Abstract
A majority of breast cancers are hormone-responsive, and require estrogen for growth, and respond to hormonal therapy that blocks estrogen receptor action. Breast tumors with low levels of or completely lacking estrogen receptor fail to respond to antiestrogen therapy yet require estrogen for tumor initiation. To address the importance of local estrogen in oncogene-mediated breast tumorigenesis, we have crossed MMTV-aromatase with MMTV-HER2/neu and examined the incidence of breast cancer in double transgenic mice in comparison with parental strains. Double transgenic mice show normal mammary development and express both transgenes at similar levels to that of parental strains. Tumor incidence in double transgenic mice (<5%) decreased compared to HER2/neu mice (> 65%). In addition to a significant decrease in tumorigenesis, these mice expressed ERα as well as high levels of ERβ along with decreased levels of cyclin D1 and phosphorylated pRb among other changes. Furthermore, experiments using THC (ERα- agonist and ERβ-antagonist) clearly demonstrate the critical role of ERβ in HER2/neu-mediated tumorigenesis. These studies provide the first genetic evidence that estrogen receptor, mainly ERβ than ERα and its dependent changes play an important role in regulating mammary tumorigenesis. These findings provide further evidence for development and testing of novel therapeutic approaches based on selective regulation of estrogen receptors (ERα and β) - dependent actions for the treatment and prevention of breast cancers.
Keywords: Aromatase, HER-2/neu, mammary tumorigenesis; Transgenic mice; Hormonal carcinogenesis
Introduction
Normal mammary development is dependent on the mitogenic effects exerted by estrogen. Alteration in the physiological levels of this steroid hormone can result in hyperplastic changes and other abnormalities in mammary glands leading to the initiation and progression of breast cancer. In the majority of primary breast tumors, the disease is hormone dependent, is characterized by the expression of the estrogen receptor (ER) and is responsive to antiestrogen treatment.
Even though the main source of estrogen is the ovaries, recent evidence has shown that estrogen produced in situ by aromatase could play an important role in mammary carcinogenesis [1]. Aromatase catalyzes the conversion of androgens to estrogen. An increase in aromatase expression in mammary tissue would therefore result in the increase of local estrogen production; estrogen in turn could affect cellular growth via autocrine or paracrine pathways [2-4].
Estrogens, progesterone, and their receptors are critical for normal mammary development as well as for induction and growth of mammary tumors. Estrogen/ERs generate multiple growth promoting signals both inside and outside the nucleus. Estrogen-induced expression of genes encoding growth factors, their receptors, and other molecules involved in signal transduction can provide cell proliferation and survival stimuli [5]. Estrogen acts through ERs by genomic (binding to DNA) as well as nongenomic (via protein-protein interactions) pathways [6, 7]. It is also very clear from several recent studies that a number of coactivators play a significant role in estrogen/ER-mediated actions [8, 9]. New evidence also suggests that ER located in or near the cell membrane can cross-talk with growth factor receptor tyrosine kinases, such as EGFR and HER-2/neu, providing another mechanism for the growth promoting effects of estrogen [10].
The majority of the breast tumors express ER. About 70% of these respond to the antiestrogen tamoxifen and prolonged treatment with tamoxifen leads to resistance to the drug despite the continued presence of estrogen and progesterone receptors. Tamoxifen and other similar compounds that are designated as “selective estrogen receptor modulator (SERM)” have variable agonistic and/or antagonistic activities depending on the type of ER (α vs β) and the coactivator and corepressor milieu that bind to ER [11]. Recent studies suggest that in breast cancer cells that express HER-2 and ER, tamoxifen acts like an estrogen agonist. These actions can be reversed by treating these cells with EGFR inhibitor that presumably inhibits HER-2- to-ER cross-talk and leads to restoration of ER antagonistic properties of tamoxifen [12]. The receptor cross-talk between the ER and growth factor receptor is bidirectional. For example, ERK1 and 2, a mitogen-activated protein kinase (MAPK) that has been activated by signaling from EGFR or HER-2 phosphorylates both ER and ER coactivators [13]. These observations raise the question of whether the results can be extrapolated to other in vitro models and, more importantly, to the vastly heterogenous clinical population.
We have developed aromatase transgenic mice that overexpress this enzyme in mammary tissue. Although the mammary glands of aromatase transgenic mice exhibit various preneoplastic changes, we have not observed the development of frank tumors [14]. This finding gives support to the hypothesis that accumulation of multiple alterations is required to develop from the preneoplastic state into tumorigenesis. In our previous study [15], we have shown that the mammary glands of these mice overexpress ER, PR, growth factors, such as TGFβ and VEGF, and cell cycle proteins. In this study, our aims were to examine the influence of aromatase overexpression on HER-2/neu-mediated tumor formation in the mammary glands of aromatase × HER-2/neu double transgenic mice and to investigate the roles of estrogen/ER in the regulation of estrogen-dependent genes that participate in the mammary tumorigenic process. For this purpose, we have generated an aromatase × HER-2/neu double transgenic strain and have examined the pathological as well as the biochemical changes to understand the interaction of these molecules in mammary tumorigenesis.
Materials and Methods
Transgenic mice
The generation of transgenic mice overexpressing aromatase in mammary glands has been previously described [14]. The aromatase transgenic mice colony was maintained by sibling mating. HER-2/neu (MMTV-neu) mice [16] were purchased from Jackson Laboratories. Both the aromatase and HER-2/neu transgenes are under the regulation of the mouse mammary tumor virus promoter. The aromatase × HER-2/neu mice were obtained by mating the parental strains. Mice positive for both HER-2/neu and aromatase transgene along with individual parental types of the same genetic background (FVB/N) were used for the various analyses. Mice were housed in a centralized animal facility accredited by the AAALAC and USDA and maintained according to the recommendations established in the NIH Guide for the Care and Use of Laboratory Animals.
Morphological and histological assessment of mammary glands
The skin containing the mammary fat pads was fixed in 10% neutral buffered formalin for at least 24 h. The mammary glands were then dissected free from skin and processed as described previously [14]. Routine sections of mammary tissues were prepared after fixation in 10% neutral buffered formalin by embedding in paraffin, sectioning at 5 μm, and staining with H&E.
RNA analysis
Total RNA from mammary glands was isolated, following homogenization of the tissue, with the Tri Reagent (Sigma, St. Louis, MO) according to the manufacturer’s instructions. Gene expression was then examined by real-time quantitative reverse transcription-PCR (RT-PCR), using the GeneAmp RNA PCR kit (Perkin Elmer, Foster City, CA) and platinum Taq polymerase (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Depending on the abundance of the specific mRNA species, 70 -250 ng of total RNA was used as starting template in the RT reaction mix. To detect amplicon synthesis in the SmartCycler real time PCR thermal cycler (Cepheid, Sunnyvale, CA) 0.25 × Cyber green dye (Roche, Indianapolis, IN) was added to the reaction mixture. For quantification, the cycle threshold number (Ct) exhibiting the maximum curve growth rate was determined. The relative gene expression of each sample, normalized to that of control (GADPH or actin), was calculated by the formula 2Ct (control) - Ct (gene).
Protein analysis
Protein extracts from mammary glands were prepared by homogenizing the tissue in lysis buffer. Equal amounts (generally 60-75 μg) of protein from each sample was separated on a denaturing polyacrylamide gel and transferred to nylon membrane. The protein-bound membranes were then incubated for at least 4 hours at room temperature with Tris-buffered saline (TBS) containing 0.05% Triton X-100 (TBST) and 5% nonfat dry milk to block non-specific antibody binding. The membranes were then incubated with respective primary antibodies in TBST-milk overnight at 4oC, and specific binding was visualized by using species-specific IgG followed by enhanced chemiluminescent detection (ECL kit; Amersham) and exposure to ECL X-ray film. Mouse specific antibodies for were obtained from different commercial sources: β-actin (Santa Cruz Biotech, Santa Cruz, CA), aromatase (Santa Cruz Biotech), (ERα (Labvision-Neomarkers, Fremont, CA), ERβ (Upstate Cell Signaling Solutions, Charlottesville, VA), PR (Santa Cruz Biotech), Cyclin D1 (Labvision-Neomarkers), pRb (BD Biosciences, San Jose, CA), ppRb (Cell Signaling Tech, Beverly, CA).
Animal Treatments
To investigate the inhibitory role of ERβ on mammary growth, we treated double transgenic mice (n=6) with THC, tetrahydrochrysene (500 μg/day/mouse) a selective ER modulator (α agonist and β antagonist) obtained from Sigma Aldrich company (St. Louis. MO) was administered daily by subcutaneus injections for three weeks beginning at the age of five weeks. Control group (n=6) received vehicle only for three weeks. At the end of experimental period mice were sacrificed and mammary tissues was used for whole mount preparation and other biochemical studies as described above.
Results
Estrogen regulates normal mammary gland development as well as the initiation and possibly the progression of tumorigenesis. Our recent results have shown that the increase of estrogen levels in the aromatase transgenic mice due to aromatase overexpression results in altered expression of growth factor genes and tumor suppressor genes [15]. To determine the effects of the presence of an additional genomic alteration, specifically the HER-2/neu transgene, in combination with the aromatase transgene, we examined the morphological and histopathological changes in the mammary glands of aromatase × HER-2/neu double transgenic animals and compared them to those of the parental strains with same genetic background. We observed persistent hyperplastic changes in the aromatase × HER-2/neu strain similar to the aromatase transgenic strain [14, 15]. Unlike the HER-2/neu parental strain (Fig.1), tumor formation was drastically decreased in the aromatase × HER-2/neu double transgenic mice (Table 1). Subsequently, we have examined whether there are biochemical changes in the mammary glands of the double transgenic strain as compared to the parental strains that may be responsible for decreased or lack of tumor formation in double transgenic mice.
Figure 1. Hyperplastic changes and mammary tumor formation in HER-2/neu × aromatase double transgenic mice and parental strains.
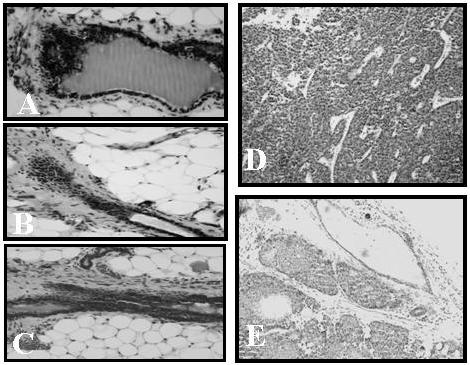
Histological sections of the fourth inguinal mammary glands from HER-2/neu (A), aromatase (B) and HER-2/neu × aromatase double transgenic mice (C) along with mammary tumors from HER-2/neu (D), and HER-2/neu × aromatase double transgenic mice (E) were stained with H&E and photographed at a magnification of × 20. Unlike rapid growing adenocarcinomas in HER-2/neu (D), the tumors formed in HER-2/neu × aromatase double transgenic mice (E) were small and not well differentiated.
Table 1.
Incidence of mammary tumors in aromatase × HER-2/neu double transgenic mice and parental strains
| Genotype/Properties | Tumor Incidence (%) | Time to tumor formation (Median weeks) | Presence of ductal hyperplasia (% of ducts) | Duration of Observation (Months) |
|---|---|---|---|---|
| Wild type (n= 65) | 0 | 0 | 0 | 24 |
| Aromatase (n=>700) | 0 | 0 | >80 | 24* |
| HER-2/neu (n=40) | 60 | 28** | >80 | 24 |
| HER-2/neu x aromatase | <5 | 42 | >45 | 24 |
No tumors are observed during the normal life span. Aromatase transgenic colony is maintained for the last ten years
Duration of spontaneous tumor formation is shorter in parous animals
To confirm that the overexpression of aromatase was persistent in the strains containing the aromatase transgene, we tested the expression of aromatase by RT-PCR as well as by western blot analysis. As expected, aromatase expression in the aromatase transgenic mouse and the aromatase × HER-2/neu cross was higher than that in the HER-2/neu and wild type mice (Fig. 2). As seen in human breast tumors [17], aromatase expression is induced in HER-2/neu mammary tissues; however it was much lower than seen in the aromatase transgenic mice (Fig.2).
Figure 2. Expression of aromatase in HER-2/neu × aromatase double transgenic mice and parental strains along with wild type control.
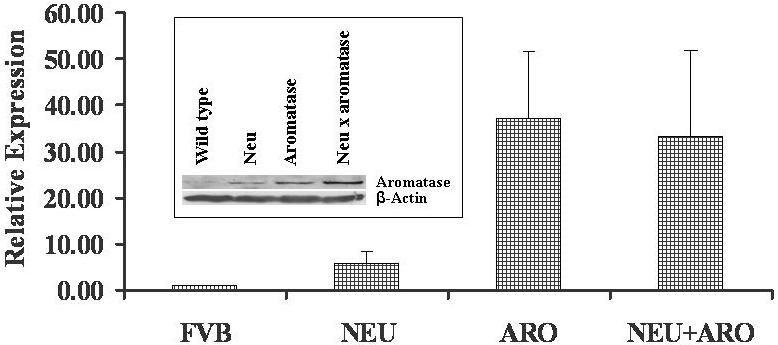
Quantitative real-time RT-PCR analysis was carried out to determine the mRNA levels of aromatase as described in Materials and Methods. For quantification, the cycle threshold number (Ct) exhibiting the maximum curve growth rate was determined. The relative gene expression of each sample, normalized to that of control (either GADPH or action), was calculated by the formula 2Ct (control) - Ct (gene). The relative expression levels (fold changes) from three independent estimations using three replicates were used for graphic representation. Inset: Figure shows a representative western blot analysis data of aromatase protein levels in HER-2/neu double transgenic mice along with parental types and wild type control. Western blot analysis of the protein extracted from different frozen mammary tissues using antibodies against aromatase (Santacurz) and β-actin (loading control). Each lane contains 60μg of protein.
Our previous studies have shown that the levels of ER and PR are increased due to aromatase overexpression in the mammary glands of aromatase transgenic mice [15]. To determine how the continuous presence of local estrogen as result of aromatase expression and HER-2/neu overexpression may affect steroidal responses, we examined the levels of ER and PR in the mammary glands of the double transgenic animals as compared to those of the parental strains using both RT-PCR and Western blot analyses. As shown in figure 3, all the mice strains express both estrogen receptors. ERα gene expression was higher in the mammary gland of the aromatase (2.0 folds) and HER-2/neu (3.5 folds) and in aromatase × HER-2/neu double transgenic mice (2.0 folds) than in the wild type (Fig. 3). ERβ expression in the aromatase × HER-2/neu double transgenic strain was higher (>8 folds) than HER-2/neu mice. The ratio of ERα/ERβ protein levels in double transgenic mice was (0.43) which was much lower than in aromatase (1.7) and HER-2/neu (2.6) parental strains. The expression of ERα and β is different in various animal groups and higher ERβ than ERα levels correlates well with decreased tumorigenesis in these animals suggesting an increased ERβ expression plays a critical role in the inhibition of hormone and/or oncogene-mediated mammary tumorigenesis. There is no significant change in the protein levels of PR among different groups.
Figure 3. Expression of estrogen and progesterone receptors in HER-2/neu × aromatase double transgenic mice and parental strains along with wild type control.
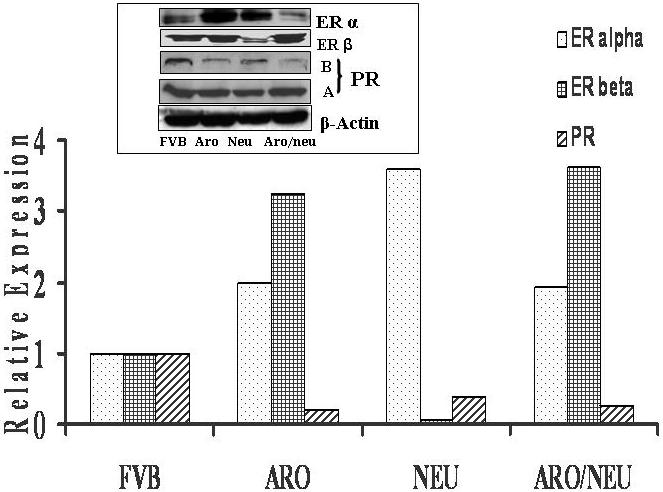
Quantitative real-time RT-PCR analysis was carried out to determine the mRNA levels of ERα and β along with progesterone receptor (PR) as described in Materials and Methods and figure 2. Inset: Figure shows a representative western blot analysis data of ERα and β and PR protein levels in HER-2/neu double transgenic mice along with parental types and wild type control. Western blot analysis of the protein extracted from different frozen mammary tissues using antibodies against ERα and β and PR along with β-actin (loading control). Each lane contains 60μg of protein.
We have also investigated the effects of aromatase overexpression on the expression of ErbB2 and EGFR in the aromatase × HER-2/neu double transgenic mice that are known to be influenced by estrogen/growth factors. Figure 4 shows the RT-PCR results for the expression of ErbB2 and EGFR in the two parental strains and in the double transgenic strain. In the aromatase × HER-2/neu double transgenic mice, the expression of EGFR is diminished (5-folds), while the expression of ErbB2 increased several folds indicating that continuous estrogenic exposure due to aromatase overexpression as well as high ligand (ErbB2) expression negatively regulates the expression of EGFR in the mammary glands.
Figure 4. Expression of ErbB2 and EGFR in HER-2/neu × aromatase double transgenic mice and parental strains along with wild type control.
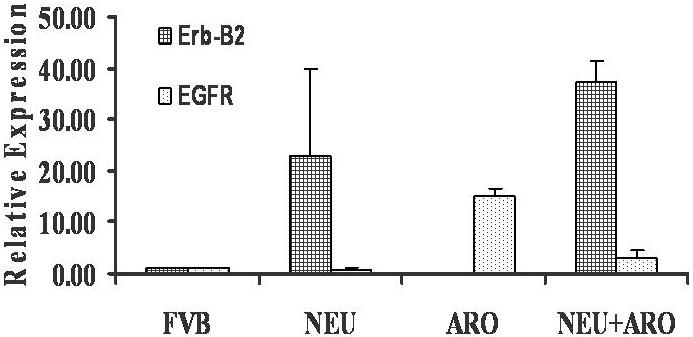
Real time RT-PCR analysis was carried out to determine the mRNA levels of the ErbB2 and EGFR genes in the mammary glands of aromatase × HER-2/neu double transgenic strain as compared to the parental types. The relative expression level (fold changes) from a representative set is used for graphic representation.
To investigate how the estrogen/ER gene regulation pathway is affected in the double transgenic mammary glands, we examined the protein levels of cyclin D1 along with retinoblastoma gene product (pRb) in transgenic mammary glands of the aromatase and HER-2/neu parental strains and the aromatase × HER-2/neu double transgenic strain. We have shown previously that the increase of ER in the aromatase transgenic mammary gland as compared to the nontransgenic gland corresponds to an increase in the expression of genes involved in the progression of cell cycle (Cyclin D1 and Cyclin E) and cellular proliferation as well as phosphorylated Rb (ppRb) levels [15]. In this study, we have examined the expression of Cyclin D1, and pRb and ppRb proteins. The Western analysis data (Fig. 5) show that the expression of Cyclin D1 was the highest in the aromatase transgenic mammary gland, followed by HER-2/neu transgenic gland, then by the aromatase × HER-2/neu double transgenic gland (∼ 5 fold lower than the aromatase). These results suggest change in the ratio of ERα/ERβ protein levels also affects the estrogen/ER-dependent actions of cyclin D1 contributing to decreased mammary proliferation and tumor formation in double transgenic.
Figure 5. Expression of cyclin D1 and pRb and ppRb in HER-2/neu × aromatase double transgenic mice and parental strains along with wild type control.
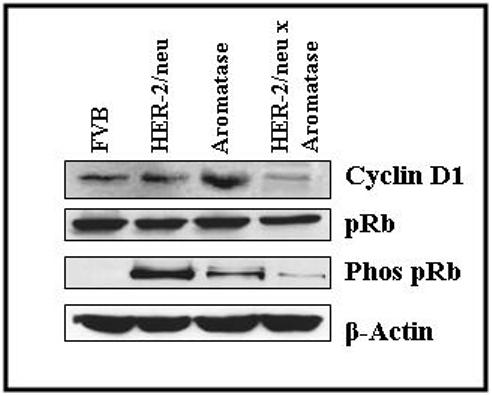
Western blot analysis of the protein extracted from different frozen mammary tissues using antibodies against cyclin D1, unphosphorylated (pRb) and phosphorylated (phos-pRb) along with β-actin (loading control). Each lane contains 75μg of protein.
The kinase activity of Cyclin/CDK complexes results in the phosphorylation and inactivation of pRb which relieves pRb inhibition of cell cycle progression from G1 to S phase. Western analysis was performed to determine the expression and phosphorylation levels of pRb in the aromatase and HER-2/neu parental strains and the double transgenic cross strain. The levels of phosphorylated pRb was ∼10 and 5 fold lower in the aromatase × HER-2/neu double transgenic mammary tissues as compared to the HER-2/neu and aromatase strains respectively (Fig. 5). The levels of pRb in the HER-2/neu and other transgenic strains are about equal. The data suggest that the kinase activity resulting in the phosphorylation of pRb is diminished in the HER-2/neu × aromatase double transgenic mammary glands compared to parental strains.
To further investigate the potential inhibitory role of ERβ on mammary growth, we have treated double transgenic mice with THC (500 μg/day/mouse) a selective ER modulator (α agonist and β antagonist) for three weeks beginning at the age of five weeks, prepubertal stage at this developmental stage the epithelial expansion into mammary fat pad is minimal or none. Mammary tissues were then examined for the change in mammary proliferation and change in the expression of estrogen receptors and estrogen dependent genes like cyclin D1. As shown in figure 6, a three week treatment with THC resulted in increased mammary ductal and labuloalveolar growth and increases in the expression of ERα and complete down regulation of ERβ as well as an increase in cyclin D1 protein levels. These observations further suggest that high ERβ activity not only affects mammary growth but also affects the regulation of estrogen-dependent genes involved in cell cycle.
Figure 6. Effect of THC on ER and cyclin D1 expression as well as on mammary growth in HER-2/meu × aromatase double transgenic mice.
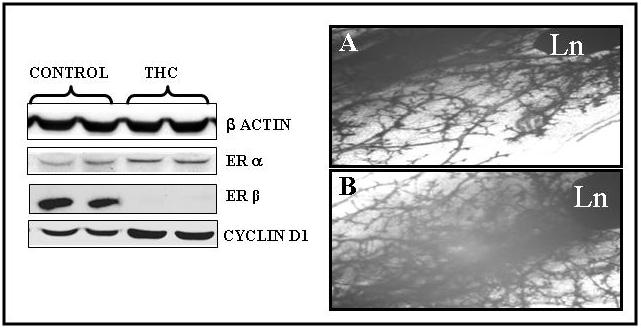
Left Panel: Expression of estrogen receptors (α and β) cyclin D1 in HER-2/neu × aromatase double transgenic mice treated with and without THC. Western blot analysis of the protein extracted from frozen mammary tissues from HER-2/neu × aromatase mice with and without THC (ERα agonist and ERβ antagonist) using mouse specific antibodies. Each lane contains 75μg of protein.
Right Panel: Whole mount analysis of mammary glands from HER-2/neu × aromatase double transgenic mice treated with and without THC. The fourth inguinal mammary glands were removed from HER-2/neu × aromatase double transgenic mice after three weeks of treatment with (A) and without THC (B), fixed and stained with hematoxylin alum stain and photographed at a magnification of 6x. Mammary epithelial expansion and ductal branching along with labuloalveolar development below lymph node (Ln) was compared. Note: Compared to untreated mice (A), increased epithelial growth along with increased ductal and lateral branching is evident in eight weeks old mice after three weeks of treatment with THC (B).
Discussion
We have established in our previous studies [14, 15] that the overexpression of aromatase in the mammary glands of transgenic mice, leading to increased estrogen synthesis, results in abnormal histological changes resembling breast preneoplasia in women. These changes are associated with altered expression in the mammary gland of genes coding for steroid receptors, cell cycle, cellular proliferation, tumor suppressor, and growth factors [15]. Despite these changes, we have not observed the onset of mammary tumors in the aromatase transgenic mice. These results suggest that aromatase overexpression and the associated biochemical changes can be responsible for the initiation of preneoplastic changes but may not be sufficient for the full progression of the disease. Current research from various laboratories suggests that multiple alterations in the genomic structure of cells are necessary for the development of the cancerous phenotype [18]. Our previous results show that treatment of aromatase transgenic mice with the carcinogen DMBA results in tumor formation, whereas no tumor formation was observed in similarly treated nontransgenic animals [19]. These results suggest that in addition to increased estrogen levels in the aromatase transgenic model, alterations in other factors are required to continue the progression from preneoplasia to tumorigenesis.
To address the importance of local estrogen in oncogene-mediated breast tumorigenesis, we have crossed MMTV-aromatase with MMTV-HER-2/neu and examined the incidence of breast cancer in double transgenic mice (MMTV-aromatase × MMTV-neu) in comparison with parental strains. Double transgenic mice show normal mammary development and express both transgenes (aromatase and HER-2/neu) at similar levels with that of parental strains. As seen in human breast tumors that overexpress HER-2/neu (17), our data also show induction of aromatase in MMTV-HER-2/neu transgenic mice; however, this expression is considerably lower than that is seen in MMTV-aromatase mice. Tumor incidence in double transgenic mice decreased to <5% compared to HER-2/neu mice (> 65%). Mammary tumors do not form in MMTV-aromatase mice without carcinogen treatment.
To investigate the underlying biochemical effects, we examined gene expression in the aromatase × HER-2/neu cross strain as compared to that in the parental strains. There is no change in the expression of transgenes in double transgenic mice compared to parental strains suggesting decreased tumorigenesis is not due to any change in the levels of expression of transgenes. In addition to significant decrease of tumorigenesis, these mice express ERα as well as high levels of ERβ. The ratio of ERα/ERβ protein levels in double transgenic mice was is much lower than in aromatase and HER-2/neu (2.6) parental strains (Fig. 3). Our data does indicate higher ERβ than ERα levels correlates well with decreased tumorigenesis in these animals suggesting an increased ERβ expression plays a critical role in the inhibition of hormone and/or oncogene-mediated mammary tumorigenesis.
Estrogens, acting through ERα [20] and ERβ [21], play a key role in mammary development and morphogenesis. Data from ERαKO, ERβKO [22, 23] and transgenic aromatase × ERαKO mice cross [24] clearly indicate that ERβ is not required for normal mouse development or function. In contrast, a different strain of ERβKO [25] mice had enlarged alveoli and reduced expression. Our own studies with aromatase transgenic mice having different genetic background shows a relatively high levels of ERβ expression in mammary tissues in response to continuous presence of local estrogen (data not shown). A number of recent studies with breast cancer cell line models suggest that concomitant activation of both estrogen receptors leads to resistance to estrogen-induced proliferation may be due opposing cellular responses with regard to proliferation and apoptosis [23]. Our data presented here (high ERβ induction in HER-2/neu × aromatase mice) not only suggest that resistance to estrogen-induced proliferation may be responsible for lack of or decreased tumorigenesis in HER-2/neu × aromatase mice, but our data using breast cancer cells with ERβ overexpression also agrees with this in vivo findings (data not shown). Consistent with our in vivo findings, treatment with THC (ERα agonist and ERβ antagonist) for three weeks beginning at the age of five weeks, a prepubertal stage of mammary development resulted in downregulation of ERβ and lead to increased mammary proliferation, indicating indeed high ERβ may be responsible for decreased mammary tumorigenesis in double transgenic mice.
Our previous results have shown that the increase in ERα expression in the aromatase mammary gland corresponds to an increase in the expression of cyclin D1 and PCNA, markers for cell cycle progression and cellular proliferation, respectively. Our current data shows a similar relationship between ERα and cyclin D1 expression in the aromatase, and HER-2/neu mice; however, in the aromatase × HER-2/neu double transgenic mice the levels of cyclin D1 was significantly decreased. For example, the lower levels of ERα in the aromatase × HER-2/neu double transgenic mice correspond to a decrease in the expression of Cyclin D1 and phosphorylation of pRb as compared to the parental strains. The decrease in both cyclin D1 and ppRb in the aromatase × HER-2/neu double transgenic mice as compared to the HER-2/neu parental strain is consistent with the absence of tumor development. In our previous work [15], we have demonstrated that cyclin D1 is increased in the aromatase transgenic mice as compared to the nontransgenic animals. In this report we have shown that the cyclin D1 levels are elevated in the aromatase and HER-2/neu parental strains; however, in contrast, the expression of cyclin D1 in the aromatase × HER-2/neu cross was dramatically reduced. Cyclin D1 is known to promote cell cycle transition from early/middle G1 phase to late G1 phase, and a decrease in cyclin D1 levels could slow cell cycle progression. In addition to its involvement in phosphorylation, the increase in cyclin D1 levels has also been shown to sequester the CDK inhibitor p27 away from cyclin E-CDK2 complexes allowing the latter to inactivate RB by phosphorylation [15, 26-28]. These observations are consistent with previous studies that found mice lacking cyclin D1 are resistant to mammary carcinomas triggered by the ErbB2 oncogene [29]. Further studies not only confirmed this observation but also suggest that in addition to cyclin D1, its dependent kinases (cdk4) are critical in this pathway [30, 31]. Our observations not only confirm the critical role of cyclin D1 in HER-2/neu-mediated oncogenesis, but further suggest a novel and important role of ERβ in this process. The expression of ppRb is higher in the HER-2/neu transgenic strain as compared to the other two strains. It is possible that the reduced phosphorylation of pRb in the aromatase transgenic strain as compared to the HER-2/neu transgenic strain could be due to the relatively higher expression of ERβ, possibly modulating cyclin D1 expression, in the aromatase transgenic strain. Combined, the data suggest that the downregulation of cyclin D1 and the regulation of pRb phosphorylation are some of the underlying factors that could account for the absence of tumor formation in the mammary gland of the aromatase × HER-2/neu cross, and these changes are mediated by the estrogen receptor. Specifically, the ratio of ERα/ERβ protein levels appears to play a critical role in mammary tumorigenesis.
Other possible reason for the lack of or reduced tumorigenesis in HER-2/neu × aromatase mice compared to HER-2/neu mice also could be due to exposure to estrogen early in the development that may mimic the protective effects of pregnancy. It is a well established fact that a full-term pregnancy early in life is associated with a long-term risk reduction for developing breast cancer [32, 33]. Pregnancy has a very similar dual effect on the etiology of mammary cancer in animal models. Parous rats and mice have a greatly reduced susceptibility to chemically-induced mammary tumorigenesis compared to their nulliparous siblings [34, 35]. Studies also using rodent models have shown that, treatment of rats with pregnancy-related hormones, such as estrogens and progesterone, appear to mimic the protective effects of pregnancy in rat mammary carcinogenesis models [36, 37]. This suggest that the mechanisms of parity-induced protection and estradiol and progesterone induced protection may be similar. Data presented here and our previous findings with aromatase tend to support this hypothesis in addition to antiproliferative role of ERβ in mammary tumorigenesis.
Our results demonstrate that continuous mammary exposure to estrogen, due to aromatase overexpression, leads to change in the ratio of ERα/ERβ protein levels and changes associated with this effect may be counteracting the oncogenic effects of HER-2/neu in the mammary glands of aromatase × HER-2/neu double transgenic mice. More detailed investigations that dissect mechanistic pathways responsible for these effects should shred more light on the antiproliferative action of ERβ.
Acknowledgment
This work was supported by the National Institutes of Health Grant CA75018 (R.R.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Tekmal RR, Santen RJ. Local estrogen production: is aromatase an oncogene? In: Manni A, editor. Contemporary Endocrinology: Endocrinology of Breast Cancer. Humana Press; New Jersey: 1999. pp. 79–89. [Google Scholar]
- [2].Santen RJ, Santner SJ, Pauley RJ, Tait L, Kaseta J, Demers LM, Hamilton C, Wang J-P. Estrogen production via the aromatase enzyme in breast cancer carcinoma: which cell type is responsible? J. Steroid Biochem. Molec. Biol. 1997;61:267–271. [PubMed] [Google Scholar]
- [3].Santner SJ, Pauley JP, Tait L, Kaseta J, Santen RJ. Aromatase activity and expression in breast cancer and benign breast tissue stromal cells. Journal of Clinical Endocrinology and metabolism. 1997;82:200–208. doi: 10.1210/jcem.82.1.3672. [DOI] [PubMed] [Google Scholar]
- [4].Anderson E, Clarke RB, Howell A. Estrogen Responsiveness and control in normal human breast proliferation. J. Mammary Gland Biol. Neoplasia. 1998;3:23–35. doi: 10.1023/a:1018718117113. [DOI] [PubMed] [Google Scholar]
- [5].Nicholson RI, McClelland RA, Robertson JF, Gee JM. Involvement of steroid hormone and growth factor cross-talk in endocrine response in breast cancer. Endocr Relat Cancer. 1999;6:373–387. doi: 10.1677/erc.0.0060373. [DOI] [PubMed] [Google Scholar]
- [6].Edwards DP. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- [7].McGuire WL, Adams DJ, Edwards EP. Estrogen-regulated protein in human breast cancer. J Steroid Biochem. 1984;20:73–81. doi: 10.1016/0022-4731(84)90191-2. [DOI] [PubMed] [Google Scholar]
- [8].O’Malley BW. Sequentiality and processivity of nuclear receptor coregulators in regulation of target gene expression. Nucl Recept Signal. 2003;1:e010–015. doi: 10.1621/nrs.01010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McKenna NJ, O’Malley BW. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell. 2002;108:465–474. doi: 10.1016/s0092-8674(02)00641-4. [DOI] [PubMed] [Google Scholar]
- [10].Razandi M, Pedram A, Park ST, Levin ER. Proximal events in signaling by plasma membrane estrogen receptors. J Biol Chem. 2003;278:2701–2712. doi: 10.1074/jbc.M205692200. [DOI] [PubMed] [Google Scholar]
- [11].Gustafsson JA. Therapeutic potential of selective estrogen receptor modulators. Curr Opin Chem Biol. 1998;2:508–511. doi: 10.1016/s1367-5931(98)80127-0. [DOI] [PubMed] [Google Scholar]
- [12].Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK. Advanced concepts in estrogen receptor biology --- estrogen receptor coregulators. Cancer Chemother Pharmacol. 2005;56(Suppl 1):10–20. doi: 10.1007/s00280-005-0108-2. [DOI] [PubMed] [Google Scholar]
- [13].Font de Mora J, Brown M. AIB1 is a conduit for kinase-mediated growth factor signaling to the estrogen receptor. Mol Cell Biol. 2000;20:5041–5047. doi: 10.1128/mcb.20.14.5041-5047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tekmal RR, Ramachandra N, Gubba S, Durgam VR, Mantione J, Toda K, Shizuta Y, Dillehay D. Overexpression of int-5/aromatase in mammary glands of transgenic mice results in the induction of hyperplasia and nuclear abnormalities. Cancer Res. 1996;56:3180–3185. [PubMed] [Google Scholar]
- [15].Kirma N, Gill K, Mandava U, Tekmal RR. Overexpression of aromatase leads to hyperplasia and changes in the expression of genes involved in apoptosis, cell cycle, growth, anti tumor suppressor functions in the mammary glands of transgenic mice. Cancer Res. 2000;61:1910–1918. [PubMed] [Google Scholar]
- [16].Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- [17].Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, hla T, Hudis C, Dannenberg AJ. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–5511. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- [18].Hannah D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [19].Keshava N, Mandava U, Kirma N, Tekmal RR. Acceleration of mammary neoplasia in aromatase transgenic mice by 7,12-Dimethylbenz [a] anthracene. Cancer Lett. 2001;167:125–133. doi: 10.1016/s0304-3835(01)00478-5. [DOI] [PubMed] [Google Scholar]
- [20].Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231:1150–1154. doi: 10.1126/science.3753802. [DOI] [PubMed] [Google Scholar]
- [21].Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hewitt SC, Harell JC, Korach KS. Lessons in estrogen biology from knockout and transgenic animals. Ann Rev Physiol. 2005;67:285–308. doi: 10.1146/annurev.physiol.67.040403.115914. [DOI] [PubMed] [Google Scholar]
- [23].Koehler KF, Helguero LA, Haldosen LA, Warner M, Gustafsson JA. Reflections on the discovery and significance of estrogen receptor beta. Endo Rev. 2005;26:465–478. doi: 10.1210/er.2004-0027. [DOI] [PubMed] [Google Scholar]
- [24].Tekmal RR, Liu YG, Nair HB, Jones J, Perla RP, Lubahn DB, Korach KS, Kirma NB. Estrogen receptor alpha is required for mammary development and the induction of mammary hyperplasia and epigenetic alterations in the aromatase transgenic mice. J Steroid Biochem Mol Biol. 2005;95:9–15. doi: 10.1016/j.jsbmb.2005.04.007. [DOI] [PubMed] [Google Scholar]
- [25].Forster C, Makela S, Warri A, Kietz S, Becker D, Hultenby K, Warner M, Gustafsson JA. Involvement of estrogen receptor beta in terminal differentiation of mammary gland epithelium. Proc Natl Acad Sci. USA. 2002;99:15578–15583. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Polyak K, Kato J, Solomon MJ, Sherr CJ, Massague J, Roberts JM, Koff A. P27kip1, a cyclin-CDK inhibitor, links transforming growth factor-β and contact inhibition to cell cycle arrest. Genes and Development. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- [27].Pestell RG, Albanese C, Reutens AT, Segall JE, Lee RJ, Arnold A. The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocrine Reviews. 1999;20:501–534. doi: 10.1210/edrv.20.4.0373. [DOI] [PubMed] [Google Scholar]
- [28].Prall OWJ, Sarcevic P, Musgrove EA, Watts CKW, Sutherland RL. Estrogen-induced activation of Cdk4 and Cdk2 during G1-S phase progression is accompanied by increased Cyclin D1 expression and decreased Cyclin-dependent kinase inhibitor association with Cyclin E-CDK2. J. Biol. Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- [29].Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
- [30].Yu Q, Sicinska E, Geng Y, Ahnstrom M, Zagozdson A, Kong Y, Gardner H, Kiyokawa H, Harris LN, Stal O, Sicinski P. Requirement for CDK4 kinase function in breast cancer. Cancer Cell. 2006;9:23–32. doi: 10.1016/j.ccr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- [31].Reddy Haritha K.D.L., Mettus RV, Rane SG, Grana X, Litvin J, Reddy EP. Cyclin-dependent kinase 4 expression is essential for neu-induced breast tumorigenesis. Cancer Res. 2005;65:10174–10178. doi: 10.1158/0008-5472.CAN-05-2639. [DOI] [PubMed] [Google Scholar]
- [32].MacMohan B, Cole P, Lin TM. Age at first birth and breast cancer risk. Bull World Health Organ. 1970;43:209–221. [PMC free article] [PubMed] [Google Scholar]
- [33].Russo J, Moral R, Balogh GA, Mallo D, Russo IH. The protective role of pregnancy in breast cancer. Breast Cancer Res. 2005;7:131–142. doi: 10.1186/bcr1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Russo IH, Russo J. Mammary gland neoplasia in long-term rodent studies. Environ Health Perspect. 1996;104:938–967. doi: 10.1289/ehp.96104938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Medina D, Smith GH. Chemical carcinogen-induced tumorigenesis in parous, involuted mouse mammary glands. J Natl Cancer Inst. 1999;91:967–969. doi: 10.1093/jnci/91.11.967. [DOI] [PubMed] [Google Scholar]
- [36].Rajkumar L, Guzman RC, Yang J, Thordarson G, Talamantes F, Nandi S. Short-term exposure to pregnancy levels of estrogen prevents mammary carcinogenesis. Proc Natl Acad Sci USA. 2001;98:11755–11759. doi: 10.1073/pnas.201393798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Guzman RC, Yang J, Rajkumar L, Thordarson G, Talamantes F, Nandi S. Hormonal prevention of breast cancer: mimicking the protective effect of pregnancy. Proc Natl Acad Sci USA. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]


