Abstract
The past 20 years have seen substantial changes in our view of the nature of the processing carried out in auditory cortex. Some processing of a cognitive nature, previously attributed to higher order “association” areas, is now considered to take place in auditory cortex itself. One argument adduced in support of this view is the evidence indicating a remarkable degree of plasticity in the auditory cortex of adult animals. Such plasticity has been demonstrated in a wide range of paradigms, in which auditory input or the behavioural significance of particular inputs is manipulated. Changes over the same time period in our conceptualization of the receptive fields of cortical neurons, and well-established mechanisms for use-related changes in synaptic function, can account for many forms of auditory cortical plasticity. On the basis of a review of auditory cortical plasticity and its probable mechanisms, it is argued that only plasticity associated with learning tasks provides a strong case for cognitive processing in auditory cortex. Even in this case the evidence is indirect, in that it has not yet been established that the changes in auditory cortex are necessary for behavioural learning and memory. Although other lines of evidence provide convincing support for cognitive processing in auditory cortex, that provided by auditory cortical plasticity remains equivocal.
Keywords: Cholinergic modulation, Classical conditioning, Cochlear lesion, Perceptual learning, Receptive field, Tonotopic organization
1. Introduction
The last 20 years or so have seen not only a massive increase in our knowledge of auditory cortical processing mechanisms but also a substantial change in the way in which we think about auditory cortical function. An assumption made by most cortical researchers in the early 1980s, so basic that it would not have had to be explicitly formulated, was that the functional response characteristics of auditory cortical neurons, and the functional organization of auditory cortex, were stable features of the adult brain. Any susceptibility of these characteristics to environmental influences was assumed, largely by analogy with the visual and somatosensory cortices, to be restricted to limited “critical” periods during development (Hensch, 2004). A related assumption was that the primary function of auditory cortex is to extract the information contained in the acoustic signal, albeit by mechanisms that are at a greater level of complexity than those at lower levels of the auditory pathway. The cortex was thus seen as the final stage of an essentially hierarchical “bottom up” signal processing system. Although our perceptual experience of the auditory world (“hearing” in the normal sense of that word) clearly involves cognitive processes by which the information provided by the environment is interpreted, it was implicitly assumed that this “higher–order” processing takes place in “association” areas beyond the auditory cortex.
A number of lines of evidence have led to substantial changes in this view of the function of auditory cortex. One has been the evidence that auditory cortical response characteristics and organization can in fact be modified in adult humans and animals as a consequence of a variety of changes in the organism’s auditory experience (see Weinberger (1995, 2004a) and Irvine and Wright (2005) for reviews). One manifestation of adult plasticity is associated with auditory learning, and suggests that the auditory cortex contains memory traces of behaviorally significant stimuli (e.g., Weinberger, 2004b). A second line of evidence is that some components of human auditory evoked potentials that are primarily generated in auditory cortex are associated with what have been thought to be higher-order processes. For example, the mismatch negativity (MMN) reflects a form of memory for previous stimuli in the generating structures (e.g., Näätänen et al., 2001). Activity generated by speech sounds in human auditory cortex has also been shown to be influenced by factors such as phonological grammar and intelligibility (e.g., Jacquemot et al., 2003; Obleser et al., 2006). A third line of evidence is that auditory cortex is involved in aspects of auditory scene analysis that have a “top down” flavour (e.g., Näätänen et al., 2001; Nelken, 2004). These (and other) findings have indicated that auditory cortex has the capacity to store some forms of memory and to carry out a range of higher order, or “cognitive”, functions (Weinberger, 2004b). As one instance of this changing view of auditory cortical function, Näätänen et al. (2001) referred to some aspects of auditory cortical processing as reflecting “primitive intelligence”.
The evidence on adult plasticity in auditory cortex has been complemented by substantial changes in the way auditory cortical receptive fields (RFs) are conceptualized, and the newly-recognized characteristics of RFs are in fact among the most important factors that contribute to adult plasticity. In this paper, the evidence for various forms of auditory cortical plasticity in adults will be reviewed, and the extent to which learning-related and other forms of plasticity support the claim that auditory cortex can be considered to carry out cognitive functions will be examined in the light of evidence as to the mechanisms involved.
2. Plasticity in adult auditory cortex
As elaborated in the following sections, plastic changes in the response characteristics of auditory cortical neurons, and in the functional organization of auditory cortical fields, have been demonstrated using a wide range of experimental interventions. Some of these interventions involve manipulation of the organism’s sensory environment or of the significance for the organism of particular stimuli in that environment. Others involve the production of changes in auditory input by modifying receptor function or the transmission of information from the receptors to the central nervous system (CNS).
Neural plasticity can be broadly defined as dynamic changes in the structural and functional characteristics of neurons that occur in response to changes in the nature or significance of their input. This definition is intended to distinguish plasticity from changes that occur as passive consequences of the altered input or as a direct consequence of changes in the organism’s state (e.g. arousal state; age). In the auditory system, for example, changes in the frequency tuning of auditory nerve fibres and central neurons occurs as a direct consequence of destruction of the outer hair cells (e.g., Dallos and Harris, 1978). These changes are passive consequences of the elimination of the cochlear amplifier rather than instances of plasticity, which involves some form of dynamic change in neural properties as a consequence of the altered input. Although the distinction between passive and plastic changes appears straightforward and is usually easily made, there are borderline cases where the distinction is difficult (see Calford (2002)).
2.1. Plasticity induced by restricted cochlear damage
Although there had been earlier reports of sensory cortical plasticity associated with behavioural conditioning, the strongest impetus to recognition that plasticity is not restricted to critical developmental periods was provided by evidence for reorganization of topographic “maps” of receptor surfaces as a consequence of elimination of input from a restricted region of the receptor epithelium. Such reorganization was first demonstrated in somatosensory cortex, but was subsequently described in both auditory and visual cortices after analogous lesions (see Buonomano and Merzenich (1998), Gilbert (1998), and Kaas and Florence (2001) for reviews). The impact of these results almost certainly derived from the fact that these maps had long been thought of as simple reflections of orderly projections from receptor surfaces to target structures in the brain, established early in development. As Calford (2002) has pointed out, the orderly nature of these maps also provided a simple metric by which the nature of the reorganization could be quantified
In the auditory system, unilateral mechanical lesions to a restricted region of the cochlea in adult guinea pigs (Robertson and Irvine, 1989) and cats (Rajan et al., 1993) result in a reorganization of the frequency map in AI contralateral to the lesioned cochlea. The general form of this reorganization is that the neurons in the region of cortex in which the lesioned section of the cochlea would normally be represented, which has been termed the lesion projection zone (LPZ; Schmid et al., 1996), have new CFs at frequencies represented at the edge(s) of the cochlear lesion. As a consequence of this change in tuning, the LPZ is wholly or partly occupied by an expanded representation of the lesion-edge frequency or frequencies.
Similar changes in frequency organization after mechanical cochlear lesions in adult cats have been shown to occur in the dorsal cochlear nucleus (DCN; Rajan and Irvine, 1998), the central nucleus of the inferior colliculus (ICC; Irvine et al., 2003), and the ventral division of the medial geniculate nucleus (MGv; Kamke et al., 2003). As discussed elsewhere, such changes in frequency organization could also occur as a passive consequence of cochlear lesions, but plastic and passive changes can readily be distinguished on the basis of the thresholds of neurons at their new CFs and of other response characteristics (see Rajan et al. (1993) and Rajan and Irvine (1998) for a detailed account). The thresholds of neurons in the LPZ in DCN, and in the majority of penetrations through that in ICC, indicate that the changes in these structures are explicable as passive consequences of the lesion. In contrast, thresholds in AI and in MGv indicate that reorganization in these structures is attributable to a dynamic process of plasticity. It therefore appears that reorganization after such lesions is a characteristic of the thalamo-cortical system.
Analogous reorganization has been described in primary auditory cortex (field AI) of adult animals of a variety of species, including non-human primates, and as a consequence of different forms of cochlear insult (e.g., ototoxic lesions; noise trauma) and of congenital sensori-neural hearing losses (see Irvine and Wright (2005) for review). As with mechanical lesions, plastic reorganization of the frequency map is not seen in the ICC after ototoxic lesions in adult chinchillas (Harrison, 2001). However, ototoxic lesions in neonatal cats result in plastic reorganization in ICC (Harrison et al., 1996), suggesting that developmental reorganization is not restricted to the thalamo-cortical system. That reorganization also occurs in auditory cortex of adult humans is indicated by magnetoencephalographic (MEG) evidence for an expanded representation of lesion-edge frequencies in humans with steeply-sloping hearing losses of the sort that produce reorganization in animals (Dietrich et al., 2001).
2.2 Plastic effects of cochlear electrical stimulation in profoundly deaf animals
The occurrence of plastic changes in the central auditory system of profoundly deaf animals (or humans) can obviously not be investigated using acoustic stimuli. However, electrical stimulation via intra-cochlear electrodes, which directly activates surviving spiral ganglion neurons, can be used to assess the responsiveness of the central auditory pathways in such cases. This stimulation is directly comparable to that provided by cochlear implants, which have been used to restore functional hearing to many thousands of profoundly deaf humans. Plasticity in the auditory cortex of congenitally-deaf or neonatally-deafened animals has been demonstrated by comparing responses to cochlear electrical stimulation in adult animals that have received chronic electrical stimulation for some specified period with those in unstimulated controls. In some cases the stimulation is presented over weeks or months prior to the final recording, in which case differences undoubtedly reflect adult plasticity. In other cases, the stimulation is initiated at an early stage of development (as soon as it is possible to carry out implant surgery), but continues during adulthood, so it is unclear to what extent the plastic changes occur during development or adulthood. Although there appear to be no studies directly comparing the effects of cochlear electrical stimulation restricted to an early developmental period with that restricted to adulthood, it is likely that the capacity for plastic change is in fact greater in the former.
Cochleotopic organization of AI has been reported to be rudimentary or absent in congenitally deaf or neonatally-deafened adult cats (Hartmann et al., 1997; Raggio and Schreiner, 1999), and we have seen no evidence of cochleotopy in the latter (Fallon, Irvine and Shepherd, unpublished observations). Chronic stimulation via a multi-channel cochlear electrode in neonatally-deafened cats, initiated as soon as implantation is possible (at around 2 months of age), results in the maintenance (or restoration) of a crude cochleotopy in the adult (Fallon, Irvine and Shepherd, unpublished observations). As noted above, it is unclear to what extent this effect is attributable to developmental or adult plasticity. When such stimulation is restricted to a single intracochlear location, expansion of the representation of that cochlear region has been reported in ICC (Snyder et al., 1990) and in AI (Dinse et al., 2003). This effect in cortex is analogous to the expansion of the representation of lesion-edge frequencies after restricted cochlear lesions. However, in contrast to the reorganization induced by restricted cochlear lesions, cochlear-stimulation induced reorganization is also seen in ICC of cats deafened as adults (Moore et al., 2002), suggesting that subcortical changes contribute in different ways to the two forms of plasticity.
The temporal pattern of AI neuronal responses to cochlear electrical stimulation is also modified by chronic stimulation. Responses in congenitally deaf adult animals are characterized by small middle-latency responses and the complete absence of long-latency responses. Chronic cochlear electrical stimulation (initiated early in development) results in increased amplitude and a broader spatial distribution of middle-latency responses, and the appearance of long-latency responses (Klinke et al., 1999).
The massive improvement in speech discrimination shown by people with cochlear implants over the months and years following implantation (e.g., McKay, 2005) is undoubtedly a manifestation of plasticity at one or more CNS locations. The extent to which it involves plasticity in auditory cortex per se, or in specialized speech processing areas, is not yet known.
2.3. Plasticity associated with behavioural conditioning or detection tasks
Studies of the effects of behavioural conditioning procedures on auditory responses date at least from the study by Galambos et al. (1955), but many of the early studies did not include the control procedures necessary to establish that the observed changes were associative in nature. The pioneering studies of Weinberger and his colleagues were the first to use appropriate controls for the general activating properties of the commonly used aversive unconditioned stimulus (UCS; e.g., foot shock) and to establish the specificity of the effects to the characteristics of the acoustic conditioned stimulus (CS). These studies indicated that conditioning produced an increase in the response at the CS frequency and a decrease in response at the pre-training best frequency (BF; that evoking maximum response) such that the BF shifted towards or became the same as the CS frequency. Suga and his colleagues have reported similar shifts in BF towards the CS frequency in auditory cortical neurons in the big brown bat as a consequence of conditioning procedures (Gao and Suga, 2000; Ji et al., 2001). The changes in frequency selectivity associated with behavioural conditioning can occur extremely rapidly, after as few as 5 training trials (over a period of approximately 10 minutes) (e.g., Edeline et al., 1993).
In a recent study of discriminative conditioning, Rutkowski and Weinberger (2005) reported that operant conditioning with a 6-kHz discriminative stimulus (SD) resulted in no change in the absolute area of the representation of the octave around 6 kHz, but in an increase in its relative area, due to a reported reduction in the total area of AI. The absence of any change in absolute area indicates that no neurons (or neuron clusters) changed their CF to the frequency of the SD (or that the CFs of equal numbers of neurons shifted to and away from that frequency). The reported reduction in the area of AI is puzzling. The criteria the authors used to distinguish between AI and belt-like sites were based on relative sensitivity to pure tones and noise and on response latency. The reported reduction in the size of AI must therefore mean that the response characteristics of neurons at many sites that would previously have satisfied the criteria for classification as AI had changed in such a way that they no longer did so. The data presented in the paper suggest a large invagination of areas classified as belt-like into the higher-frequency regions of AI, such that the high-frequency area was substantially reduced. It could be that the tuning characteristics of many high-frequency neurons changed in such a fashion that they became more responsive to noise than to CF tones, but the nature of the tuning (or other) changes of neurons in these areas was not described.
Although a selective increase in the responses to the CS (or to the paired stimulus (CS+) in classical discrimination learning tasks) has been commonly reported, there have been other reports of decreases in the response to the CS (or CS+). Notably, Ohl and Scheich (1996) studied the effects of discrimination training on the responses of AI neurons in unanesthetized gerbils, using a procedure in which all the frequencies (other than that used as CS+) that were presented in determining the pre-conditioning frequency receptive field (FRF) were used as unpaired stimuli (CS−s) during training. Although individual neurons showed either increases or decreases in spike rate at CS+ at different times during the response, the pooled data from the entire sample of neurons showed a decrease in the early (onset) part of the response at the CS+ frequency, whereas increases in response at this time were greatest at frequencies above and below the CS frequency, such that the CS+ frequency came to lie at a local minimum. Whether this different pattern of results is attributable to differences in task requirements or in other experimental procedures is unclear (see Weinberger (2004a,b) and the on-line correspondence associated with Weinberger (2004b) for discussion).
Recently Fritz and his colleagues have reported changes in the spectro-temporal RFs (STRFs) of neurons in AI of unanaesthetized ferrets during training either to detect a target tone of a particular frequency (Fritz et al., 2003, 2005a) or to discriminate between two tone frequencies (Fritz et al., 2005b). On the former task, they described a population pattern of enhancement of the response to the target tone and suppression at adjacent frequencies that is remarkably similar to that described by Weinberger and his colleagues. On the discrimination task, the responses of individual neurons varied, but population responses showed a potentiation of response at the target frequency (viz., that associated with reinforcement) and a depression of response at the reference frequency. On both tasks, the STRF returned to its pre-training form within a relatively short period in some neurons, while in others the changes persisted for some time after training. Like the conditioning studies, these studies establish that changes in the frequency selectivity of AI neurons can occur very rapidly (within a few minutes) when a behavioural task confers significance on a particular frequency or frequencies.
In what appears to be the only study of frequency-specific changes in human auditory cortex associated with behavioural conditioning, Morris et al. (1998) used positron emission topography (PET) to investigate the effects of discriminatory classical conditioning using a noxious intense noise as the unconditioned stimulus (UCS) and tones of different frequencies as CS+ and CS−. They reported that behavioural conditioning of the skin conductance response was associated with a decrease in the response to the CS+ frequency, a result apparently in accord with Ohl and Scheich’s (1996) report of a decreased response to the CS+.
The different patterns of results reported in various studies of the effects of behavioural conditioning and related procedures on the frequency selectivity of auditory cortical neurons undoubtedly reflect methodological, contextual, and task-related factors that remain incompletely understood (see Weinberger (2004a) for a detailed discussion). It is also unclear what feature or features of the training procedures are responsible for the neural changes (e.g., the association of that stimulus with reinforcement per se or the learned importance of that stimulus). Nevertheless, the conditioning, target detection, and frequency discrimination data constitute one of the strongest lines of evidence that the frequency selectivity of individual neurons, and hence the way in which spectral information is represented by populations of neurons, exhibits a high degree of plasticity in adult animals. The possible mechanisms responsible for these changes, and the extent to which they reflect cognitive processes, will be considered in a later section.
2.4. Plasticity associated with auditory perceptual learning
Another form of auditory learning that has been postulated to involve auditory cortical plasticity is perceptual learning, the improvement in discriminative capacity with training that is a common observation in all sensory modalities both in psychophysical experiments and in everyday life. The specificity of many forms of visual perceptual learning to the region of the receptor surface to which the training stimuli are presented, or to particular parameters of the stimuli, has prompted the proposal that the learning might reflect plastic changes at early stages of cortical processing (see Karni and Bertini (1997) for review). However, direct evidence from studies of the response characteristics of neurons in primary visual cortex (V1) of monkeys exhibiting perceptual learning on various discrimination tasks have shown either no changes or relatively subtle changes in RFs and no changes in cortical topography (Crist et al., 2001; Schoups et al., 2001; Ghose et al., 2002).
Two studies of auditory perceptual learning on frequency discrimination tasks, in which auditory cortex was mapped in detail in acute experiments at the completion of training, have yielded conflicting results. Recanzone et al. (1993) reported that in owl monkeys the area of representation in AI of the frequencies used in training was enlarged (by a factor of ~7 to ~9, depending on frequency) relative to untrained (control) monkeys, and that larger areas of representation were associated with superior discrimination performance. In contrast to these results, Brown et al. (2004) found no change in the frequency organization of AI in cats that were trained and showed perceptual learning on a somewhat different frequency discrimination task. The only change in neural response characteristics was a tendency for neurons with CF immediately above the training frequency to have slightly broader tuning in the trained cats than in controls. This pattern of results is similar to those reported in the studies of visual perceptual learning referred to above, but the reasons for the differences between Recanzone et al.’s (1993) and Brown et al.’s (2004) results remain unclear (see Brown et al. (2004) and Irvine et al. (2005) for discussion).
Blake et al. (2002) studied the effects of frequency discrimination training in two owl monkeys in which chronic recordings were made from neurons at the same cortical loci throughout the course of training. Although these experiments were directed at elucidating the neural correlates of reinforcement rather than those of perceptual learning per se, they bear on the latter issue. In both animals, the summed responses of all neurons sampled by the electrode array decreased during training, but the decrease was non-monotonic: at the time at which the monkey’s performance began to improve, responses to both the target frequency and control frequencies transiently increased, with a greater increase in the response to the target frequency. The method of data summation does not allow it to be determined whether this increase reflected a change in the frequency selectivity of some or all of the neurons, or simply a greater increase in responsiveness of those neurons tuned to the target frequency. Response strength declined over further training trials, while behavioural discrimination continued to improve, suggesting that the period of increased responsiveness was not the substrate of the improved discrimination but was a correlate of the behavior coming under the control of the reinforcement contingency. A similar decrease in AI responses to target stimuli was observed by Beitel et al. (2003) in a study in which owl monkeys were trained to detect an increase in the envelope frequency of an amplitude modulated tone. The extent to which the discriminative ability of the animals improved with practice is not clear from their report, however, so it is unclear whether the neural changes they observed reflect perceptual learning.
There is a large body of human imaging data indicating differences between musicians and non-musicians in auditory cortical responses, which might reflect perceptual learning by the former (see Irvine and Wright (2005) for review). Although the correlations reported in some of these studies could indicate either that musical training had produced plastic changes in auditory cortical responses or that individuals with these traits are more likely to pursue musical careers, a number of lines of evidence indicate that the changes are likely to be the product of training.
The longer-latency responses enhanced by training in these studies are localized to belt and parabelt areas of auditory cortex, whereas animal studies of perceptual learning have been largely restricted to AI. In the visual system, neural plasticity associated with perceptual learning on an orientation discrimination task is seen in V4 but not in V1 (Yang and Maunsell, 2004), and it seems reasonable to assume that auditory belt and parabelt areas would have greater capacity for plastic change than AI.
Finally, two forms of auditory perceptual learning that are of great practical significance relate to speech perception. The first is the effect of language experience on the perception of speech sounds, and thus on language acquisition, during a critical period of development (e.g., Kuhl et al., 1992; Kuhl, 2000). There is a growing body of imaging evidence indicating language-specific modification of the responses of belt and parabelt areas of human auditory cortex to speech sounds (e.g., Näätänen et al., 1997; Jacquemot et al., 2003). The second is the improvements in speech perception shown by cochlear implantees over the post-implantation period; as noted in Section 2.2, there appears to be no evidence to indicate the extent to which this form of learning reflects plasticity in auditory cortex itself or in higher-level areas.
2.5. Plasticity associated with environmental enrichment
One of the most intensively studied aspects of structural brain plasticity over more than 50 years has been the effects of so-called environmental “enrichment”: the brains of rats raised in enriched environments differ structurally in numerous ways from those of rats raised in (admittedly often impoverished) standard conditions (van Praag et al., 2000; Nithianantharajah and Hannan, 2006). There has been only limited investigation of the effects of environmental enrichment on the response characteristics of auditory cortical neurons, but Kilgard and his colleagues (Engineer et al., 2004; Percaccio et al., 2005) have reported that a combination of generalized and specifically acoustic enrichment results in changes in the response strength, RF characteristics, and temporal processing characteristics of AI neurons in rats. In a recent intriguing study of the effects of an enhanced acoustic environment in adult cats, Noreña et al. (2006) reported that an environment consisting of tone pips of 32 different frequencies in the range 5–20 kHz resulted in a reduction in short-latency neuronal responses to, and in the area of representation of, those frequencies, and to over-representation of frequencies neighbouring those in the enhanced acoustic environment. It appears that this exposure resulted in reduced input to that area (which could be regarded as a functional lesion) and thus in changes similar to those produced by cochlear lesions (see Eggermont (2007) for discussion).
2.6. Plasticity induced by cortical microstimulation
Changes in the frequency selectivity of AI neurons (and consequently in the cortical frequency map) as a consequence of focal electrical stimulation of the cortex have been observed in a number of studies. In both anesthetized (Maldonado and Gerstein, 1996) and awake (Talwar and Gerstein, 2001) rats, stimulation at a specific AI location changes the FRFs of nearby neurons (within ~300 μm) such that they become more responsive to the BF of the cells at the stimulation site and (in some cases) their BF shifted towards that at the stimulation site. This response enhancement occurred rapidly (in the course of a few hours of stimulation) and lasted for a period of some hours. Some rats were tested within that period on a frequency discrimination task at the BF of neurons at the stimulation site, but the enlarged area of strong responses at that frequency did not result in better discrimination (Talwar and Gerstein, 2001). More dramatic effects of microstimulation in AI of gerbils and echolocating bats have been reported by Suga and his colleagues (see Suga and Ma (2003) for review). They found that the BF of neurons surrounding the stimulation site shifted towards that of the neurons at the stimulation site, resulting in an enlarged representation of that frequency. In a smaller area surrounding the region in which BF shifted toward that at the stimulating site, smaller shifts in BF away from that at the stimulating site were observed. In the experiments with echolocating bats, the frequency selectivity of neurons in the tonotopically corresponding area of the ICC were also found to change as a consequence of cortical stimulation, presumably as a consequence of the activation of corticofugal fibres (Suga and Ma, 2003).
2.7. Plasticity induced by direct activation of neuromodulatory systems
The neocortex receives diffuse extrathalamic projections from five different subcortical cell groups, which act to modulate cortical sensitivity and have been implicated in cortical plasticity (e.g., Gu, 2002). The most extensively studied of these systems is the system of cholinergic fibres originating in the basal forebrain. A number of lines of evidence indicate that acetylcholine (ACh) and the cholinergic basal forebrain are involved in at least some forms of auditory cortical plasticity
Pairing a tonal stimulus of a particular frequency with direct application of either cholinergic agents (e.g., McKenna et al., 1989; Metherate and Weinberger, 1990) or of acetylcholine-esterase inhibitors (Ashe et al., 1989) alters the FRFs of a large proportion of auditory cortical neurons by selectively modifying the response to tones at or near the paired frequency. These observations imply that endogenous ACh has a role in modulating the frequency selectivity of auditory cortical neurons. This interpretation is supported by data obtained under more physiological conditions by pairing electrical stimulation of the basal forebrain with tonal stimulation. Such pairing has been shown to result in frequency-selective changes in FRFs in a number of species (see Weinberger (2003) and Ma and Suga (2003) for reviews) and in an enlarged representation of the paired frequency in AI of the rat (Kilgard and Merzenich, 1998a). When paired with tone-pip stimulation at particular rates, basal forebrain stimulation also modifies the temporal information processing characteristics of auditory cortical neurons (Kilgard and Merzenich, 1998b). Although such stimulation also activates non-cholinergic basal forebrain neurons (notably the large population of GABAergic neurons that also project to the cortex), the fact that these effects were in a number of cases shown to be blocked by application of ACh antagonists to the cortex indicates that they were mediated by activation of cholinergic neurons. These effects of experimental activation of the BF suggest that it might be involved in the range of injury- and use-related plasticity in auditory cortex described in previous sections. This possibility is examined in the following section
3. Mechanisms of auditory cortical plasticity
All of the various forms of plasticity described in the previous sections involve changes in the efficacy of particular stimuli in “driving” (i.e., producing action potentials in) auditory cortical neurons. In some cases, stimuli that are normally ineffective in driving the neuron become effective; in others the relative efficacy of different stimuli is altered. An explanation of the former cases requires an account of the source of the apparently “new” input, and of the mechanisms by which it becomes effective. Explanation of the latter cases requires an account of the change in the relative efficacy of the inputs. As elaborated in the following section, evidence that has required substantial revision of classical ideas about neuronal RFs provides at least part of the answer (and perhaps the entire answer) to the question concerning the source of new inputs. This evidence will be reviewed in the following section, before consideration of the mechanisms by which new inputs become effective or the efficacy of existing inputs is modified.
3.1 Sources of “new” input: Changes in the conceptualization of cortical receptive fields
In the early period of sensory neuroscience, RFs were described simply in terms of the range of the given stimulus parameter/s that evoked an increase in action potential frequency above the background resting activity. In the visual cortex literature, the “classical” RF so defined has been referred to as the “minimum discharge field” (MDF) (e.g., Bringuier et al., 1999). An auditory cortical neuron’s MDF in the frequency domain comprises the range of frequencies and sound pressure levels (SPLs) that evoke an increased discharge (i.e., the area delimited by its tuning curve). In the absence of spontaneous activity, these studies could not identify inhibitory components of RFs, but studies using forward masking paradigms (e.g., Calford and Semple, 1995), or the iontophoretic application of inhibitory transmitter antagonists, revealed that many neurons at all levels of the central auditory system receive inhibitory input at a range of frequencies and SPLs outside the MDF, often as flanking or “side-band” inhibition (but see Wehr and Zador, 2003). The immediate expansion of the RFs of some auditory cortical neurons following iontophoretic application of the GABA-A receptor antagonist bicuculline (e.g., Wang et al., 2002; Foeller et al., 2001) indicates that in these neurons suprathreshold excitatory input outside the MDF is masked by inhibition. The complex combinations of excitatory and inhibitory regions comprising the suprathreshold RFs of auditory cortical neurons, and the way in which these components vary over time, are particularly clearly illustrated in STRFs (e.g., Sutter, 2005; Fritz et al., 2003, 2005a).
These accounts of auditory cortical RFs are based on the spiking activity of cortical neurons, however, and undoubtedly the most dramatic changes in our conceptualization of RFs have been those produced by in vivo intracellular recordings. Such recordings in V1 have revealed that many neurons receive subthreshold excitatory and inhibitory input over an area (the “synaptic integration field”) many times larger than the MDF (e.g., Bringuier et al., 1999; Frégnac et al., 1996). Similarly, intracellular data indicate that the RFs of AI neurons defined in terms of subthreshold excitatory post-synaptic potentials (EPSPs) are much broader than those defined in terms of spike responses (Ojima & Murakami, 2002; Wehr and Zador, 2003), extending over 5 octaves or more at moderate SPLs (Kaur et al., 2004; Metherate et al., 2005) (Fig. 1). As in the visual system, this synaptic integration field reflects input derived from intrinsic cortico-cortical connections over horizontal fibers (Kaur et al., 2004; Metherate et al., 2005). Finally, there is now overwhelming evidence for the existence of “silent” synapses which can be rapidly activated by patterns of stimulation than evoke long-term potentiation (LTP) (see Atwood and Wojtowicz (1999) for review). It is possible that such silent inputs could be derived from points beyond the synaptic integration field of AI neurons, thus increasing even further the range of locations within the cortical tonotopic map from which input can be derived.
Figure 1.
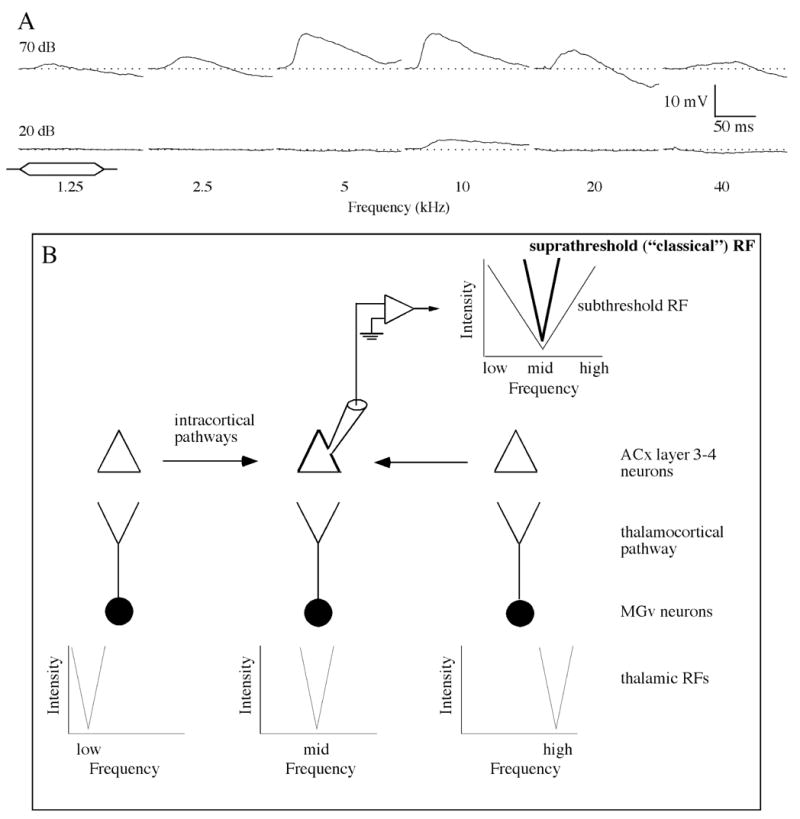
The synaptic integration field (the combination of supra- and sub-threshold RFs) of an AI neuron, and a schematic illustration of the derivation of the classical and subthreshold RFs. (a) Intracellular synaptic potentials evoked in response to a 5-octave range of frequencies at 20 dB and 70 dB SPL. Tone duration 100 ms, beginning at trace onset (10-ms rise times). Synaptic potentials were obtained using whole-cell recording from a layer 3 neuron in AI of a urethane anesthetized rat; resting potential ~−60 mV. (b) Schematic illustration of the hypothesis that direct thalamo-cortical inputs to auditory cortical neurons convey information about stimuli within the classical RF and intracortical horizontal projections carry information mostly about spectrally distant non-CF stimuli. ACx, auditory cortex. Reproduced with permission from Metherate et al. (2005).
3.2. Mechanisms by which the efficacy of particular inputs is modified
The supra- and sub-threshold inputs outside the classic RF revealed by the studies reviewed in the previous section provide a broad matrix of inputs from which the short- and long-term RF changes observed in various plasticity paradigms could potentially be sculpted. Given this broad range of potential inputs, the major question raised by the various forms of plasticity concerns the nature of the “sculpting” processes involved: What are the mechanisms by which the efficacy of various inputs is modified (i.e., by which excitatory and inhibitory synaptic weights are changed)? Direct evidence bearing on this question in the case of auditory plasticity is limited, and the following account is leavened by evidence from other sensory systems and by a fair measure of speculation. It should also be noted that different forms of plasticity are likely to involve different mechanisms or combinations of mechanisms with different time courses.
Undoubtedly the best established mechanism for the strengthening of synaptic connections is correlated pre- and post-synaptic activity, as originally postulated by Hebb (1949), and realised in the phenomenon of LTP (see Buonomano and Merzenich (1998), Bi and Poo (2001), and Dan and Poo (2006) for reviews). Auditory cortical plasticity produced by cortical micro-stimulation and the preservation/restoration of cochleotopicity by electrical stimulation at discrete cochlear places would appear to be readily explicable in terms of this mechanism. In the former case, cortical microstimulation would be expected to activate nearby neurons via intrinsic intracortical connections, such that these inputs to those neurons would be strengthened. In the latter case, the pathways projecting from the cochlear regions activated by intracochlear stimulation to (more or less) discrete cortical regions would also be selectively strengthened. Although this mechanism is intuitively plausible, it must be acknowledged that there is currently no direct evidence for its involvement in these forms of plasticity.
Synaptic LTP and long-term depression (LTD) would also appear to be strong candidates for the processes involved in changes in synaptic weights in injury-induced reorganization. Evidence from the somatosensory system indicates that the first stage of reorganization after digit amputation is an immediate expansion onto peri-lesion skin areas of the RFs of neurons in the LPZ (Calford and Tweedale (1988, 1991); see Calford (2002) for review). The immediacy of this expansion can only be explained in terms of the “unmasking” (presumably, the release from inhibition) of pre-existing suprathreshold excitatory inputs. Over a period of weeks after the lesion, the expanded RFs gradually reduced in size, a process which Calford and Tweedale (1988) attributed to a re-establishment of excitatory-inhibitory balance. LTP and LTD are likely to be involved in this second phase of plastic change, and there is evidence for the involvement of NMDA receptor-mediated LTP in lesion-induced (and other forms) of somatosensory cortical plasticity (e.g., Kano et al., 1991; see Calford (2002) for review).
The role of unmasking of previously inhibited excitatory inputs in auditory cortical lesion-induced plasticity has not been established with certainty. Such unmasking as a consequence of loud-tone induced hearing losses has been demonstrated in the immediate expansion of the frequency-SPL response areas of some AI neurons and associated changes in CF (Calford et al., 1993; Seki and Eggermont, 2002; Noreña et al., 2003). In the only study of the acute effects of mechanical cochlear lesions, Robertson and Irvine (1989) did not see an immediate expansion of RFs. Rather, the post-lesion tuning curves appeared to be explicable as the residue of pre-lesion responses. However, interpretation of this observation is qualified by the fact that the mechanical lesioning technique used in that study almost certainly produced short-term disruptions of cochlear processes at regions around the area of permanent damage. Snyder and his colleagues have reported that restricted spiral ganglion lesions (which would not be expected to have these short-term consequences) result in the immediate post-lesion appearance of low-threshold excitation at frequencies below (or, in fewer cases, above) the borders of the pre-lesion frequency tuning curve in neurons in the LPZ in AI (Snyder and Sinex, 1998) and in ICC (Snyder et al., 2000; Snyder and Sinex, 2002). While such unmasking presumably contributes to injury-induced auditory cortical reorganization, it does not seem to be sufficient for its occurrence. Although acute unmasking occurs in ICC after restricted spiral ganglion cell lesions, long-term reorganization after mechanical cochlear lesions either does not occur or occurs only in restricted regions of the nucleus (Irvine et al., 2003).
In the visual system, there is direct evidence that input derived over long-range horizontal intracortical connections is necessary for reorganization in V1 after restricted retinal lesion in adults (Calford et al., 2003). This conclusion is supported by the fact that only minimal reorganization is seen in the visual thalamus after such lesions (Gilbert and Wiesel, 1992). In the auditory system, however, cochlear lesions in adult cats result in reorganization in the MGV that is indistinguishable from that in AI (Kamke et al., 2003). If the reorganization in cortex partially or wholly reflects that in MGV, it would indicate that reorganization in AI involves mechanisms other than changes in intracortical circuitry. However, it could be that the cortical changes are primary and are transferred to the thalamus via corticofugal projections. The possible importance of corticofugal projections in subcortical plasticity is indicated by the demonstration by Suga and his colleagues that changes in the frequency organization of ICC induced by conditioning and by cortical microstimulation depend on corticofugal projections from AI (see Suga and Ma (2003) for review). In the somatosensory system, there is extensive reorganization in the thalamus after peripheral lesions, and corticofugal projections contribute substantially to thalamic plasticity (see Calford (2002) for review). Despite the similarities between lesion-induced reorganization in the major sensory systems, that in the auditory and somatosensory systems seems to be a characteristic of the entire thalamo-cortico-thalamic system, while that in the visual system seems to depend more on intrinsically cortical mechanisms.
In both microstimulation- and injury-induced plasticity, the nature of the intervention is such as to result in substantial changes in the relative frequency with which different inputs activate the AI neuron and thus in changes in synaptic strength. In the case of conditioning and other forms of behavioural training, however, the training procedure itself would be expected to involve only a slight increase in the frequency of activation of inputs associated with the training or discrimination frequency. In these forms of plasticity, the critical point seems to be that the training stimulus is associated with reinforcement, and thus acquires enhanced behavioural significance for the organism. Under these conditions, it is widely held that the modulating influence of the cholinergic projections to AI is the critical factor in modifying the strength of inputs associated with that stimulus.
This view is supported by the vast body of evidence reviewed above for the effects of cholinergic agents and of stimulation of the basal forebrain on the response characteristics of auditory cortical neurons. ACh antagonists have also been shown to block the changes in auditory cortical fMRI responses in humans (Thiel et al., 2002), and the BF changes in auditory cortical neurons in bats (Ji et al., 2001), that are produced by classical conditioning procedures. However, neither of these studies directly established the role of the cholinergic projections from the basal forebrain in producing those changes. In what appears to be the only study of the effects of inactivation of this system on auditory learning, Kudoh et al. (2004) found that immunotoxic injections into the auditory cortex in rats disrupted the learning of a sound-sequence discrimination, but in that study no evidence on plasticity of neuronal response characteristics was presented. Thus, although there is a vast body of indirect evidence pointing to the role of the cholinergic basal forebrain in learning-induced auditory cortical neural plasticity, it has not been established directly that it is necessary for such changes. What is required is a study in which the effects of appropriate training procedures on auditory cortical neurons are determined in animals in which cholinergic terminals in auditory cortex have been eliminated.
In the only study of the role of the basal forebrain in injury-induced auditory cortical plasticity, Kamke et al. (2005) found that immunotoxic lesions of the cholinergic basal forebrain regions projecting to AI did not prevent cochlear-lesion induced reorganization in AI of adult cats. If one assumes that injury-induced reorganization in different sensory cortices involves the same mechanisms, this result is apparently at variance with evidence from the somatosensory system that injury-induced reorganization is blocked by lesions of the basal forebrain (Webster et al., 1991; Juliano et al., 1991). That effect cannot unequivocally be attributed to destruction of the cholinergic system, however, as the neurotoxic lesions used in those studies would also have destroyed non-cholinergic neurons in the basal forebrain. The evidence indicating that the cholinergic basal forebrain is not involved in cochlear-lesion induced plasticity in AI (which presumably depends on the strengthening of intra-cortical connections), but does appear to be involved in learning-related plasticity (which presumably depends on the strengthening of particular thalamo-cortical connections) is in accord with Hasselmo’s (1995) hypothesis that neuromodulators such as ACh enhance the influence of synapses from extracortical afferent fibres relative to those of intrinsic intracortical fibers.
Finally, on a longer time scale, structural changes have been shown to be associated with some forms of cortical plasticity. Axonal sprouting of horizontal fibers in the superficial layers of the cortex are associated with visual cortical plasticity after retinal lesions in adults (Darian-Smith and Gilbert, 1994). In the barrel cortex of adult rats, vibrissal deafferentation, which results in an expansion of the influence of intact vibrissae into the area in which the deafferented vibrissae were represented, results in changes in the dendritic orientation of neurons in this area toward the sources of this new input (Tailby et al., 2005). Structural changes of this sort have not yet been shown in injury-induced auditory plasticity in adults, but the similarity of many forms of adult plasticity in different sensory systems suggests that such changes could well be involved.
4. Functional significance of central auditory system plasticity
The plastic changes in the frequency selectivity of auditory cortical neurons that have been described in various learning and discrimination studies are clearly adaptive, in that they result in a stronger response to, or better discrimination of, acoustic stimulus characteristics of behavioural significance. It is not clear, however, that the plastic changes seen after cochlear lesions are in any sense adaptive. Although it is tempting to think of the reorganization as a central compensation for the peripheral loss, the fact remains that sensitivity to the frequencies that would normally produce activation in the part of the cochlea damaged by the lesion is in no way restored by central reorganization.
Although they do not appear to be compensatory, the marked changes in the pattern of cortical activation produced by peri-lesion frequencies would be expected to have perceptual consequences. A number of studies in humans with steeply sloping hearing losses of the type that would be expected to result in auditory cortical reorganization have found that listeners with such losses exhibited improved frequency difference limens (DLFs) in the region of the cut-off (edge) frequency of their hearing loss (McDermott et al. 1998; Thai-Van et al., 2002, 2003; Kluk and Moore, 2006). The improved DLFs are not attributable to loudness cues, and do not reflect spontaneous otoacoustic emissions at or near the edge frequency, which might interact with external tones in that frequency range and provide additional discrimination cues (Thai-Van et al., 2003). These observations strengthen the possibility that the enhanced DLFs might reflect cortical reorganization, but it must be emphasised that there is no direct evidence of such reorganization’s having occurred in the participants in these studies.
It is also possible that some forms of cortical plasticity might have pathological consequences. In the somatosensory system, there is evidence that cortical reorganization might be responsible for some features of phantom limb experiences in amputees (e.g., Ramachandran and Hirstein, 1998). It has similarly been suggested that tinnitus might be a consequence of cortical reorganization consequent on a cochlear lesion (Mühlnickel et al., 1998)
Finally, the possibility that some forms of learning impairment might involve deficiencies in aspects of auditory processing that exhibit plasticity, such that these deficiencies can be modified by training, has resulted in the recent development of a number of auditory training software packages (e.g., Merzenich et al., 1996; Hayes et al., 2003; Warrier et al., 2004)
5. Does auditory cortical plasticity provide evidence of cognitive processing in auditory cortex?
The answer to this question depends, of course, on how cognitive processing is defined, and the meaning of the term is unfortunately both diffuse and constantly evolving. In an early definition, Neisser (1967) stated that “cognition refers to all the processes by which the sensory input is transformed, reduced, elaborated, stored, recovered, and used” (p.4). On this definition, auditory cortex undoubtedly carries out cognitive processing, but it would also be hard to deny that status to the cochlear nucleus and other auditory brainstem nuclei. Without getting bogged down in questions of precise definition, there is probably general consensus that cognitive processes are those mental processes that are involved in the interpretive aspects of perception, and in memory, learning, reasoning, and attention etc.
In the context of this sort of broad conceptualization, there would seem to be little reason to assign cognitive status to the processes involved in a number of the forms of auditory cortical plasticity reviewed here. As argued above, it is probable that plasticity produced by cortical microstimulation in hearing animals, by cochlear electrical stimulation in deafened animals, and following cochlear lesions producing partial deafness, is explicable in terms of changes in synaptic strength produced by changes in the relative frequency of activation of different inputs to cortical neurons. Even if such changes are adaptive, which they would seem to be in the case of increased efficacy of input from a cochlear prosthesis, but which I have argued is probably not the case with cochlear-lesion induced plasticity, the adaptive process is not a cognitive one.
Although the effects of environmental enrichment reported by Kilgard and his colleagues (Engineer et al., 2004; Percaccio et al., 2005) might also be explicable in terms of relatively simple strengthening of connections, those reported by Noreña et al. (2006) are not. But whatever the mechanisms involved, and even if enrichment results in improved cognitive performance (van Praag et al., 2000; Nithianantharajah and Hannan, 2006), there would seem to be little reason to identify the processes involved as cognitive in nature.
Of the various forms of auditory cortical plasticity reviewed here, that with the strongest claim to be considered cognitive in nature is the plasticity associated with various forms of learning, and this evidence has frequently been cited as establishing that specific memory traces are stored in primary auditory cortex (e.g., Weinberger, 2004b). This view is strengthened by the fact that many forms of auditory cortical plasticity seem to be associated with the effects of the cholinergic basal forebrain, and by the vast body of evidence for the involvement of this modulatory system in memory, learning and attention (e.g., Hasselmo, 1995; Sarter et al., 2001). The storage of auditory memory traces in auditory cortex (whether primary, belt, or parabelt) is also in accord with the view, long held in the memory literature, that memories are likely to be stored in the same neural systems that are involved in the processing and analysis of the information. While this evidence is cumulatively extremely strong, it must nevertheless be acknowledged that it remains correlational. That is, training that results in behavioural learning and memory also results in changes in RFs and in other response properties in auditory cortex, of a nature that would seem appropriate to underlie the learning. It has not yet been directly established, however, that those auditory cortical changes are necessary for the establishment of the behavioural memory; as Weinberger (2003) has acknowledged, “RF plasticity and behavioural memory …. have not yet been tightly linked” (p280). Establishing this linkage presents a daunting experimental challenge. Lesion studies are not appropriate because they do not allow the roles of auditory cortex in the processing of the auditory information and the storage of the auditory memory to be separated, and because in the absence of the cortex its function might be taken over by other structures. A possible solution might be to determine whether blocking RF changes in cortex during training (perhaps by blocking locally the effects of the cholinergic basal forebrain) prevents the establishment of the behavioural memory.
In conclusion, while many of the other aspects of auditory cortical function that were mentioned in the Introduction provide compelling evidence of cognitive processing by auditory cortex, the evidence provided by plasticity would seem to be limited to those forms involved in learning and memory, and would still appear to require conclusive evidence that RF plasticity is a necessary condition of behavioural memory.
Acknowledgments
I am grateful to Marc Kamke and to two anonymous reviewers for comments on earlier versions of this manuscript and to Deafness Research (UK) for their support of the conference. The research by the author and his colleagues cited here was supported by grants from the National Health and Medical Research Council of Australia and by a contract from the National Institute of Deafness and Communication Disorders (NIH-N01-DC-3-1005)
Abbreviations
- AI
Primary auditory cortex
- ACh
Acetylcholine
- BF
Best frequency
- CF
Characteristic frequency
- CNS
Central nervous system
- CS
Conditioned stimulus
- DCN
Dorsal cochlear nucleus
- DLF
Frequency difference limen
- EPSP
Excitatory post-synaptic potential
- fMRI
Functional magnetic resonance imaging
- ICC
Central nucleus of inferior colliculus
- LTD
Long-term depression
- LTP
Long-term potentiation
- LPZ
Lesion projection zone
- MEG
Magnetoencephalography
- MDF
Minimum discharge field
- MGV
Ventral division of medial geniculate nucleus
- MMN
Mismatch negativity
- NMDA
N-methyl-D-aspartate
- PET
Positron emission topography
- RF
Receptive field
- SD
Discriminative stimulus
- SPL
Sound pressure level
- STRF
Spectro-temporal receptive field
- UCS
Unconditioned stimulus
- V1
Primary visual cortex
References
- Ashe JH, McKenna TM, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: II Frequency-specific effects of anticholinesterases provide evidence for a modulatory action of endogenous ACh. Synapse. 1989;4:44–54. doi: 10.1002/syn.890040106. [DOI] [PubMed] [Google Scholar]
- Atwood HL, Wojtowicz JM. Silent synapses in neural plasticity: Current evidence. Learn Mem. 1999;6:542–571. doi: 10.1101/lm.6.6.542. [DOI] [PubMed] [Google Scholar]
- Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci, USA. 2003;100:11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi G-q, Poo M-m. Synaptic modification by correlated activity: Hebb's postulate revisited. Ann Rev Neurosci. 2001;24:139–166. doi: 10.1146/annurev.neuro.24.1.139. [DOI] [PubMed] [Google Scholar]
- Blake DT, Strata F, Churchland AK, Merzenich MM. Neural correlates of instrumental learning in primary auditory cortex. Proc Natl Acad Sci USA. 2002;99:10114–10119. doi: 10.1073/pnas.092278099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bringuier V, Chavane F, Glaeser L, Frégnac Y. Horizontal propagation of visual activity in the synaptic integration field of area 17 neurons. Science. 1999;283:695–699. doi: 10.1126/science.283.5402.695. [DOI] [PubMed] [Google Scholar]
- Brown M, Irvine DRF, Park VN. Perceptual learning on an auditory frequency discrimination task by cats: association with changes in primary auditory cortex. Cereb Cortex. 2004;14:952–965. doi: 10.1093/cercor/bhh056. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Calford MB. Dynamic representational plasticity in sensory cortex. Neuroscience. 2002;111:709–738. doi: 10.1016/s0306-4522(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Calford MB, Rajan R, Irvine DRF. Rapid changes in the frequency tuning of neurons in cat auditory cortex resulting from pure-tone-induced temporary threshold shift. Neuroscience. 1993;55:953–964. doi: 10.1016/0306-4522(93)90310-c. [DOI] [PubMed] [Google Scholar]
- Calford MB, Semple MN. Monaural inhibition in cat auditory cortex. J Neurophysiol. 1995;73:1876–1891. doi: 10.1152/jn.1995.73.5.1876. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Immediate and chronic changes in responses of somatosensory cortex in adult flying-fox after digit amputation. Nature. 1988;332:446–448. doi: 10.1038/332446a0. [DOI] [PubMed] [Google Scholar]
- Calford MB, Tweedale R. Acute changes in cutaneous receptive fields in primary somatosensory cortex after digit denervation in adult flying fox. J Neurophysiol. 1991;65:178–187. doi: 10.1152/jn.1991.65.2.178. [DOI] [PubMed] [Google Scholar]
- Calford MB, Wright LL, Metha AB, Taglianetti V. Topographic plasticity in primary visual cortex is mediated by local corticocortical connections. J Neurosci. 2003;23:6434–6442. doi: 10.1523/JNEUROSCI.23-16-06434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nature Neurosci. 2001;4:519–525. doi: 10.1038/87470. [DOI] [PubMed] [Google Scholar]
- Dallos P, Harris D. Properties of auditory nerve responses in absence of outer hair cells. J Neurophysiol. 1978;41:365–383. doi: 10.1152/jn.1978.41.2.365. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity: from synapse to perception. Physiol Rev. 2006;86:1033–1048. doi: 10.1152/physrev.00030.2005. [DOI] [PubMed] [Google Scholar]
- Darian-Smith C, Gilbert CD. Axonal sprouting accompanies functional reorganization in adult cat striate cortex. Nature. 1994;368:737–740. doi: 10.1038/368737a0. [DOI] [PubMed] [Google Scholar]
- Dietrich V, Nieschalk M, Stoll W, Rajan R, Pantev C. Cortical reorganization in patients with high frequency cochlear hearing loss. Hear Res. 2001;158:1–7. doi: 10.1016/s0378-5955(01)00282-9. [DOI] [PubMed] [Google Scholar]
- Dinse HR, Godde B, Reuter G, Cords SM, Hilger T. Auditory cortical plasticity under operation: reorganization of auditory cortex induced by electrical cochlear stimulation reveals adaptation to altered sensory input statistics. Speech Communication. 2003;41:201–219. [Google Scholar]
- Edeline JM, Pham P, Weinberger NM. Rapid development of learning-induced receptive field plasticity in the auditory cortex. Behav Neurosci. 1993;107:539–551. doi: 10.1037//0735-7044.107.4.539. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Correlated neural activity as the driving force for functional changes in auditory cortex. Hear Res. 2007 doi: 10.1016/j.heares.2007.01.008. In press. [DOI] [PubMed] [Google Scholar]
- Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. J Neurophysiol. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- Foeller E, Vater M, Kössl M. Laminar analysis of inhibition in the gerbil primary auditory cortex. J Assoc Res Otolaryngol. 2001;2:279–296. doi: 10.1007/s101620010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frégnac Y, Bringuier V, Chavane F, Glaeser L, Lorenceau J. An intracellular study of space and time representation in primary visual cortical receptive fields. J Physiol (Paris) 1996;90:189–197. doi: 10.1016/s0928-4257(97)81422-2. [DOI] [PubMed] [Google Scholar]
- Fritz J, Elhilali M, Shamma S. Task-dependent adaptive plasticity of receptive fields in primary auditory cortex of the ferret. In: Koenig R, Heil P, Budinger E, Scheich H, editors. Auditory Cortex: Towards a Synthesis of Human and Animal Res. Lawrence Erlbaum; Mahwah, New Jersey: 2005a. pp. 445–466. [Google Scholar]
- Fritz JB, Elhilali M, Shamma SA. Differential dynamic plasticity of A1 receptive fields during multiple spectral tasks. J Neurosci. 2005b;25:7623–7635. doi: 10.1523/JNEUROSCI.1318-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nature Neurosci. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- Galambos R, Sheatz G, Vernier VG. Electrophysiological correlates of a conditioned response in cats. Science. 1955;123:376–377. doi: 10.1126/science.123.3192.376. [DOI] [PubMed] [Google Scholar]
- Gao EQ, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci USA. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose GM, Yang T, Maunsell JHR. Physiological correlates of perceptual learning in monkey V1 and V2. J Physiol. 2002;87:1867–1888. doi: 10.1152/jn.00690.2001. [DOI] [PubMed] [Google Scholar]
- Gilbert CD. Adult cortical dynamics. Physiol Rev. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Wiesel TN. Receptive field dynamics in adult primary visual cortex. Nature. 1992;356:150–152. doi: 10.1038/356150a0. [DOI] [PubMed] [Google Scholar]
- Gu Q. Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience. 2002;111:815–835. doi: 10.1016/s0306-4522(02)00026-x. [DOI] [PubMed] [Google Scholar]
- Harrison RV. Age-related tonotopic map plasticity in the central auditory pathways. Scand Audiol. 2001;30:8–14. doi: 10.1080/010503901750166529. [DOI] [PubMed] [Google Scholar]
- Harrison RV, Ibrahim D, Stanton SG, Mount RJ. Reorganization of frequency maps in chinchilla auditory midbrain after long-term basal cochlear lesions induced at birth. In: Salvi RJ, Henderson D, Fiorino F, Colletti V, editors. Auditory System Plasticity and Regeneration. Thieme Medical; New York: 1996. pp. 238–255. [Google Scholar]
- Hartmann R, Shepherd RK, Heid S, Klinke R. Response of the primary auditory cortex to electrical stimulation of the auditory nerve in the congenitally deaf white cat. Hear Res. 1997;112:115–133. doi: 10.1016/s0378-5955(97)00114-7. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. Neuromodulation and cortical function: Modeling the physiological basis of behavior. Behav Brain Res. 1995;67:1–27. doi: 10.1016/0166-4328(94)00113-t. [DOI] [PubMed] [Google Scholar]
- Hayes EA, Warrier CM, Nicol TG, Zecker SG, Kraus N. Neural plasticity following auditory training in children with learning problems. Clinical Neurophysiol. 2003;114:673–684. doi: 10.1016/s1388-2457(02)00414-5. [DOI] [PubMed] [Google Scholar]
- Hebb DO. Organization of Behavior. Wiley; New York: 1949. [Google Scholar]
- Hensch TK. Critical period regulation. Annu Rev Neurosci. 2004;27:549–579. doi: 10.1146/annurev.neuro.27.070203.144327. [DOI] [PubMed] [Google Scholar]
- Irvine D, Brown M, Martin R, Park V. Auditory perceptual learning and cortical plasticity. In: Koenig R, Heil P, Budinger E, Scheich H, editors. Auditory Cortex: Towards a Synthesis of Human and Animal Research. Lawrence Erlbaum; Mahwah, New Jersey: 2005. pp. 409–428. [Google Scholar]
- Irvine DRF, Rajan R, Smith S. Effects of restricted cochlear lesions in adult cats on the frequency organization of the inferior colliculus. J Comp Neurol. 2003;467:354–374. doi: 10.1002/cne.10921. [DOI] [PubMed] [Google Scholar]
- Irvine DRF, Wright BA. Plasticity in spectral processing. In: Malmierca M, Irvine DRF, editors. Auditory Spectral Processing. Elsevier; San Diego: 2005. pp. 435–472. [Google Scholar]
- Jacquemot C, Pallier C, LeBihan D, Dehaene S, Dupoux E. Phonological grammar shapes the auditory cortex: A functional magnetic resonance imaging study. J Neurosci. 2003;23:9541–9546. doi: 10.1523/JNEUROSCI.23-29-09541.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji WQ, Gao EQ, Suga NB. Effects of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. J Neurophysiol. 2001;86:211–225. doi: 10.1152/jn.2001.86.1.211. [DOI] [PubMed] [Google Scholar]
- Juliano SL, Ma W, Eslin D. Cholinergic depletion prevents expansion of topographic maps in somatosensory cortex. Proc Natl Acad Sci. 1991;88:780–784. doi: 10.1073/pnas.88.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Florence SL. Reorganization of sensory and motor systems in adult mammals after injury. In: Kaas JH, editor. The Mutable Brain. Harwood; Amsterdam: 2001. pp. 165–242. [Google Scholar]
- Kamke MR, Brown M, Irvine DRF. Plasticity in the tonotopic organization of the medial geniculate body in adult cats following restricted unilateral cochlear lesions. J Comp Neurol. 2003;459:355–367. doi: 10.1002/cne.10586. [DOI] [PubMed] [Google Scholar]
- Kamke MR, Brown M, Irvine DRF. Basal forebrain cholinergic input is not essential for lesion-induced plasticity in mature auditory cortex. Neuron. 2005;48:675–686. doi: 10.1016/j.neuron.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Kano M, Lino K, Kano M. Functional reorganization of adult cat somatosensory cortex is dependent on NMDA receptors. Neuroreport. 1991;2:77–80. doi: 10.1097/00001756-199102000-00003. [DOI] [PubMed] [Google Scholar]
- Karni A, Bertini G. Learning perceptual skills: behavioral probes into adult cortical plasticity. Curr Opinion Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- Kaur S, Lazar R, Metherate R. Intracortical pathways determine breadth of subthreshold frequency receptive fields in primary auditory cortex. J Neurophysiol. 2004;91:2551–2567. doi: 10.1152/jn.01121.2003. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998a;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nature Neurosci. 1998b;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinke R, Kral A, Heid S, Tillein J, Hartmann R. Recruitment of the auditory cortex in congenitally deaf cats by long-term cochlear electrostimulation. Science. 1999;285:1729–1733. doi: 10.1126/science.285.5434.1729. [DOI] [PubMed] [Google Scholar]
- Kluk K, Moore BCJ. Dead regions in the cochlea and enhancement of frequency discrimination: Effects of audiogram slope, unilateral versus bilateral loss, and hearing- aid use. Hear Res. 2006;222:1–15. doi: 10.1016/j.heares.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Kudoh M, Seki K, Shibuki K. Sound-sequence discrimination learning is dependent on cholinergic inputs to the rat auditory cortex. Neuroscience Res. 2004;50:113–123. doi: 10.1016/j.neures.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. A new view of language acquisition. Proc Natl Acad Sci. 2000;97:11850–11857. doi: 10.1073/pnas.97.22.11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl P, Williams K, Lacerda F, Stevens K, Lindblom B. Linguistic experience alters phonetic perception in infants by 6 months of age. Science. 1992;255:606–608. doi: 10.1126/science.1736364. [DOI] [PubMed] [Google Scholar]
- Maldonado PE, Gerstein GL. Reorganization in the auditory cortex of the rat induced by intracortical microstimulation: A multiple single-unit study. Exp Brain Res. 1996;112:420–430. doi: 10.1007/BF00227948. [DOI] [PubMed] [Google Scholar]
- McDermott HJ, Lech M, Kornblum MS, Irvine DRF. Loudness perception and frequency discrimination in subjects with steeply sloping hearing loss: Possible correlates of neural plasticity. J Acoust Soc Am. 1998;104:2314–2325. doi: 10.1121/1.423744. [DOI] [PubMed] [Google Scholar]
- McKay CM. Spectral processing in cochlear implants. In: Malmierca M, Irvine DRF, editors. Auditory Spectral Processing. Elsevier; San Diego: 2005. pp. 473–509. [Google Scholar]
- McKenna TM, Ashe JH, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: I. Frequency-specific effects of muscarinic agonists. Synapse. 1989;4:30–43. doi: 10.1002/syn.890040105. [DOI] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM, Johnston P, Schreiner C, Miller SL, Tallal P. Temporal processing deficits of language-learning impaired children ameliorated by training. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- Metherate R, Kaur S, Kawai H, Lazar R, Liang K, Rose HJ. Spectral integration in auditory cortex: Mechanisms and modulation. Hear Res. 2005;206:146–158. doi: 10.1016/j.heares.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Metherate R, Weinberger NM. Cholinergic modulation of responses to single tones produces tone-specific receptive field alterations in cat auditory cortex. Synapse. 1990;6:133–145. doi: 10.1002/syn.890060204. [DOI] [PubMed] [Google Scholar]
- Moore CM, Vollmer M, Leake PA, Snyder RL, Rebscher SJ. The effects of chronic intracochlear electrical stimulation on inferior colliculus spatial representation in adult deafened cats. Hear Res. 2002;164:82–96. doi: 10.1016/s0378-5955(01)00415-4. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Dolan RJ. Experience-dependent modulation of tonotopic neural responses in human auditory cortex. Proc Roy Soc Lond, Series B. 1998;265:649–657. doi: 10.1098/rspb.1998.0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näätänen R, Tervaniemi M, Sussman E, Paavilainen P, Winkler I. 'Primitive intelligence' in the auditory cortex. Trends in Neurosciences. 2001;24:283–288. doi: 10.1016/s0166-2236(00)01790-2. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Lehtokoski A, Lennes M, Cheour M, Huotilainen M, Iivonen A, Vainio M, Alku P, Ilmoniemi RJ, Luuk A, Allik J. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385:432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- Neisser U. Cognitive Psychology. Appleton Century Crofts; NY: 1967. [Google Scholar]
- Nelken I. Processing of complex stimuli and natural scenes in the auditory cortex. Curr Opin Neurobiol. 2004;14:474–480. doi: 10.1016/j.conb.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature Rev Neurosci. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Noreña AJ, Gourevich B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nature Neurosci. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- Noreña A, Tomita M, Eggermont JJ. Neural changes in cat auditory cortex after a transient pure-tone trauma. J Neurophysiol. 2003;90:2387–2401. doi: 10.1152/jn.00139.2003. [DOI] [PubMed] [Google Scholar]
- Obleser J, Scott SK, Eulitz C. Now you hear it, now you don't: Transient traces of consonants and their nonspeech analogues in the human brain. Cereb Cortex. 2006;16:1069–1076. doi: 10.1093/cercor/bhj047. [DOI] [PubMed] [Google Scholar]
- Ohl FW, Scheich H. Differential frequency conditioning enhances spectral contrast sensitivity of units in auditory cortex (field Al) of the alert Mongolian gerbil. Eur J Neurosci. 1996;8:1001–1017. doi: 10.1111/j.1460-9568.1996.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Ojima H, Murakami K. Intracellular characterization of suppressive responses in supragranular pyramidal neurons of cat primary auditory cortex in vivo. Cereb Cortex. 2002;12:1079–1091. doi: 10.1093/cercor/12.10.1079. [DOI] [PubMed] [Google Scholar]
- Percaccio CR, Engineer ND, Pruette AL, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment increases paired-pulse depression in rat auditory cortex. J Neurophysiol. 2005;94:3590–3600. doi: 10.1152/jn.00433.2005. [DOI] [PubMed] [Google Scholar]
- Raggio MW, Schreiner CE. Neuronal responses in cat primary auditory cortex to electrical cochlear stimulation. III Activation patterns in short- and long-term deafness. J Neurophysiol. 1999;82:3506–3526. doi: 10.1152/jn.1999.82.6.3506. [DOI] [PubMed] [Google Scholar]
- Rajan R, Irvine DRF. Absence of plasticity of the frequency map in dorsal cochlear nucleus of adult cats after unilateral partial cochlear lesions. J Comp Neurol. 1998;399:35–46. [PubMed] [Google Scholar]
- Rajan R, Irvine DRF, Wise LZ, Heil P. Effect of unilateral partial cochlear lesions in adult cats on the representation of lesioned and unlesioned cochleas in primary auditory cortex. J Comp Neurol. 1993;338:17–49. doi: 10.1002/cne.903380104. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W. The perception of phantom limbs. Brain. 1998;121:1603–1630. doi: 10.1093/brain/121.9.1603. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D, Irvine DRF. Plasticity of frequency organization in auditory cortex of guinea pigs with partial unilateral deafness. J Comp Neurol. 1989;282:456–471. doi: 10.1002/cne.902820311. [DOI] [PubMed] [Google Scholar]
- Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci USA. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Revs. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Schmid LM, Rosa MGP, Calford MB, Ambler JS. Visuotopic reorganization in the primary visual cortex of adult cats following monocular and binocular retinal lesions. Cereb Cortex. 1996;6:388–405. doi: 10.1093/cercor/6.3.388. [DOI] [PubMed] [Google Scholar]
- Schoups A, Vogels R, Quian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature. 2001;412:549–553. doi: 10.1038/35087601. [DOI] [PubMed] [Google Scholar]
- Seki S, Eggermont JJ. Changes in cat primary auditory cortex after minor-to-moderate pure-tone induced hearing loss. Hear Res. 2002;173:172–186. doi: 10.1016/s0378-5955(02)00518-x. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Rebscher SJ, Cao K, Leake PA, Kelly K. Chronic intracochlear electrical stimulation in the neonatally deafened cat. I: Expansion of central representation. Hear Res. 1990;50:7–34. doi: 10.1016/0378-5955(90)90030-s. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Sinex D. Tonotopic reorganization of cat primary auditory cortex (AI) after acute lesions of restricted sectors of the spiral ganglion. Soc Neurosci Abstr. 1998;24:904. [Google Scholar]
- Snyder RL, Sinex DG. Immediate changes in tuning of inferior colliculus neurons following acute lesions of cat spiral ganglion. J Neurophysiol. 2002;87:434–452. doi: 10.1152/jn.00937.2000. [DOI] [PubMed] [Google Scholar]
- Snyder RL, Sinex DG, McGee JA, Walsh EW. Acute spiral ganglion lesions change the tuning and tonotopic organization of cat inferior colliculus neurons. Hear Res. 2000;147:200–220. doi: 10.1016/s0378-5955(00)00132-5. [DOI] [PubMed] [Google Scholar]
- Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nature Rev Neurosci. 2003;4:783–794. doi: 10.1038/nrn1222. [DOI] [PubMed] [Google Scholar]
- Sutter ML. Spectral processing in the auditory cortex. In: Malmierca M, Irvine DRF, editors. Auditory Spectral Processing. Elsevier; San Diego: 2005. pp. 253–298. [Google Scholar]
- Tailby C, Wright LL, Metha AB, Calford MB. Activity-dependent maintenance and growth of dendrites in adult cortex. Proc Natl Acad Sci USA. 2005;102:4631–4636. doi: 10.1073/pnas.0402747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talwar SK, Gerstein GL. Reorganization in awake rat auditory cortex by local microstimulation and its effect on frequency-discrimination behavior. J Neurophysiol. 2001;86:1555–1572. doi: 10.1152/jn.2001.86.4.1555. [DOI] [PubMed] [Google Scholar]
- Thai-Van H, Micheyl C, Moore BCJ, Collet L. Enhanced frequency discrimination near the hearing loss cut-off: a consequence of central auditory plasticity induced by cochlear damage? Brain. 2003;126:2235–2245. doi: 10.1093/brain/awg228. [DOI] [PubMed] [Google Scholar]
- Thai-Van H, Micheyl C, Norena A, Collet L. Local improvement in auditory frequency discrimination is associated with hearing-loss slope in subjects with cochlear damage. Brain. 2002;125:524–537. doi: 10.1093/brain/awf044. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Friston KJ, Dolan RJ. Cholinergic modulation of experience-dependent plasticity in human auditory cortex. Neuron. 2002;35:567–574. doi: 10.1016/s0896-6273(02)00801-2. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Wang X, McFadden SL, Caspary D, Salvi R. Gamma-aminobutyric acid circuits shape response properties of auditory cortex neurons. Brain Res. 2002;944:219–231. doi: 10.1016/s0006-8993(02)02926-8. [DOI] [PubMed] [Google Scholar]
- Warrier CM, Johnson KL, Hayes EA, Nicol T, Kraus N. Learning impaired children exhibit timing deficits and training-related improvements in auditory cortical responses to speech in noise. Exp Brain Res. 2004;157:431–441. doi: 10.1007/s00221-004-1857-6. [DOI] [PubMed] [Google Scholar]
- Webster HH, Hanisch UK, Dykes RW, Biesold D. Basal forebrain lesions with or without reserpine injection inhibit cortical reorganization in rat hindpaw primary somatosensory cortex following sciatic nerve section. Somat Mot Res. 1991;8:327–346. doi: 10.3109/08990229109144756. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM. Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature. 2003;426:442–446. doi: 10.1038/nature02116. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Dynamic regulation of receptive fields and maps in the adult sensory cortex. Annu Rev Neurosci. 1995;18:129–158. doi: 10.1146/annurev.ne.18.030195.001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger NM. The nucleus basalis and memory codes: auditory cortical plasticity and the induction of specific, associative behavioral memory. Neurobiol Learn Mem. 2003;80:268–284. doi: 10.1016/s1074-7427(03)00072-8. [DOI] [PubMed] [Google Scholar]
- Weinberger NM. Experience-dependent response plasticity in the auditory cortex: issues, characteristics, mechanisms, and functions. In: Parks TN, Rubel EW, Popper AN, Fay RR, editors. Plasticity of the Auditory System. Springer; New York: 2004a. pp. 173–227. [Google Scholar]
- Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nature Rev Neurosci. 2004b;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Maunsell JHR. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci. 2004;24:1617–1626. doi: 10.1523/JNEUROSCI.4442-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


