, revealing a striking and previously unappreciated homology to T lymphocytes of the adaptive immune system.
Introduction
Natural killer (NK) cells are effector lymphocytes of the rapidly acting innate immune system that mediate cellular cytotoxicity, produce cytokines, and chemokines (Trinchieri, 1989). NK cells play an essential role in the recognition and eradication of virally infected cells and tumors (Lodoen and Lanier, 2006; Trinchieri, 1989). In contrast to T lymphocytes of the adaptive immune system, which need to be primed for effector functions by cognate interaction with antigen-presenting dendritic cells (DC) in lymph nodes (LN) (Banchereau and Steinman, 1998; Mempel et al., 2004), NK cells were originally identified as blood borne or tissue-resident, ‘naturally active’ cells that readily display effector functions upon encountering infected or transformed cells (Herberman et al., 1975; Kiessling et al., 1975; Trinchieri, 1989). However, NK cells from mice and humans show minimal effector functions (cytotoxicity, cytokine production) when incubated in vitro with tumor target cells or when directly triggered via their stimulatory receptors, suggesting that resting NK cells depend on additional signals for their activation (Bryceson et al., 2006). Indeed, most investigators treat mice with Toll-like receptor (TLR) ligands or cytokines (e.g., type I interferons, IFN-I or their synthetic inducers) or culture NK cells ex vivo in the presence of cytokines (e.g., IL-2, IL-15) to elicit detectable effector functions prior to in vitro analyses of NK cell responses (Bryceson et al., 2006; Djeu et al., 1979; Gidlund et al., 1978).
Given the increasingly appreciated role of innate immune cells and NK cells for protective therapeutic strategies, it is of considerable interest to establish a functional hierarchy of the molecular and cellular requirements that lead to NK cell activation in vivo. Some reports have suggested that NK cells, like myeloid cells, directly recognize pathogen-associated molecules via NK cell-expressed TLR (Hart et al., 2005; Schmidt et al., 2004; Sivori et al., 2004). Numerous in vitro studies have focused on a potential role of myeloid cells for the activation of NK cells and demonstrated that TLR-stimulated bone marrow (BM)- or monocyte-derived DC or macrophages from humans or mice contribute to NK cell activation (Andoniou et al., 2005; Baratin et al., 2005; Carbone et al., 1999; Chambers et al., 1996; Ferlazzo et al., 2004; Fernandez et al., 1999; Gerosa et al., 2002; Jinushi et al., 2003a; Krug et al., 2004; Piccioli et al., 2002). Both cytokines and NK cell receptor/ligand interactions have been implicated in the in vitro activation of NK cells by BM- or monocyte-derived DC. Most reports suggest that IFN-γ production of mouse and human NK cells after co-culture with pathogen-stimulated DC is diminished in the absence of IL-12 whereas cytotoxicity is dependent on the presence of IFN-I (Andoniou et al., 2005; Ferlazzo et al., 2004).
IL-15 is an important factor for the survival and the proliferation of NK cells during co-culture with DC (Ferlazzo et al., 2004). Ma and colleagues have suggested a pivotal role of DC-expressed IL-15Rα trans-presenting IL-15 both for killing and IFN-γ production by NK cells in vitro (Koka et al., 2004). It is currently unknown whether IL-15 mediates NK cell activation in vivo. In addition to cytokines, ligands for the stimulatory NKG2D receptor expressed by myeloid cells have been implicated in NK cell stimulation by DC (Andoniou et al., 2005; Hamerman et al., 2004; Jinushi et al., 2003b). It is unknown whether all these factors contribute to NK cell activation depending on the type of infection, the availability of cytokines, the expression of stimulatory ligands or whether some of these signals are non-redundant requirements for the in vivo induction of NK cell effector functions.
A role for myeloid cells in the induction of NK cell responses in vivo has been considered in previous reports (Andrews et al., 2003; Cudkowicz and Yung, 1977; Djeu et al., 1979; Fernandez et al., 1999; Shah et al., 1985). However, previous data were based on unspecific approaches to deplete DC (e.g., depletion of all CD8α+ cells including CD8αα+ DC) and did not analyze NK cell effector functions in the absence of DC (Andrews et al., 2003; Fernandez et al., 1999). Thus, the role of DC or other myeloid cells for the acquisition of NK cell effector responses (cytokine production, cytotoxicity and control of pathogens) in vivo remains undefined because until recently, the specific ablation of DC or macrophages was not feasible (Jung et al., 2002).
The spatiotemporal organisation of NK cell responses and of potential NK cell/DC interaction in vivo is unknown. It has been proposed that NK cells interact with DC in inflamed peripheral tissues (Moretta, 2002). Others have shown that NK cells are in intimate contact with DC in secondary lymphoid organs (Bajenoff et al., 2006; Ferlazzo et al., 2004) and that naïve NK cells can be recruited to the draining LN (DLN) after local injection of BM-derived TLR-stimulated DC or tumors (Chen et al., 2005; Martin-Fontecha et al., 2004). It is unknown whether the interaction of resting NK cells with DC in secondary lymphoid organs is required for the emergence of peripheral effector NK cells.
Using a genetic approach for the inducible ablation of DC, we investigated the role of DC in the acquisition of NK cell effector functions in vivo. Here we show that NK cell responses to viral and bacterial pathogens in vivo depend on their interaction with CD11chigh DC and on IFN-I signals received by DC. Marginal zone and/or metallophilic macrophages, which are also ablated in these mice, were dispensable. IFN-I experienced DC produced and trans-presented IL-15 in vivo, a signal necessary and sufficient for NK cell priming. Our data indicate that NK cells are not constitutively braced to respond according to the balance between activating and inhibiting signals. Instead, naïve NK cells need to acquire effector functions by interacting with DC in secondary lymphoid organs revealing a striking homology to T lymphocytes of the adaptive immune system.
Results
NK cell priming requires the presence of CD11chigh DC
To assess whether the acquisition of NK cell effector functions requires the presence of DC we employed a transgenic (tg) mouse model allowing the inducible ablation of all conventional CD11chigh DC including the CD8αα+ subset (Jung et al., 2002). In these mice, the simian diphtheria toxin receptor (DTR) is expressed as a fusion protein with green fluorescent protein (GFP) under the transcriptional control of the CD11c promoter. Injection of diphtheria toxin (DT) led to the ablation of all CD11chigh DC (Figure S1A) but did not affect relative (Figure S1A) or absolute NK cell numbers (Figure S1B) or the composition of NK cell subsets (Figure S1C). CD11cint cells, like plasmacytoid (p)DC (Figure S1A), and CD11cneg cells (T cells, B cells) were also unaffected in CD11c DTR tg mice after DT injection (Jung et al., 2002; Probst et al., 2005). To formally exclude any direct functional impact of DT on NK cells in CD11c DTR tg mice, we generated mixed BM chimeric mice in which the NK cell compartment was derived in equal parts from CD11c DTR tg and non-tg littermate BM cells. After DT injection and TLR stimulation, NK cells derived from CD11c DTR tg-derived stem cells were functionally indistinguishable from NK cells derived from the BM of littermate control mice (Figure S1D). Thus, CD11c DTR tg mice are a suitable in vivo model system to study NK cell activation in the absence of CD11chigh DC.
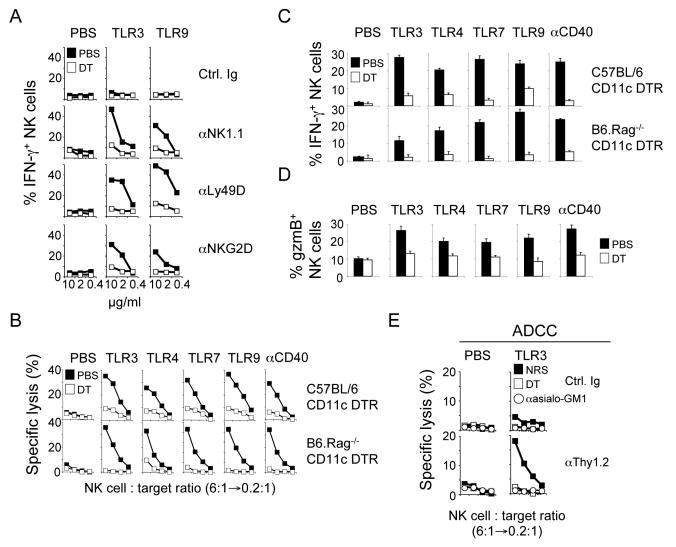
It is widely appreciated that infections strongly enhance NK cell effector functions but it remained unknown whether DC are required in this process in vivo. Innate immune recognition of viruses and other microbes is mediated to a large extent by TLR interacting with conserved microbial molecules (Beutler et al., 2006; Medzhitov and Janeway, 2002). As an initial assessment of the contribution of CD11chigh DC to NK cell responses following microbial infections in vivo, we injected TLR ligands into DC-ablated and control mice and analyzed NK cell effector functions. NK cells isolated from TLR-stimulated mice produced IFN-γ when directly triggered by the activating NK cell receptors NKR-P1C/NK1.1, NKG2D or Ly49D (Figure 1A) and killed (Figure 1B) or produced cytokines (Figure 1C) in response to tumor target cells expressing a stimulatory NKG2D ligand. Strikingly, NK cells isolated from CD11chigh DC-ablated mice displayed strongly reduced effector functions following TLR stimulation in vivo (Figures 1A-C). Similar results were obtained with various other NK cell targets including YAC-1 or RMA-S cells (data not shown). Following TLR stimulation, NK cells isolated from CD11chigh DC-ablated mice did not accumulate granzyme B (Figure 1D) and showed impaired upregulation of CD69 (Figure S2). Notably, non-microbial stimulation of CD11chigh DC by injection of an agonistic antibody specific for CD40 but not an isotype control antibody (data not shown) stimulated NK cell activation that was DC-dependent (Figures 1B-D and S2). Similar results were obtained with CD11c DTR tg mice deficient for recombination-activating gene 1 (RAG1-/-), which lack all T and B cells but have a functional NK and myeloid cell compartment (Figures 1B and 1C). These data demonstrate that B and/or T cells do not significantly contribute to DC-mediated NK cell activation.
Figure 1. Dendritic cells are required for the priming of NK cells.
(A-D) CD11c DTR tg mice or RAG1-/- CD11c DTR tg were injected with diphtheria toxin (DT) to ablate all CD11chigh DC (open symbols). Control mice received PBS injections (solid symbols). One day after DT injection, mice were injected i.p. with the indicated TLR ligands or an agonistic anti-CD40 antibody or with control injections of PBS or control Ig (not shown). NK cell effector functions were determined 18h after stimulation. IFN-γ production by highly purified splenic NK cells in response to immobilized anti-NKR-P1C/NK1.1, anti-Ly49D and anti-NKG2D antibodies (A). Cytotoxicity (B) and IFN-γ production (C) of NK cells against RMA-S/H60 targets. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). The percentage of NK1.1+CD3− cells in the lymphocyte populations was determined prior to the cytotoxicity assay and lymphocyte numbers were adjusted to contain the same number of NK cells. Thus, an NK cell:target ratio of 6:1 is equivalent to a 100-200:1 splenocyte:target ratio. Granzyme B (D) expression by NK cells. IFN-γ production and granzyme B expression were determined by intracellular cytokine staining and electronic gating on NK cells. Error bars display s.d. (n=3) and results are representative of at least three separate experiments.
(E) Groups of CD11c DTR tg mice were injected with DT (open squares). Control mice received PBS injections (solid squares). Some mice received two injections of anti-asialo GM1 antiserum to deplete all NK cells (open circles) and control mice received equal amounts of normal rabbit serum (NRS). One day later, mice were injected with TLR3 ligand or PBS. Antibody-dependent cytotoxicity (ADCC) of NK cells was evaluated against RMA cells pre-incubated with anti-Thy1.2 or control antibodies 18h after TLR stimulation.
NK cells isolated from TLR-stimulated mice fail to lyse RMA cells (Karre et al., 1986) but further enhancement of the stimulatory signal by pre-incubating RMA cells with anti-Thy1 antibodies led to NK cell-mediated antibody-dependent cellular cytotoxicity (ADCC), which required the presence of DC during TLR stimulation in vivo (Figure 1E). To formally exclude a contribution of CD11c+ cells of non-hematopoietic origin, we lethally irradiated non-tg B6 mice (CD45.1) and reconstituted them with BM from CD11c DTR tg mice (CD45.2) (Zammit et al., 2005). As in CD11c DTR tg mice, TLR or anti-CD40 stimulation of the BM-reconstituted mice failed to induce NK cell effector functions when CD11chigh DC were ablated (data not shown).
In most cases, NK cells from TLR-stimulated mice did not spontaneously produce IFN-γ ex vivo or in situ (Figure 1A and data not shown). However, these NK cells displayed potent effector functions when their stimulatory receptors were triggered demonstrating that TLR-stimulated DC ‘prime’ (rather than activate) NK cells in vivo. Thus, the presence of DC is required for the priming of NK cells, endowing them with effector functions in response to TLR stimulation or CD40-mediated stimulation of DC.
We considered a role for macrophages in the priming of NK cells as the analysis of DT-injected CD11c DTR tg mice revealed that, in addition to CD11chigh DC, splenic ER-TR9+ marginal zone (MZM) and MOMA-1+ metallophilic macrophages (MM) as well as their sinusoidal counterparts in LN are ablated. CD11b+ F4/80+ macrophages are unlikely to contribute to the priming of NK cells because they were not affected in DT-treated CD11c DTR tg mice, although a transient downregulation of the F4/80 antigen was observed (Probst et al., 2005). CD11chigh DC were restored 3-4 days after DT injection, whereas MZM/MM remain absent for more than 7 days (Jung et al., 2002; Probst et al., 2005). This differential kinetics of repopulation allowed us to experimentally address the question whether MZM and/or MM are involved in NK cell priming (Figure 2A). At day 2 after DT injection, both DC and MZM/MM were ablated whereas red pulp macrophages were unaffected (Figure 2B, C) and NK cells cannot be primed for effector responses following TLR stimulation (Figure 2D, E). In contrast, NK cells were primed at day 5 after DT injection (Figure 2D, E), when CD11chigh DC had repopulated the spleen but MZM/MM were still absent (Figure 2B, C). These data exclude an important role of these macrophage populations for NK cell priming. In agreement with these data, NK cell effector functions in DC-ablated mice could be restored by a single injection of 3-10×106 non-transgenic DC two days prior to microbial stimulation (Figure 2F). In contrast, the same number of macrophages was unable to rescue NK cell priming under these experimental conditions (Figure 2F). Collectively, our data demonstrate that CD11chigh DC but not macrophages play a central and non-redundant role for the priming of NK cell responses in vivo.
Figure 2. CD11chigh DC but not macrophages are required for NK cell priming.
(A-E) Groups of CD11c DTR tg mice were injected with DT (open symbols). Control mice received PBS injections (solid symbols). One day or 4 days later, mice were injected with the indicated TLR ligand and analyzed 18-24h later (A). Immunophosphatase staining of splenic sections from TLR stimulated mice for the indicated cell surface markers 2 or 5 days after DT injection. Magnification, 200x (B). The percentage of CD11chigh MHC-II+ cells in the spleen was evaluated by flow cytometry analysis at the indicated time points after DT injection (C). Cytotoxicity (D) and IFN-γ production (E) of splenic NK cells were determined at the indicated timepoints after DT injection against RMA-S/H60 targets. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=3) and results are shown from two independent experiments, which are representative of three experiments performed.
(F) Dendritic cells but not macrophages rescue NK cell priming. CD11c DTR tg mice were injected i.v. with 3-10×106 BM-derived DC or macrophages one day prior to DC ablation. One day later, mice were injected with TLR3 ligand and cytotoxicity (left) and IFN-γ (right) production of NK cells were assayed against RMA-S/H60 cells 18h after TLR stimulation. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of five (cytotoxicity data) or three (IFN-γ data) separate experiments. nd, not done.
(G) NK cells do not require cell-autonomous TLR-signaling for priming. Mixed BM chimeric mice were generated by injecting lethally irradiated mice with BM cells derived from the indicated TLR signaling-deficient mouse strains or from B6 control mice expressing the indicated congenic markers. BM chimeric mice were injected 8-12 weeks later with the indicated TLR ligands. IFN-γ production of CD45.1+ (solid bars) and CD45.2+ NK cells (open bars) was assayed in response to RMA-S/H60 target cells 18h after TLR stimulation. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of two separate experiments.
NK cell-autonomous TLR signaling is not required for NK cell priming
Recent in vitro studies using human NK cells suggested that NK cell activation might be mediated by direct interaction with microbial products via NK cell-expressed TLR (Hart et al., 2005; Schmidt et al., 2004; Sivori et al., 2004). We investigated the contribution of NK cell-autonomous TLR signals for the priming of NK cells in response to TLR ligands in mixed BM chimeric mice, in which the NK cell compartment was derived in equal parts from TLR signaling-deficient (myeloid differentiation factor 88, MyD88-/- or Toll-interleukin 1 receptor domain-containing adaptor inducing IFN-β, TRIF-/-) or competent hematopoietic stem cells, which were distinguishable by a congenic marker. Injection of TLR ligands including those entirely dependent on MyD88 (TLR9) or TRIF (TLR3) signaling led to efficient priming of both B6-derived and TLR signaling-deficient NK cells demonstrating that NK cell-autonomous TLR signaling is not a requirement for NK cell priming (Figure 2G). These data also rule out an important contribution of IL-1 or IL-18 because their cytokine receptors require MyD88 for signaling (Adachi et al., 1998).
NK cell responses to pathogens require the presence of CD11chigh DC
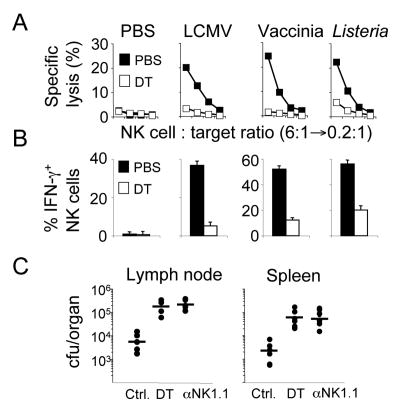
These findings led us to ask whether NK cell responses to pathogens would also require the presence of CD11chigh DC. Infection of mice with viruses (lymphocytic choriomeningitis virus, LCMV and vaccinia virus, VV) and local infection with bacteria (Listeria monocytogenes) that do not depend on DC for their replication, resulted in potent priming of NK cells. NK cell priming was strongly reduced in mice ablated of CD11chigh DC (Figures 3A and 3B). During the first days, local Listeria infection is controlled by NK cell-derived IFN-γ production (Dunn and North, 1991). Ablation of CD11chigh DC or depletion of all NK cells led to a similar increase in pathogen load (Figure 3C) demonstrating that potent NK cell responses to pathogens in vivo are critically dependent on the presence of CD11chigh DC.
Figure 3. NK cell responses to pathogens require DC.
(A and B) DC-ablated (open symbols) and control mice (solid symbols) were infected with the indicated pathogens 12h after DT injection. NK cell activation was analyzed 24h (Listeria) or 48h (LCMV and Vaccinia) later by determining cytotoxicity (A) and IFN-γ production (B) in response to RMA-S/H60 target cells. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of three separate experiments.
(C) NK cell-mediated control of local Listeria infection requires the presence of DC. Mice were depleted of NK cells by injection anti-NK1.1 antibody two days prior to and at the day of infection. Control mice received the same amount of mouse Ig. DC were ablated by DT injections one day before infection. Mice were infected subcutaneously with Listeria and bacterial titers from the draining LN and spleen were determined one day later. Each symbol represents results from an individual mouse and bars represent the arithmetic mean.
Emergence of peripheral effector NK cells after local infection
Our in vivo findings are reminiscent of T cell priming, which requires cognate interactions between T cells and DC in the LN draining infections (Banchereau and Steinman, 1998; Mempel et al., 2004). After this interaction, T cells leave the LN via the efferent lymph and emerge as effector T cells in peripheral organs where they can effectively battle infected cells (Masopust et al., 2001; Reinhardt et al., 2001). It is unknown where the functionally relevant NK cell/DC interactions take place during an infection and whether an interaction of NK cells with DC in LN or other secondary lymphoid organs (i.e., spleen) would lead to the priming of effector NK cells migrating to the periphery. To investigate this issue, we studied NK cell recruitment and priming following peripheral microbial challenge in an example secondary lymphoid organ, the LN, because it allows us to specifically interfer with NK cell recruitment and to locally restrict the microbial stimulus.
Using adoptive transfer of naïve CD45.1+ splenocytes into B6 mice (CD45.2+), we show that in the absence of inflammation (not shown) or in NDLN, less than 1% of donor-derived lymphocytes are NK cells (Figure S3A). During the first four hours after local TLR stimulation, the proportion of donor-derived NK cells in the DLN strongly increased whereas the proportion of T cells did not significantly change (Figure S3A). Local TLR stimulation led to an increase in LN cellularity (Figure S3B, upper panel). While the total number of donor-derived lymphocytes and T cells in the DLN increased proportionally (8-15-fold), the total number of NK cells increased by one order of magnitude more (80-200-fold), demonstrating that NK cells are strongly recruited to DLN early after infection (Figure S3B). Similar results were obtained by injection of TLR4, 7, 9 ligands or Listeria (data not shown) and are supported by previous data investigating the recruitment of NK cells to LN following local application of TLR stimulated BM-DC (Martin-Fontecha et al., 2004).
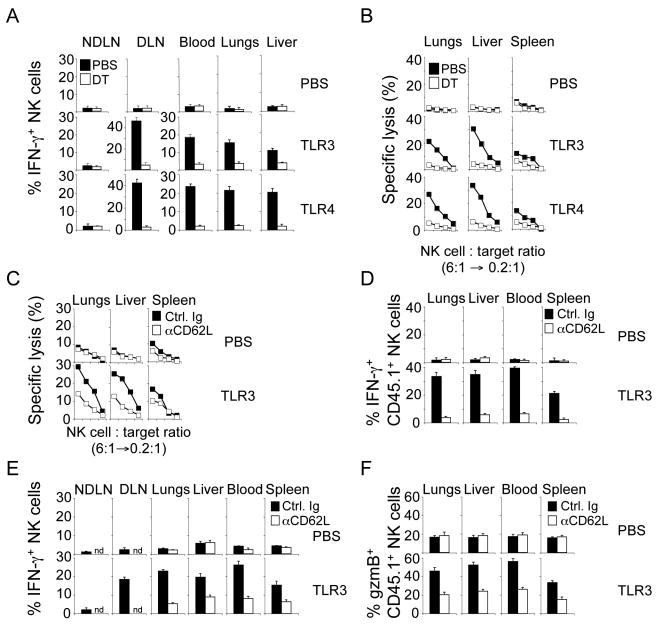
Does local TLR stimulation lead to the DC-dependent emergence of effector NK cells in the periphery? Similar to splenic NK cells (Figures 1 and S2), resting NK cells isolated from LN, blood, liver, lungs and bone marrow had a naïve phenotype (CD69low, CD25neg, granzyme Blow) and did not display any effector functions when incubated with target cells (Figure 4 and data not shown). Similar to the i.p. injection of TLR ligands, local TLR stimulation led to the CD11chigh DC-dependent priming of NK cells in the DLN but not the NDLN (Figure 4A). Interestingly, hours after local TLR stimulation primed NK cells emerged in blood and peripheral organs (Figures 4A and 4B). The emergence of primed NK cells in the periphery did not occur in the absence of CD11chigh DC. Collectively, these data demonstrate that NK cells are recruited to secondary lymphoid organs draining microbial stimuli where they interact with DC and emerge after this interaction as effector cells in the periphery.
Figure 4. Local priming of NK cells requires interaction with DC and requires the recruitment of NK cells to lymph nodes.
(A and B) One day after DT injection, B6 mice (solid symbols) or CD11c DTR tg mice (open symbols) were injected subcutaneously (s.c.) into one footpad with the indicated TLR ligand. NK cells were purified from the indicated organs and IFN-γ production (A) and cytotoxicity (B) was determined in response to RMA-S/H60 cells 18-24h later. Similar results were obtained with YAC-1 and RMA-S target cells (data not shown). Error bars display s.d. (n=3-4) and results are representative of three separate experiments.
(C and D) Blocking entry of NK cells into LN results in dramatically decreased numbers of effector NK cells in peripheral tissues. B6 mice were injected with control Ig (Ctrl. Ig; solid symbols) or an anti-CD62L antibody (open symbols) blocking entry of NK cells into the LN. One day later, mice were injected with the indicated TLR ligands. Cytotoxicity (C) and IFN-γ production (D) of NK cells purified from the indicated tissues in response to RMA-S/H60 cells was determined 18-24h after local TLR stimulation. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of three separate experiments.
(E and F) Circulating naïve NK cells need to enter the draining LN for priming. B6 mice (CD45.2+) were injected i.p. with control Ig (solid symbols) or an anti-CD62L antibody (open symbols). Two hours later, 3×106 purified NK cells (CD45.1+) were injected i.v. followed by TLR stimulation 12h later. IFN-γ production (E) and granzyme B expression (F) of transferred CD45.1+ NK cells isolated from the indicated tissues in response to RMA-S/H60 cells was determined by intracellular cytokine staining and electronic gating on NK cells 18-24h after local TLR stimulation. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of two separate experiments. nd, not done.
NK cell priming requires entry of naïve NK cells into secondary lymphoid organs
To directly address the question whether the emergence of peripheral effector NK cells requires entry of naïve NK cells into secondary lymphoid organs, we evaluated the function of peripheral NK cells under conditions that prevented NK cells from entering the DLN. NK cell entry into LN is dependent on the interaction of L-selectin (CD62L) with its addressins on high endothelial venules (Bajenoff et al., 2006; Chen et al., 2005). Blocking entry of NK cells into LN due to treatment with an anti-CD62L antibody prior to local microbial stimulation resulted in dramatically decreased numbers of effector NK cells in peripheral tissues (Figures 4C and 4D). To directly demonstrate that circulating naïve NK cells must enter the draining LN for priming, we adoptively transferred highly purified, naïve NK cells (CD45.1+) into syngeneic CD45.2+ B6 mice prior to local TLR stimulation. In control mice, transferred CD45.1+ NK cells emerged as effector cells (IFN-γ production, granzyme B upregulation) in peripheral tissues whereas NK cells prevented from migrating to the DLN remained naïve (Figures 4E and 4F). We demonstrate that NK cells are recruited to the LN draining microbial infections and that LN entry and NK cell/DC interaction is required for the emergence of primed NK cells in the periphery.
IFN-I signals are required for the priming of NK cells
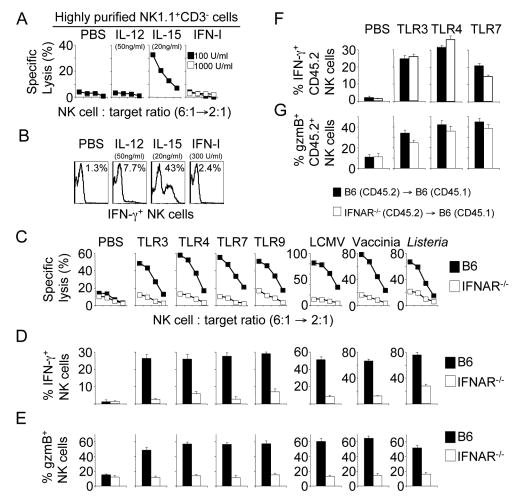
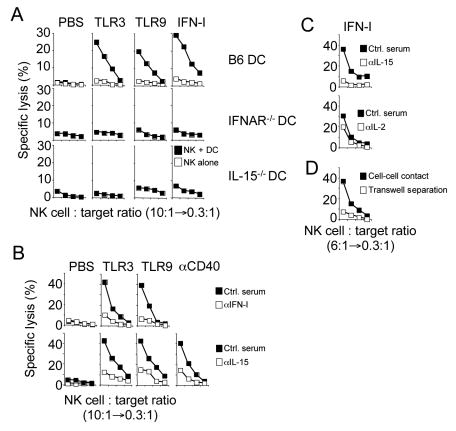
Previous studies employing in vitro co-culture of NK cells with BM-DC identified a variety of soluble factors produced by DC that were able to enhance NK cell functions (Andoniou et al., 2005; Fernandez et al., 1999; Krug et al., 2004). In order to identify potential non-redundant signals for the priming of NK cells in vivo, we investigated NK cell priming in various mouse strains genetically deficient for cytokines or cytokine signaling. NK cell priming in response to all TLR ligands tested was unaffected by the absence of IFN-γ, the IFN-γ receptor or CD40 (data not shown). Although IFN-γ production was reproducibly reduced in response to some microbial stimuli (TLR4, TLR9, Listeria), IL-12-deficient mice allowed for substantial priming of NK cell responses (Figure S4). In addition, stimulation of highly purified NK cells with recombinant IL-12 in vitro did not lead to efficient priming of NK cells for killing (Figure 5A) or IFN-γ production (Figure 5B). Collectively, these data suggest that IL-12 is not required for NK cell priming but can potently enhance IFN-γ production of primed NK cells.
Figure 5. Priming of NK cells in response to infections requires IFN-I signaling and IL-15.
(A and B) IL-15 is sufficient to prime NK cells in vitro. Highly purified NK cells were incubated overnight with the indicated cytokines. Cytotoxic activity of NK cells (A) and IFN-γ production (B) was determined in response to RMA-S/H60 target cells. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). The numbers in the histograms (B) represent the percentage of IFN-γ+ NK cells as determined by intracellular cytokine staining.
(C-E) NK cell priming requires IFN-I signaling. B6 (solid symbols) or IFNAR-/- mice (open symbols) were injected with the indicated TLR ligands or pathogens and cytotoxicity (C) or IFN-γ production (D) in response to RMA-S/H60 target cells and granzyme B expression (E) by NK cells were assayed 18h later. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=4) and results are representative of five separate experiments.
(F and G) NK cell priming does not require NK cell-autonomous IFN-I signaling. Purified CD45.2+ B6 (solid bars) or CD45.2+ IFNAR-/- NK cells (open bars) were transferred i.v. into CD45.1+ B6 recipients. One day later, mice received i.p. injections of PBS or the indicated TLR ligands and IFN-γ production (F) of CD45.2+ NK cells in response to RMA-S/H60 cells and granzyme B expression (G) by donor NK cells was assayed 18-24h after TLR stimulation. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of three separate experiments.
In contrast, the IFN-I receptor (IFNAR) was required for the priming of NK cells in response to all TLR ligands and pathogens tested, demonstrating a central role of this signaling pathway (Figures 5C-E). Whereas IFNAR was absolutely required for NK cell cytotoxicity (Figure 5C) and the upregulation of granzyme B expression (Figure 5E), residual, although strongly reduced, IFN-γ responses could be elicited in response to Listeria infection and to TLR4 and TLR9 stimulation (Figure 5D). Thus, NK cell priming largely depends on a functional IFN-I signaling pathway.
NK cell-autonomous IFN-I signalling is not required for NK cell priming
Surprisingly, IFNAR-/- NK cells (CD45.2+) transferred into IFNAR+/+ mice (CD45.1+) were primed to the same extent as transferred IFNAR+/+ NK cells (Figures 5F and 5G) and incubation of highly purified naïve NK cells with graded doses of IFN-I in vitro failed to enhance NK cell effector functions (Figures 5A and 5B). Thus, IFN-I signals are required for NK cell priming in response to pathogens, but IFN-I do not prime NK cells directly.
To investigate IFN-I effects on DC leading to the priming of NK cells, we employed an in vitro co-culture system of BM-derived DC and highly purified NK cells. In extension of previous work (Andoniou et al., 2005; Ferlazzo et al., 2004), we found that TLR- (Figure 6A) and anti-CD40-stimulated (Figure 6B), but not unstimulated BM-DC prime NK cells whereas these stimuli had no direct effect on NK cells (Figure 6A). Confirming our in vivo findings, priming of NK cells by stimulated DC depended on the presence of the IFN-I receptor on DC but not NK cells (Figure 6A) and was abrogated in the presence of blocking antibodies to IFN-I (Figure 6B, top). IFN-I stimulation of BM-DC was sufficient to prime IFNAR-/- NK cells (Figure 6A). These data demonstrate that TLR stimulation of BM-DC induces IFN-I production and the recognition of the IFN-I signal by DC (but not NK cells) is required for the priming of NK cells (Figures 5 and 6A, B).
Figure 6. TLR stimulation of DC induces IFN-I production and the reception of the IFN-I signal by DC (but not NK cells) is required for the priming of NK cells.
(A) Highly purified IFNAR-/- NK cells were either cultured in the presence (solid squares) or absence (open squares) of BM-DC from the indicated mouse strains stimulated as indicated. Lysis of RMA-S/H60 cells by NK cells was determined 12h later. Similar results were obtained with RMA-S and YAC-1 targets (data not shown).
(B and C) BM-DC from B6 mice were stimulated with the indicated TLR ligands, an agonistic anti-CD40 antibody (B) or with IFN-I (C) and co-cultured with NK cells in the presence of control serum (solid squares) or antisera specific for the indicated cytokines (open squares). NK cell cytotoxicity against RMA-S/H60 target cells was determined 12h later. Similar results were obtained with RMA-S and YAC-1 targets (data not shown).
(D) Cytotoxicity of NK cells either co-cultured with IFN-I stimulated DC in the same well (solid squares) or spatially separated (open squares) by a membrane (0.4μm pores) was assayed against RMA-S/H60 cells. Similar results were obtained with RMA-S and YAC-1 targets (data not shown).
IL-15 is required and sufficient for NK cell priming in vivo
As IFN-I did not directly prime NK cells, we wanted to identify the molecular signal(s) induced by IFN-I leading to NK cell priming. It has been suggested that IFN-I and CD40 stimulation induce the production of IL-15 by DC (Jinushi et al., 2003b; Koschella et al., 2004; Mattei et al., 2001; Nguyen et al., 2002; Zhang et al., 1998). In support of a central role for IL-15 in the priming of NK cells, TLR- or IFN-I stimulated BM-DC derived from IL-15-/- mice were unable to prime NK cells (Figure 6A, bottom) and neutralization of IL-15 (but not IL-2) during co-culture of TLR-, anti-CD40- (Figure 6B) or IFN-I-stimulated DC (Figure 6C) with NK cells strongly reduced NK cell priming. Thus, IFN-I induced IL-15 is required for the priming of NK cell responses.
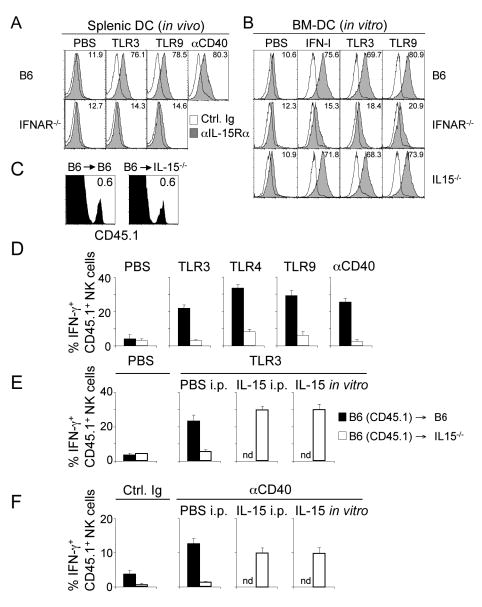
Previous work showed that IL-15 is trans-presented on the surface of IL-15-producing cells in complex with the IL-15 receptor α chain (IL-15Rα) creating an IL-15 gradient and confining the action of IL-15 (Dubois et al., 2002; Koka et al., 2004). This is in agreement with our observation that IL-15-dependent priming of NK cells by DC in vitro was cell-cell contact dependent under our experimental conditions (Figure 6D). TLR or IFN-I stimulation led to the IFN-I-dependent upregulation of IL-15Rα expression by DC in vivo (Figure 7A) and in vitro (Figure 7B) (Koka et al., 2004). Direct stimulation of DC with an anti-CD40 antibody in vivo also resulted in the IFN-I-dependent upregulation of IL-15Rα (Figure 7A).
Figure 7. Trans-presented IL-15 is a non-redundant priming signal for NK cells.
(A and B) IL-15Rα expression (shaded histograms) by splenic DC 24h after TLR or anti-CD40 stimulation in vivo (A) or by BM-DC 24h after in vitro stimulation (B). Open histograms represent staining with control Ig. The numbers indicate the percentage of IL-15Rα+ CD11chigh MHC-II+ cells. Data are representative of at least three independent experiments.
(C) Highly purified CD45.1+ NK cells were injected into either CD45.2+ B6 or CD45.2+ IL-15-/- mice. The percentage of annexin V- CD45.1+ NK cells (in the lymphocyte gate) recovered from the spleens of B6 or IL15-/- mice 6h after transfer are indicated. Data are representative of five independent experiments.
(D-F) Highly purified CD45.1+ NK cells were injected i.v. into either CD45.2+ B6 (solid bars) or CD45.2+ IL-15-/- mice (open bars). Mice were injected 2h later with the indicated TLR ligands or anti-CD40. Control mice received injections of PBS or control Ig (Ctrl. Ig), respectively. IFN-γ production of gated CD45.1+ NK cells against RMA-S/H60 target cells was determined 4h after TLR stimulation by intracellular cytokine staining and electronic gating on donor NK cells. Some IL-15-/- mice received i.p. injections of 4μg IL-15 at the time of TLR stimulation (IL-15 i.p.). In some experiments, 10ng/ml IL-15 was added during the in vitro restimulation of donor NK cells with RMA-S/H60 target cells required for the determination of intracellular cytokine expression (IL-15 in vitro). Similar results were obtained with RMA-S or YAC-1 target cells (data not shown). Error bars display s.d. (n=3) and data are representative of at least three independent experiments. nd, not done.
Whereas NK cells (CD45.1) transferred into an IL-15–competent environment (CD45.2) were primed after TLR3, 4 or 9 stimulation, or direct triggering of the CD40 receptor (Figure 7D), NK cells transferred into IL-15–deficient mice (CD45.2) were not. NK cell responses to Listeria and LCMV infection also required IL-15 production (data not shown). Priming of NK cells in IL-15-/- mice could be completely restored in vivo by i.p. injection of IL-15 at the time of TLR or anti-CD40 stimulation (Figures 7E, F, IL-15 i.p.). Although IL-15 is required for the survival of NK cells (Cooper et al., 2002; Kennedy et al., 2000; Prlic et al., 2003), the number of donor-derived functional and non-apoptotic NK cells recovered from the IL-15-/- environment remained comparable to the number of donor-derived NK cells from an IL-15+/+ environment (Figure 7C) if the duration of the experiment was short (4-8 hours). In addition, donor-derived NK cells isolated from IL-15-/- mice were fully functional in vitro when IL-15 was added to the cultures during the IFN-γ assay (Figures 7E, F, IL-15 in vitro) providing further evidence that the lack of NK cell priming in IL-15-/- mice is not reflective of defective NK cells. Our data demonstrate that NK cells are primed by IL-15 trans-presented on IL-15Rα+ DC, expression of which is induced by IFN-I produced in response to microbial stimulation. As IL-15 was the only cytokine able to efficiently prime NK cells in vitro (Figures 5A, B and 6A-C), we conclude that IFN-I induced IL-15 constitutes the central signal required for the priming of NK cells in response to infections.
Discussion
Here we report that potent antimicrobial NK cell responses to all viral and bacterial infections tested require the interaction of NK cells with CD11chigh DC in secondary lymphoid organs whereas macrophages cannot compensate for a complete lack of DC. Our data support a concept in which NK cells undergo a process of priming that rapidly initiates a molecular program endowing NK cells with effector functions. We chose to term this process ‘priming’ because in most cases NK cells were not activated when interacting with DC in situ (i.e., they do not produce IFN-γ) but, unlike naïve NK cells, were able to recognize diseased cells. We have carefully compared the functional properties of freshly isolated NK cells from primary (bone marrow) and secondary lymphoid organs (spleen, LN), blood and peripheral tissues (lung, liver). NK cells from all organs tested displayed a naïve phenotype and the fraction of NK cells directly triggered by various potent target cells in vitro was negligible (< 3%) contradicting the idea of a sizeable population of locally restricted ‘naturally active’ NK cells. Although triggering of a single stimulatory receptor (with the notable exception of CD16) did not activate resting human NK cells in redirected lysis assays, they could be activated for redirected lysis by co-engagement of two or more stimulatory receptors (Bryceson et al., 2006). In contrast, naïve NK cells from mice are not appreciably activated by target cells (e.g., YAC-1) known to express ligands for at least two stimulatory immunoreceptors (Jamieson et al., 2002; Pessino et al., 1998). It is unknown whether naïve NK cells can be triggered in redirected lysis assays after crosslinking of two or more stimulatory receptors. However, our ADCC experiments employing CD16-mediated amplification of NK cell responses did not lead to activation of naïve NK cells and ADCC required priming by DC. Our data strongly support the concept that resting NK cells in mice are naïve lymphocytes that require a priming event to obtain full effector functions.
A potential role of DC for NK cell activation was inferred from in vitro co-culture experiments employing TLR stimulated or infected BM- or monocyte-derived DC or macrophages which were able to activate NK cells (Andoniou et al., 2005; Carbone et al., 1999; Chambers et al., 1996; Ferlazzo et al., 2004; Fernandez et al., 1999; Gerosa et al., 2002; Jinushi et al., 2003a; Koka et al., 2004; Krug et al., 2004; Piccioli et al., 2002). Although a potential role of endogenous DC for the in vivo activation of NK cells has been extensively discussed on the basis of these in vitro data, no data was available analyzing NK cell effector functions in vivo (cytotoxicity, cytokine production, antimicrobial activity) in the absence of DC. A previous report using depletion of all CD8α+ cells including CD8αα+ DC found that at day 6 after MCMV infection the accumulation of Ly49H+ NK cells was reduced (Andrews et al., 2003) but did not analyze NK cell effector functions. No differences in the expansion of Ly49H+ NK cells were found at earlier stages of infection with MCMV (day 2 or 4) when NK cells are known to be activated for killing and cytokine production (Scalzo et al., 1992). In addition, NK cell priming and acquisition of NK cell effector functions do not generally require proliferation of distinct NK cell clones (M.L. and A.D., unpublished data). DC-ablated CD11c DTR tg mice infected with herpes simplex virus type 1 (HSV-1) showed decreased survival implicating defective T and NK cell responses (Kassim et al., 2006). Our data are the first to provide definitive evidence that NK cell effector responses require the in vivo interaction of NK cells with CD11chigh DC.
It was unknown where the priming of NK cells by DC occurs. Two concepts have been advanced predicting either an interaction between NK cells and DC in peripheral tissues (Cooper et al., 2004; Moretta, 2002) or in secondary lymphoid organs (Bajenoff et al., 2006; Chen et al., 2005; Ferlazzo et al., 2004; Martin-Fontecha et al., 2004). Infections start with the local invasion of pathogens and the initiation of an immune response in the local LN or in the spleen after hematogenous spreading of the pathogen. Our data demonstrate that following local infection or TLR stimulation, NK cells are recruited to the DLN where they are primed by CD11chigh DC. Once primed in the local LN, NK cells emerge as effector cells in blood and peripheral tissues. Blocking of LN entry abrogated the appearance of primed NK cells in the periphery, ruling out the possibility that systemic TLR effects are responsible for the priming of peripheral NK cells. It must be noted that NK cell priming is likely to also occur in other secondary lymphoid organs (e.g., spleen), but we chose to use the LN as a model, because we can experimentally interfer with NK cell recruitment to the LN and locally restrict the microbial stimulus. Our data indicate that NK cells, like T cells, leave secondary lymphoid organs after their interaction with DC and preferentially migrate to peripheral organs to battle diseased cells. Studies are under way to further analyze the migratory pattern of NK cells and to elucidate the molecular program regulating this process.
The precise mechanism by which DC prime NK cells was unknown. A previous in vitro study by Koka et al. indicated that LPS stimulation of BM-derived DC led to increased expression of IL-15Rα which was required for IFN-γ production and cytotoxicity by NK cells following in vitro co-culture with BM-DC (Koka et al., 2004). We provide evidence that IL-15 produced by DC in response to microbial stimulation or direct CD40 triggering and its presentation on IL-15Rα to NK cells is required and sufficient for NK cell priming in vivo. Our data clarify that the reported TLR-induced IL-15 production by DC is indirect and requires IFN-I production and signaling. Our findings are supported by data showing that NK cells derived from uninfected mice constitutively overexpressing IL-15 (IL-15 tg mice) display a primed phenotype whereas NK cells from non-tg littermates are naïve (Fehniger et al., 2001). It is intriguing that IL-15-expressing CD11chigh DC are required for NK cell priming, although IL-15 can be expressed by many cells including other leukocytes and epithelial cells (Mention et al., 2003; Shinozaki et al., 2002; Waldmann et al., 2001). The central role of CD11chigh DC for NK cell priming is likely attributable to their unique ability to create a locally confined niche of intimate NK cell/DC contact in secondary lymphoid organs (Bajenoff et al., 2006) and to their immediate upregulation of both IL-15 and IL-15Rα expression following infections. Our data do not rule out that IL-15 expressing cells other than DC might play an important role for NK cell homeostasis or for the maintenance of NK cell responses under conditions of chronic inflammation which is frequently associated with increased IL-15 expression by epithelial cells (Mention et al., 2003; Shinozaki et al., 2002). An interesting question is, whether in situ production of IL-15 by DC is involved in the maturation of human LN-resident CD34+ hematopoietic precursor cells into CD56bright NK cells (Freud et al., 2005; Freud et al., 2006). Our data indicate that the IL-15/IL-15Rα system functions as a rheostat to regulate distinct aspects of NK cell function. Constitutive expression of IL-15/IL-15Rα is required for the survival of NK cells while IFN-I induced upregulation of IL-15/IL-15Rα by DC is indispensable for their priming. Intriguingly, very high levels of DC-derived IL-15 have been recently implied in causing immunopathology (Ohteki et al., 2006).
We have identified IFN-I as the principal inducer of IL-15 production by DC following TLR stimulation or bacterial and viral infections in vivo. This is supported by previous studies suggesting that IFN-I can induce IL-15 production by myeloid cells (Jinushi et al., 2003b; Mattei et al., 2001) and by data indicating that IFN-I-dependent IL-15 production is required for the accumulation of NK cells following MCMV infection (Nguyen et al., 2002). Although IFN-I are long known to be potent inducers of NK cell activation (Djeu et al., 1979; Gidlund et al., 1978), our data clarify that NK cell-autonomous IFN-I signalling is not required for NK cell priming. Instead, priming of NK cells for cytotoxicty and cytokine production requires a functional IFN-I signalling pathway within DC. An important and unresolved question is the source of IFN-I following TLR stimulation or infection. Previous work suggested an important role for IFN-I production by pDC for NK cell activation following MCMV infection (Dalod et al., 2003; Krug et al., 2004). Although specific depletion of pDC in vivo led to reduced IFN-I production following MCMV infection, NK cell responses were not impacted because increased IL-12 production by myeloid DC could compensate for the reduced availability of IFN-I (Krug et al., 2004). Our data demonstrate that the central role of CD11chigh DC for NK cell priming is their unique ability to quickly respond to pathogen-induced IFN-I production with enhanced expression of IL-15Rα and IL-15.
Other cytokines, although not absolutely required for NK cell priming, might play an important role to further increase NK cell activity when IL-15/IL-15Rα levels are limiting, which is supported by the observation that IL-12 and IL-15/IL-15Rα act synergistically in activating cytokine production by NK cells in vitro (Koka et al., 2004). Specifically, the limited role of DC-derived IL-12 for IFN-γ production in vivo was unexpected but the majority of data indicating a non-redundant role of IL-12 for IFN-γ production by NK cells were obtained from in vitro experiments (Andoniou et al., 2005; Ferlazzo et al., 2004; Krug et al., 2004). In agreement with previous reports, we detected a reproducible reduction of IFN-γ production in IL-12-deficient mice in response to certain stimuli (TLR4, TLR9, Listeria) (Tripp et al., 1994; Tripp et al., 1993). However, when compared to IFN-I or IL-15, the requirement of IL-12 for IFN-γ production by NK cells was less absolute. This is supported by our in vitro data showing that direct stimulation of highly purified naïve NK cells with IL-12 did not elicit substantial IFN-γ responses.
In many ways, NK cell priming resembles the priming of T lymphocytes. However, NK cell priming does not generally require proliferation of distinct NK cell clones (data not shown). The molecular signals required for NK cell priming and bystander proliferation of T lymphocytes after viral infection or CD40-stimulation are strikingly similar (Koschella et al., 2004; Lodolce et al., 2001; Tough et al., 1996; Zhang et al., 1998). These findings suggest that IFN-I induced expression of IL-15/IL-15Rα is an ancient molecular program common to all lymphocytes. While IL-15 is indispensable for NK cell priming it might be vestigial for the priming of T lymphocytes. Together with data from other groups demonstrating that NK cells undergo an education process that has been compared by some to “positive selection” of T cells (Anfossi et al., 2006; Fernandez et al., 2005; Kim et al., 2005), and a recent report indicating that NK cells are capable of immunological “memory” (O'Leary et al., 2006), NK cells appear to be much more related to lymphocytes of the adaptive immune system than previously appreciated. These results have important implications for the development of immunotherapeutic strategies aiming to boost NK cell effector functions.
Experimental Procedures
Mice
C57BL/6 (B6), BALB/c and B6-Ly5.2/Cr (CD45.1+) mice were obtained from the NCI-Frederick Animal Production Program. IL-15-/- mice (Kennedy et al., 2000) were purchased from Taconic. RAG1-/-, MyD88-/- (Adachi et al., 1998) and CD11c DTR tg mice (Jung et al., 2002) (all on a C57BL/6 background) were a kind gift of Dr. Dan Littman. CD11c DTR tg mice were backcrossed 10-15 generations to C57BL/6. RAG1-/- CD11c DTR tg mice were generated by crossing RAG1-/- mice to CD11c DTR tg mice. IFNAR-/- mice (Muller et al., 1994) backcrossed 12 generations to C57BL/6 were generated by Drs. Jon Sprent and Mark Rubinstein (The Scripps Research Institute, La Jolla, CA) and kindly provided by Drs. Joel Ernst and Andrea Wolf. IL-12p35-/-p40-/- mice on a B6 background and IL-12p35-/- mice (Magram et al., 1996) on a BALB/c background were kindly provided by Dr. Christian Bogdan (Universität Freiburg, Germany). Mice were used at 8-16 weeks of age. All experiments were approved by and in accordance with the local animal care and use committees.
Stimulation of DC and determination of bacterial loads
Mice were injected i.p. or s.c. with TLR ligands (TLR3: 50μg or 5 μg poly(I:C) (Sigma), TLR4: 25μg or 1μg ultrapure LPS from E. coli O111:B4 (Invivogen), TLR7: 250μg or 25μg R837 (Invivogen), TLR9: 125μg or 10μg CpG1668 synthesized by Invitrogen) or with an agonistic antibody specific for CD40 (100μg 1C10; eBioscience). Control mice received injections of PBS or 100μg isotype control antibody (rat IgG2a), respectively. Mice were injected with 104 cfu L. monocytogenes strain EGD i.v. or s.c. To determine bacterial titers in LN and spleen, organs were homogenized in 1ml of PBS, and serial dilutions of the homogenates were plated on BHI agar plates. Colonies were counted after incubation at 37°C for 24h. For viral infections, mice were infected i.v. with 200 pfu of LCMV or 5×104 pfu vaccinia virus (kindly provided by Dr. Hanspeter Pircher, Universität Freiburg).
DC ablation
CD11chigh DC were ablated by a single injection of 2-4ng DT (Sigma or Calbiochem)/g body weight (Jung et al., 2002). Ablation of DC was confirmed by staining for CD11chigh MHC-II+ cells in spleen and LN. Routinely, we observed a >95% reduction of CD11chigh DC. In some experiments, CD11c DTR tg mice were injected i.v. with 3-10×106 BM-DC or peritoneal macrophages from non-tg mice one day prior to DT application.
Preparation of lymphocyte and myeloid cell populations
Blood samples were collected by heart bleed. Red blood cells were lysed using the BD PharmLyse solution (BD Biosciences). For liver lymphocyte preparation, total liver cells were resuspended in 40% isotonic Percoll (GE Healthcare), layered upon an 80% Percoll solution and centrifuged at 900 × g. Mononuclear cells were harvested from the 40/80% interphase and extensively washed thereafter. For lymphocyte isolation from the lungs, organs were perfused through the right ventricle with 20ml PBS. Lung tissue was cut into small pieces and incubated for 45min at 37°C in Hank's balanced salt solution (HBSS; Invitrogen) containing 1mg/ml collagenase D (Roche). Total lung cells were layered upon Lympholyte (Cedarlane), centrifuged at 720 × g and lymphocytes were recovered at the interphase. Where indicated, NK cells were positively selected by magnetic enrichment of DX5+ cells (Miltenyi Biotec) followed by sorting of DX5+CD3− cells on a MoFlo cell sorter (Dako). The purity of NK cells was generally between 95-99%. BM-DC were obtained by culture of BM cells for 5-6 days in 10ng/ml GM-CSF (Peprotech). Peritoneal exudate macrophages were isolated 4 days after i.p. injection of 1.5 ml of 4% Brewer's thioglycolate broth (Difco).
Immunohistology
Cryostat tissue sections (5μm) were thawed onto slides, air-dried and fixed with acetone. Sections were blocked (10% FCS and 10μg/ml anti-FcγRI/II) and stained with biotinylated antibodies specific for CD11c (N418, BD Biosciences), ER-TR9, MOMA-1 (both BMA Biomedicals) visualizing marginal zone and metallophilic macrophages, respectively or F4/80 (Caltag), CD11b (M1/70, BD Biosciences) visualizing red pulp macrophages. Immunophosphatase stain was performed by adding streptavidin-alkaline phosphatase and Vector Red Alkaline Phosphatase reagent (Vector Laboratories). Sections were counterstained with hematoxylin.
Antibody blocking and depletion
NK cells were depleted by injection of 200μg of purified anti-NK1.1 antibody or by injection of 50μl anti-asialo GM1 antiserum (Wako Chemicals) two days prior and at the day of microbial stimulation. The anti-NK1.1 antibody was purified from hybridoma supernatants (PK136, ATCC HB-191) in our laboratory using standard procedures. Control mice received injections of the same amount of normal mouse IgG or rabbit IgG (Jackson ImmunoResearch Laboratories). Depletion of NK cells was confirmed at the day of the experiment by flow cytometry analysis using non-competing antibodies (co-staining with DX5/CD49b, CD122 and CD3). In general, less than 2% of the depleted cell population could be detected. For blocking of lymphocyte entry into LN, mice were injected with 200μg anti-CD62L antibody (Mel14; eBioscience) one day prior to the injection of microbial stimuli or transfer of cell populations. Control animals received the same amount of normal IgG. One day later, mice were injected with the indicated TLR ligands and NK cell priming was evaluated.
Ex vivo analysis of NK cell function
IFN-γ production by NK cells was determined by intracellular cytokine staining. Highly purified NK cells (0.1-0.2×106) or splenocytes (0.25-1×106 ) were incubated on plates coated overnight with the indicated concentrations of purified antibodies (all from eBioscience) or at a 1:1 ratio with the indicated target cell lines for 4-6h in the presence of 2μM monensin (GolgiStop, BD Biosciences) or 10μg/ml brefeldin A (Sigma). After cell surface staining using monoclonal antibodies specific for NK1.1 or DX5/CD49b and CD3, cells were fixed and permeabilized using the Cytofix/Cytoperm reagent (BD Biosciences) followed by intracellular IFN-γ staining. Results represent percent of gated NK1.1+ or CD49b/DX5+ CD3− cells producing IFN-γ. Accumulation of granzyme B was analyzed by intracellular staining with a 1:750 dilution of a rabbit serum specific for mouse granzyme B or rabbit pre-immune serum (kind gift of Dr. Markus Simon, Max-Planck-Institut für Immunbiologie, Freiburg, Germany) followed by a fluorochrome labeled goat anti-rabbit antibody (2.5-5μg/ml, Molecular Probes). Cytotoxicity of NK cells against the indicated target cells was determined in a standard 4h 51chromium release assay (Diefenbach et al., 2001). For the ADCC assays, 51Cr labeled RMA cells were incubated for 30min on ice with 20-50μg/ml anti-Thy1.2 (BD Biosciences) or isotype control antibody (rat IgG2b, eBioscience). Target cells were washed and mixed with effector cells. The percentage of NK1.1+CD3− cells in the lymphocyte populations was determined prior to the cytotoxicity assays and lymphocyte numbers were adjusted to contain the same number of NK cells. Thus, an NK cell:target ratio of 6:1 is equivalent to a 100-200:1 splenocyte:target ratio. Data from all assays are given as the mean of triplicate measurements. Standard deviations were generally less than 6% and were in most cases omitted from the figures for reasons of clarity.
In vitro assays for NK cell priming
Highly purified NK cells were stimulated for 12-18h with the indicated concentrations of cytokines (IL-12: R&D Systems, IFN-I: IFN-αA and IFN-β, PBL Biomedical Laboratories, IL-15: Peprotech). BM-DC were stimulated with TLR ligands (TLR3: 25-50μg/ml poly(I:C), TLR9: 25-50μg/ml CpG1668), IFN-I (100-1,000 U/ml purified IFN-I; kind gift of Dr. Ion Gresser), a 1:1 mixture of recombinant mouse IFN-αA and IFN-β (100-1,000 U/ml each; PBL Biomedical Laboratories) or anti-CD40 (20-50μg/ml). Highly purified NK cells (1-2×106) were cultured for 12h alone or with 106 stimulated BM-DC as indicated. In some experiments, co-culture was performed by spatially separating BM-DC and NK cells in transwell plates (Costar). DC-derived IFN-I were neutralized by using 100-1,000 neutralizing units of sheep anti-mouse IFN-α/β immunoglobulin (Gidlund et al., 1978; Gresser et al., 1988) (kind gift of Dr. Ion Gresser) or 10-50μg/ml each of neutralizing rat monoclonal antibodies to IFN-α and IFN-β (PBL Biomedical Laboratories). Control wells contained control sheep immunoglobulin or an isotype control antibody (rat IgG1, eBioscience). IL-15 and IL-2 were neutralized by using goat antisera against mouse IL-15 (50μg/ml) or mouse IL-2 (50μg/ml) (R&D Systems). After 12-18h of stimulation, NK cells were harvested and analyzed in a standard 4h 51Cr release assay.
Bone marrow chimeric mice
Mice were lethally irradiated (12 Gy) and injected i.v. with 2-5×106 BM cells derived from the indicated mouse strains. BM chimeric mice were analyzed 8-12 weeks after transplantation. Chimerism was evaluated prior to the experiments by analyzing blood leukocytes. Chimeras used in the functional experiments had < 2% of remaining host leukocytes and showed even engraftment (50 ± 12%) for the mixed BM chimeras. The observed differences in engraftment following transfer of mixed BM were random demonstrating that BM cells from the indicated mouse strains were not genetically impaired in reconstituting the hematopoietic pool.
NK cell transfers
For the transfer of NK cells, 2-5×106 purified splenic NK cells were injected i.v. into the indicated hosts. In some of the experiments using IL-15-/- mice as hosts, 4μg of recombinant IL-15 (Peprotech) was injected at the time of TLR stimulation to rescue NK cell function.
Recruitment assays
To assess the recruitment of circulating lymphocytes to LN, 20-50×106 CD45.1+ splenocytes were injected i.v. into CD45.2+ B6 mice. Two hours later, mice were challenged s.c. into the footpad with the indicated microbial stimuli. At the indicated time points, LN were harvested and the proportion of donor-derived NK cells and T cells in the LN was determined by electronical gating on the CD45.1+NK1.1+CD3− or CD45.1+NK1.1−CD3+ population, respectively. Absolute numbers of donor-derived T and NK cells were calculated based on the total number of cells per LN.
Flow cytometry analysis
Staining of cells was performed with FITC-conjugated antibodies specific for mouse CD3ε (145-2C11), CD25 (PC61.5), Ly49A (A1), Ly49C/I (5E6), Ly49D (4E5), Ly49G2 (Cwy-3), NKG2A/C/E (20d5), CD122 (TM-β1), CD45.1 (A20), CD45.2 (104), class II MHC (M5/114.15.2), IFN-γ (XMG1.2), PE-conjugated antibodies specific for mouse CD19 (6D5), CD69 (H1.2F3), NKR-P1B/C (PK136), CD45.1 (A20), CD45.2 (104), CD49b (DX5), CD122 (TM-β1), PDCA-1 (Miltenyi) or class II MHC (M5/114.15.2), PerCP-conjugated antibody to mouse CD3ε (145-2C11) or CD45R/B220 (RA3-6B2, BD Biosciences) or allophycocyanin (APC)-conjugated antibodies specific for mouse CD3ε (145-2C11), CD11b (M1/70), CD11c (N418), CD45.1 (A20), NKR-P1B/C (PK136) and IFN-γ (XMG1.2) (all from eBioscience unless stated differently). IL-15Rα expression by DC was analyzed by staining with a biotinylated goat anti-mouse IL-15Rα antibody (R&D Systems) or a biotinylated normal goat Ig and electronical gating on CD11chigh MHC-II+ cells. When staining cells from CD11c DTR tg mice FITC-conjugated antibodies were excluded from the characterization of GFP+ cells (CD11chigh DC). All flow cytometry data were acquired on a FACSCalibur cytometer (BD Biosciences) using CellQuest software (BD Biosciences) and analyzed using FloJo software (Tree Star, Inc).
Supplementary Material
Figure S1: NK cells and plasmacytoid (p) DC are not directly affected by DT injection into CD11c DTR tg mice.
(A) Non-tg littermates or CD11c DTR tg mice were injected with PBS (solid bars) or DT (open bars). The percentage of NK1.1+ CD3− (NK cells), CD11chigh MHC-II+ cells (CD11chigh DC) and of PDCA-1+ B220+ CD11b− pDC in the spleen was evaluated two days later. Error bars display s.d. (n=3-4) and results are representative of five separate experiments.
(B and C) Non-tg littermates (solid bars) or CD11c DTR tg mice (open bars) were injected with DT. One day later mice received i.p. injections of the indicated TLR ligands and the absolute number of NK cells per spleen was evaluated in a large cohort (n=36) of mice 18h later (B). The percentage of splenic NK cells (NK1.1+CD122+CD3−) co-expressing the indicated receptors was evaluated (C). Error bars display s.d. (n=36 or n=3-4, respectively) and results are representative of two (B) or five (C) separate experiments.
(D) Mixed BM chimeric mice were generated by injecting lethally irradiated mice with BM cells derived from CD11c DTR tg mice (CD45.2+) or from B6 control mice expressing the indicated congenic markers. BM chimeric mice were injected 8-12 weeks later with the indicated TLR ligands. IFN-γ production of CD45.1+ (solid bars) and CD45.2+ NK cells (open bars) in response to RMA-S/H60 cells was determined by intracellular cytokine staining and electronic gating on donor NK cells 24h following TLR stimulation. Similar results were obtained with YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of three separate experiments.
Figure S2: Dendritic cells are required for the priming of NK cells.
CD11c DTR tg mice were injected with diphtheria toxin (DT) to ablate all CD11chigh DC (open histograms). Control mice received PBS injections (grey histograms). One day later, mice were injected i.p. with the indicated TLR ligands or an agonistic anti-CD40 antibody. Control groups of mice received injections of PBS or control Ig (not shown). CD69 expression by NK cells was evaluated 18h after TLR stimulation. Dotted histograms represent staining with an isotype control antibody. Results are representative of at least three separate experiments.
Figure S3: NK cells are recruited to the LN draining microbial stimuli.
(A and B) B6 mice (CD45.2+) were injected i.v. with 30×106 splenocytes (CD45.1+). Two hours later, mice were injected s.c. with the indicated TLR ligands. Percentages of donor-derived (CD45.1+) cells constituting the NK or T cell compartment in the draining (solid squares) and non-draining LN (open squares) were determined at the indicated time points after local TLR stimulation (A). Absolute numbers of donor-derived cells homing to DLN or NDLN are depicted as fold increase (log scale) compared to the absolute number of donor-derived cells homing to PBS-draining LN (B). Error bars display s.d. (n=4) and data are representative of three independent experiments.
Figure S4: NK cell priming does not require IL-12 production.
(A-D) B6, BALB/c (solid symbols) or the indicated IL-12-deficient strains (open symbols) were injected with TLR ligands or pathogens. One day later (36h for LCMV injection), cytotoxicity (A and C) or IFN-γ production (B and D) by NK cells in response to RMA-S/H60 cells was analyzed. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). One representative experiment of three is shown.
Acknowledgments
We thank Christian Bogdan for support and reagents, Nina Bhardwaj, Michael Dustin, Dan Littman and Hanspeter Pircher for critical comments on the manuscript and Markus Schnare for discussions. The granzyme B antibody was a kind gift of Markus Simon (Max-Planck-Institut für Immunbiologie, Freiburg, Germany). Work in the Diefenbach lab is supported by grants from the National Institutes of Health (R01-AI059758), Deutsche Forschungsgemeinschaft (Di 764/2-2), The Irene Diamond Foundation and a Whitehead Fellowship for Junior Faculty (all to A.D.).The authors declare that they have no competing financial interests.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Andoniou CE, van Dommelen SL, Voigt V, Andrews DM, Brizard G, Asselin-Paturel C, Delale T, Stacey KJ, Trinchieri G, Degli-Esposti MA. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–1019. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- Andrews DM, Scalzo AA, Yokoyama WM, Smyth MJ, Degli-Esposti MA. Functional interactions between dendritic cells and NK cells during viral infection. Nat Immunol. 2003;4:175–181. doi: 10.1038/ni880. [DOI] [PubMed] [Google Scholar]
- Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25:331–342. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med. 2006;203:619–631. doi: 10.1084/jem.20051474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Baratin M, Roetynck S, Lepolard C, Falk C, Sawadogo S, Uematsu S, Akira S, Ryffel B, Tiraby JG, Alexopoulou L, et al. Natural killer cell and macrophage cooperation in MyD88-dependent innate responses to Plasmodium falciparum. Proc Natl Acad Sci USA. 2005;102:14747–14752. doi: 10.1073/pnas.0507355102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler B, Jiang Z, Georgel P, Crozat K, Croker B, Rutschmann S, Du X, Hoebe K. Genetic analysis of host resistance: toll-like receptor signaling and immunity at large. Annu Rev Immunol. 2006;24:353–389. doi: 10.1146/annurev.immunol.24.021605.090552. [DOI] [PubMed] [Google Scholar]
- Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107:159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Terrazzano G, Ruggiero G, Zanzi D, Ottaiano A, Manzo C, Karre K, Zappacosta S. Recognition of autologous dendritic cells by human NK cells. Eur J Immunol. 1999;29:4022–4029. doi: 10.1002/(SICI)1521-4141(199912)29:12<4022::AID-IMMU4022>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Chambers BJ, Salcedo M, Ljunggren HG. Triggering of natural killer cells by the costimulatory molecule CD80 (B7-1) Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- Chen S, Kawashima H, Lowe JB, Lanier LL, Fukuda M. Suppression of tumor formation in lymph nodes by L-selectin-mediated natural killer cell recruitment. J Exp Med. 2005;202:1679–1689. doi: 10.1084/jem.20051473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Fuchs A, Colonna M, Caligiuri MA. NK cell and DC interactions. Trends Immunol. 2004;25:47–52. doi: 10.1016/j.it.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Cudkowicz G, Yung YP. Abrogation of resistance to foreign bone marrow grafts by carrageenans. I. Studies with the anti-macrophage agent seakem carrageenan. J Immunol. 1977;119:483–487. [PubMed] [Google Scholar]
- Dalod M, Hamilton T, Salomon R, Salazar-Mather TP, Henry SC, Hamilton JD, Biron CA. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J Exp Med. 2003;197:885–898. doi: 10.1084/jem.20021522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach A, Jensen ER, Jamieson AM, Raulet DH. Rae1 and H60 ligands of the NKG2D receptor stimulate tumour immunity. Nature. 2001;413:165–171. doi: 10.1038/35093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu JY, Heinbaugh JA, Holden HT, Herberman RB. Role of macrophages in the augmentation of mouse natural killer cell activity by poly I:C and interferon. J Immunol. 1979;122:182–188. [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity. 2002;17:537–547. doi: 10.1016/s1074-7613(02)00429-6. [DOI] [PubMed] [Google Scholar]
- Dunn PL, North RJ. Early gamma interferon production by natural killer cells is important in defense against murine listeriosis. Infect Immun. 1991;59:2892–2900. doi: 10.1128/iai.59.9.2892-2900.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehniger TA, Suzuki K, Ponnappan A, VanDeusen JB, Cooper MA, Florea SM, Freud AG, Robinson ML, Durbin J, Caligiuri MA. Fatal leukemia in interleukin 15 transgenic mice follows early expansions in natural killer and memory phenotype CD8+ T cells. J Exp Med. 2001;193:219–231. doi: 10.1084/jem.193.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, Bougras G, Muller WA, Moretta L, Munz C. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci USA. 2004;101:16606–16611. doi: 10.1073/pnas.0407522101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez NC, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med. 1999;5:405–411. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, Hughes TL, Marburger TB, Sung J, Baiocchi RA, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304. doi: 10.1016/j.immuni.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, Caligiuri MA. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–1043. doi: 10.1084/jem.20052507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal Activating Interaction between Natural Killer Cells and Dendritic Cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidlund M, Orn A, Wigzell H, Senik A, Gresser I. Enhanced NK cell activity in mice injected with interferon and interferon inducers. Nature. 1978;273:759. doi: 10.1038/273759a0. [DOI] [PubMed] [Google Scholar]
- Gresser I, Maury C, Vignaux F, Haller O, Belardelli F, Tovey MG. Antibody to mouse interferon alpha/beta abrogates resistance to the multiplication of Friend erythroleukemia cells in the livers of allogeneic mice. J Exp Med. 1988;168:1271–1291. doi: 10.1084/jem.168.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamerman JA, Ogasawara K, Lanier LL. Cutting edge: Toll-like receptor signaling in macrophages induces ligands for the NKG2D receptor. J Immunol. 2004;172:2001–2005. doi: 10.4049/jimmunol.172.4.2001. [DOI] [PubMed] [Google Scholar]
- Hart OM, Athie-Morales V, O'Connor GM, Gardiner CM. TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production. J Immunol. 2005;175:1636–1642. doi: 10.4049/jimmunol.175.3.1636. [DOI] [PubMed] [Google Scholar]
- Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer. 1975;16:216–229. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Kanto T, Tatsumi T, Groh V, Spies T, Miyagi T, Suzuki T, Sasaki Y, Hayashi N. Critical role of MHC class I-related chain A and B expression on IFN-alpha-stimulated dendritic cells in NK cell activation: impairment in chronic hepatitis C virus infection. J Immunol. 2003a;170:1249–1256. doi: 10.4049/jimmunol.170.3.1249. [DOI] [PubMed] [Google Scholar]
- Jinushi M, Takehara T, Tatsumi T, Kanto T, Groh V, Spies T, Suzuki T, Miyagi T, Hayashi N. Autocrine/paracrine IL-15 that is required for type I IFN-mediated dendritic cell expression of MHC class I-related chain A and B is impaired in hepatitis C virus infection. J Immunol. 2003b;171:5423–5429. doi: 10.4049/jimmunol.171.10.5423. [DOI] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In Vivo Depletion of CD11c+ dendritic cells Abrogates Priming of CD8+ T Cells by Exogenous Cell-Associated Antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- Kassim SH, Rajasagi NK, Zhao X, Chervenak R, Jennings SR. In vivo ablation of CD11c-positive dendritic cells increases susceptibility to herpes simplex virus type 1 infection and diminishes NK and T-cell responses. J Virol. 2006;80:3985–3993. doi: 10.1128/JVI.80.8.3985-3993.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol. 1975;5:112–117. doi: 10.1002/eji.1830050208. [DOI] [PubMed] [Google Scholar]
- Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- Koka R, Burkett P, Chien M, Chai S, Boone DL, Ma A. Cutting edge: murine dendritic cells require IL-15R alpha to prime NK cells. J Immunol. 2004;173:3594–3598. doi: 10.4049/jimmunol.173.6.3594. [DOI] [PubMed] [Google Scholar]
- Koschella M, Voehringer D, Pircher H. CD40 ligation in vivo induces bystander proliferation of memory phenotype CD8 T cells. J Immunol. 2004;172:4804–4811. doi: 10.4049/jimmunol.172.8.4804. [DOI] [PubMed] [Google Scholar]
- Krug A, French AR, Barchet W, Fischer JA, Dzionek A, Pingel JT, Orihuela MM, Akira S, Yokoyama WM, Colonna M. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Burkett PR, Boone DL, Chien M, Ma A. T cell-independent interleukin 15Ralpha signals are required for bystander proliferation. J Exp Med. 2001;194:1187–1194. doi: 10.1084/jem.194.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFN gamma production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Mattei F, Schiavoni G, Belardelli F, Tough DF. IL-15 is expressed by dendritic cells in response to type I IFN, double-stranded RNA, or lipopolysaccharide and promotes dendritic cell activation. J Immunol. 2001;167:1179–1187. doi: 10.4049/jimmunol.167.3.1179. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Mention JJ, Ben Ahmed M, Begue B, Barbe U, Verkarre V, Asnafi V, Colombel JF, Cugnenc PH, Ruemmele FM, McIntyre E, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology. 2003;125:730–745. doi: 10.1016/s0016-5085(03)01047-3. [DOI] [PubMed] [Google Scholar]
- Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- Muller U, Steinhoff U, Reis LF, Hemmi S, Pavlovic J, Zinkernagel RM, Aguet M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei XQ, Liew FY, Caligiuri MA, Durbin JE, Biron CA. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J Immunol. 2002;169:4279–4287. doi: 10.4049/jimmunol.169.8.4279. [DOI] [PubMed] [Google Scholar]
- O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- Ohteki T, Tada H, Ishida K, Sato T, Maki C, Yamada T, Hamuro J, Koyasu S. Essential roles of DC-derived IL-15 as a mediator of inflammatory responses in vivo. J Exp Med. 2006;203:2329–2338. doi: 10.1084/jem.20061297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188:953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent Stimulation and Inhibition of Dendritic Cells by Natural Killer Cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst HC, Tschannen K, Odermatt B, Schwendener R, Zinkernagel RM, Van Den Broek M. Histological analysis of CD11c-DTR/GFP mice after in vivo depletion of dendritic cells. Clin Exp Immunol. 2005;141:398–404. doi: 10.1111/j.1365-2249.2005.02868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- Scalzo AA, Fitzgerald NA, Wallace CR, Gibbons AE, Smart YC, Burton RC, Shellam GR. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J Immunol. 1992;149:581–589. [PubMed] [Google Scholar]
- Schmidt KN, Leung B, Kwong M, Zarember KA, Satyal S, Navas TA, Wang F, Godowski PJ. APC-independent activation of NK cells by the Toll-like receptor 3 agonist double-stranded RNA. J Immunol. 2004;172:138–143. doi: 10.4049/jimmunol.172.1.138. [DOI] [PubMed] [Google Scholar]
- Shah PD, Gilbertson SM, Rowley DA. Dendritic cells that have interacted with antigen are targets for natural killer cells. J Exp Med. 1985;162:625–636. doi: 10.1084/jem.162.2.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinozaki M, Hirahashi J, Lebedeva T, Liew FY, Salant DJ, Maron R, Kelley VR. IL-15, a survival factor for kidney epithelial cells, counteracts apoptosis and inflammation during nephritis. J Clin Invest. 2002;109:951–960. doi: 10.1172/JCI14574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivori S, Falco M, Della Chiesa M, Carlomagno S, Vitale M, Moretta L, Moretta A. CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells. Proc Natl Acad Sci USA. 2004;101:10116–10121. doi: 10.1073/pnas.0403744101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1949. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp CS, Gately MK, Hakimi J, Ling P, Unanue ER. Neutralization of IL-12 decreases resistance to Listeria in SCID and C.B-17 mice. Reversal by IFN-gamma. J Immunol. 1994;152:1883–1887. [PubMed] [Google Scholar]
- Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90:3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Dubois S, Tagaya Y. Contrasting roles of IL-2 and IL-15 in the life and death of lymphocytes: implications for immunotherapy. Immunity. 2001;14:105–110. [PubMed] [Google Scholar]
- Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: NK cells and plasmacytoid (p) DC are not directly affected by DT injection into CD11c DTR tg mice.
(A) Non-tg littermates or CD11c DTR tg mice were injected with PBS (solid bars) or DT (open bars). The percentage of NK1.1+ CD3− (NK cells), CD11chigh MHC-II+ cells (CD11chigh DC) and of PDCA-1+ B220+ CD11b− pDC in the spleen was evaluated two days later. Error bars display s.d. (n=3-4) and results are representative of five separate experiments.
(B and C) Non-tg littermates (solid bars) or CD11c DTR tg mice (open bars) were injected with DT. One day later mice received i.p. injections of the indicated TLR ligands and the absolute number of NK cells per spleen was evaluated in a large cohort (n=36) of mice 18h later (B). The percentage of splenic NK cells (NK1.1+CD122+CD3−) co-expressing the indicated receptors was evaluated (C). Error bars display s.d. (n=36 or n=3-4, respectively) and results are representative of two (B) or five (C) separate experiments.
(D) Mixed BM chimeric mice were generated by injecting lethally irradiated mice with BM cells derived from CD11c DTR tg mice (CD45.2+) or from B6 control mice expressing the indicated congenic markers. BM chimeric mice were injected 8-12 weeks later with the indicated TLR ligands. IFN-γ production of CD45.1+ (solid bars) and CD45.2+ NK cells (open bars) in response to RMA-S/H60 cells was determined by intracellular cytokine staining and electronic gating on donor NK cells 24h following TLR stimulation. Similar results were obtained with YAC-1 targets (data not shown). Error bars display s.d. (n=3-4) and results are representative of three separate experiments.
Figure S2: Dendritic cells are required for the priming of NK cells.
CD11c DTR tg mice were injected with diphtheria toxin (DT) to ablate all CD11chigh DC (open histograms). Control mice received PBS injections (grey histograms). One day later, mice were injected i.p. with the indicated TLR ligands or an agonistic anti-CD40 antibody. Control groups of mice received injections of PBS or control Ig (not shown). CD69 expression by NK cells was evaluated 18h after TLR stimulation. Dotted histograms represent staining with an isotype control antibody. Results are representative of at least three separate experiments.
Figure S3: NK cells are recruited to the LN draining microbial stimuli.
(A and B) B6 mice (CD45.2+) were injected i.v. with 30×106 splenocytes (CD45.1+). Two hours later, mice were injected s.c. with the indicated TLR ligands. Percentages of donor-derived (CD45.1+) cells constituting the NK or T cell compartment in the draining (solid squares) and non-draining LN (open squares) were determined at the indicated time points after local TLR stimulation (A). Absolute numbers of donor-derived cells homing to DLN or NDLN are depicted as fold increase (log scale) compared to the absolute number of donor-derived cells homing to PBS-draining LN (B). Error bars display s.d. (n=4) and data are representative of three independent experiments.
Figure S4: NK cell priming does not require IL-12 production.
(A-D) B6, BALB/c (solid symbols) or the indicated IL-12-deficient strains (open symbols) were injected with TLR ligands or pathogens. One day later (36h for LCMV injection), cytotoxicity (A and C) or IFN-γ production (B and D) by NK cells in response to RMA-S/H60 cells was analyzed. Similar results were obtained with RMA-S and YAC-1 targets (data not shown). One representative experiment of three is shown.