Abstract
Background
Neuropeptide Y (NPY) is a neuromodulator with anxiolytic properties. Recent evidence suggests that NPY modulates neurobiological responses to ethanol. Because withdrawal from ethanol is associated with elevated anxiety-like behavior, and because central NPY modulates anxiety, we assessed anxiety-like behavior in mutant mice lacking normal production of NPY (NPY−/−) and in normal wild-type mice (NPY+/+) 6-hours after removal of a liquid diet containing 4.5% ethanol.
Methods
NPY−/− and NPY+/+ mice on a pure 129/SvEv genetic background were given 6-days of access to a liquid ethanol diet (ED) or control diet (CD). Six-hours before elevated plus maze (EPM) testing, ED was replaced with CD in the ethanol withdrawn group.
Results
Ethanol withdrawn NPY−/− mice showed significantly less open arm time and total proportion of time spent in the open arm of the EPM relative to ethanol-withdrawn NPY+/+ mice and when compared to NPY−/− and NPY+/+ mice that had access to the CD. On the other hand, ethanol withdrawn NPY+/+ mice did not show altered EPM behavior relative to controls.
Conclusions
Central NPY is protective against anxiety-like behavior stemming from exposure to and/or withdrawal from ethanol. Targets aimed at NPY receptors may be useful compounds for treating anxiety associated with ethanol dependence.
Keywords: Neuropeptide Y, Alcohol, Anxiety, Withdrawal, Mice
1. Introduction
Factors that may contribute to the initiation of ethanol consumption and/or continued use of this drug are high basal levels of anxiety and increased anxiety associated with ethanol withdrawal (Bibb and Chambless, 1986; Breese et al., 2005; Cappell and Herman, 1972; Cornelius et al., 2003; Koob, 2003; Schuckit and Hesselbrock, 1994). Viewed this way, excessive ethanol consumption and relapse drinking results from an attempt to self-medicate against the negative emotional responses that accompany ethanol withdrawal. Thus, identifying the neurochemical substrates that modulate withdrawal-induced anxiety may reveal pharmacological targets for treating alcohol abuse and relapse.
An interesting candidate is neuropeptide Y (NPY), a 36-amino-acid neuromodulator belonging to the PP-fold family of peptides (Berglund et al., 2003; Colmer and Wahlestedt, 1993; Dumont et al., 1992) that is expressed throughout the central nervous system (Gray and Morley, 1986) and has been shown to modulate neurobiological responses to ethanol (Badia-Elder et al., 2001; Pandey et al., 2003a; Thiele et al., 2002; Thiele et al., 1998; Thiele et al., 2004). There are several observations that make NPY a likely candidate for modulating withdrawal-induced anxiety. First, NPY possesses anxiolytic properties when infused into the brain (Heilig et al., 1993; Heilig et al., 1989). Second, twenty-four hours after withdrawal from an ethanol-containing diet, rats show decreased NPY immunoreactivity in several brain regions including the central and medial nuclei of the amygdala (Roy and Pandey, 2002). Third, infusion of a protein kinase A (PKA) activator into the central nucleus of the amygdala, a treatment that causes increases of amygdalar NPY levels (Pandey et al., 2005), protects against withdrawal-induced anxiety in rats (Pandey et al., 2003b). The purpose of the present experiment was to use a genetic approach to study the role of NPY in modulating anxiety-like behavior stemming from exposure to and/or withdrawal from ethanol using mutant mice lacking production of NPY (NPY−/−) and normal wild-type mice (NPY+/+).
2. Material and methods
Male and female NPY−/− and littermate NPY +/+ mice were maintained on an inbred pure 129/SvEv background and were developed as described elsewhere (Erickson et al., 1996). Because there were no significant differences between male and female mice, data are collapsed across sex within each of the analyses below. All mice were individually housed in plastic mouse cages with free access to standard rodent chow (Teklad, Madison, WI) and water except were noted. Mice were approximately 16 weeks of age at the start of each experiment. The colony room was maintained at approximately 22° C with a 12-hour light/dark cycle and lights off at 6:00 a.m. All procedures used in the present studies were in compliance with the National Institute of Health guidelines, and all procedures were approved by the University of North Carolina Institutional Animal Care and Use Committee (IACUC).
The diet used was a lactalbumin/dextrose-based, nutritionally complete diet with concentrations of vitamins, minerals and other nutrients derived from ICN Research Diets (Moy et al., 1997; Moy et al., 2000). Dextrose calories in the control diet (CD) were equated with ethanol calories in the ethanol diet (ED, 4.5%, w/v). Normal rodent chow was removed from the mouse cages during access to diet and water was provided in a second bottle. To reduce spillage, diet was presented to the mice in drinking bottles fitted with ball-point sipper tubes. Mice were first habituated with 3-days of access to CD and were given 6-days access to ED (NPY−/−, n = 7; NPY+/+, n = 8) or CD (NPY−/−, n = 8; NPY+/+, n = 8). Six-hours before testing, ED was replaced with CD in the ethanol-withdrawn groups (ED-WD). We chose to assess anxiety-like behavior 6-hours after removal of ethanol because we have found withdrawal-induced anxiety at this time point using the current diet protocol in rats (Knapp et al., 2004; Overstreet et al., 2002).
To assess anxiety-like behavior, mice were individually tested using elevated plus maze (EPM) procedures. Testing began at approximately 9:00 a.m., during the dark cycle. The plus maze (MED Associates, Inc., St. Albans, Vermont) was positioned in the center of the room directly below a ceiling-mounted lamp fitted with a single 25-watt red light bulb which provided the only light for the room. Each mouse was placed onto the center square of the plus maze with its nose pointing towards one of the open arms. The 5-min test session was video recorded with a tripod-mounted camcorder. Sessions were scored by genotype-blind investigators for time spent (min), and the proportion of total time spent, in the open arm defined as open arm time divided by total time spent in both arms. An animal was considered to have entered an arm of the plus maze if all four paws had left the center square. Open and closed arm time was considered terminated once a single paw was placed back into the center square. To determine possible group differences in locomotor activity, the total number of arm entries (open and closed) was also assessed. All data in this report are presented as mean ± S. E. M. We used 2 × 2 (genotype x diet) analyses of variance (ANOVAs) to assess main effects and conducted t-tests (Winer et al., 1991) for planned comparisons. Significance was accepted at P < 0.05.
3. Results
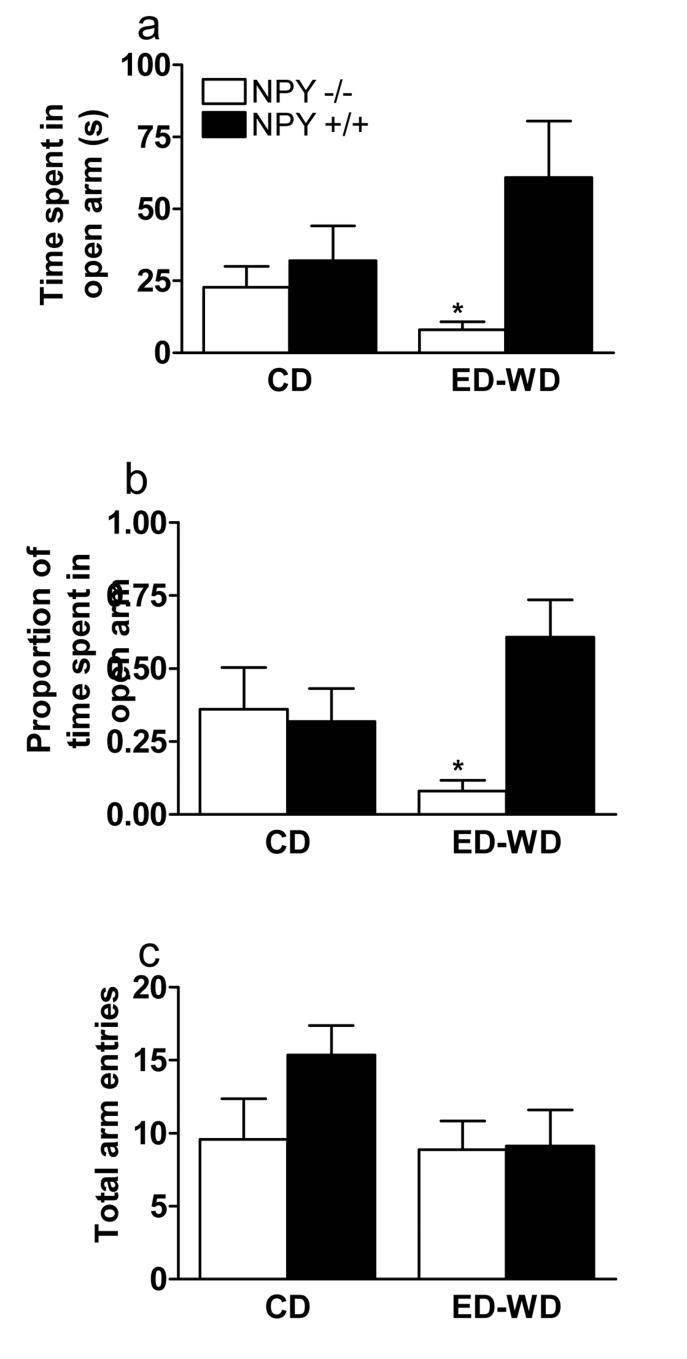
NPY−/− and NPY+/+ mice that drank ED consumed 15.99 ± 0.72 and 15.13 ± 0.65 g ethanol/kg per day, respectively. On the day of testing, ED-WD groups (NPY−/−, 22.72 ± 1.13 g; NPY+/+, 22.78 ± 0.76 g) had similar body weight compared to the CD groups (NPY−/−, 24.62 ± 1.91 g; NPY+/+, 23.71 ± 1.01 g). Figure 1 shows EPM data collected on time spent in open arms, proportion of time in spent open arms, and total arm entries. ANOVA performed on EPM data (Figure 1A) revealed a significant main effect of genotype {F(1, 27) = 6.20} on the time spent in the open arm of the plus maze, while an ANOVA performed on data representing the proportion of time spent in the open arm (Figure 1B) revealed a significant main effect of genotype {F(1, 27) = 4.81} and a significant genotype by diet interaction effect {F(1, 27) = 6.57}. While NPY−/− mice that were withdrawn from ethanol (ED-WD) showed significantly less open arm time and proportion of open arm time relative to NPY−/− mice that drank the CD, there were no significant differences in anxiety-like behavior between NPY+/+ mice given CD or ED-WD treatment. Additionally, NPY−/− and NPY+/+ mice showed significant differences in open arm time and in the proportion of time spent in the open arm following the ED-WD treatment. However, there were no genotype differences in mice that had access to the CD. An ANOVA performed on total open arm entry data (Figure 1C) revealed no significant effects.
Fig. 1.
Elevated plus maze performance by NPY−/− and NPY+/+ mice following 6-days of access to a control diet (CD) or 6-days of access to a 4.5% ethanol diet that was withdrawn and replaced with CD 6-hours before testing (ED-WD). Data from 5-min test sessions are expressed as time in seconds (a), the proportion of total time that was spent in the open arm (b), and the total number of arm entries (c). All values reported are mean ± SEM. *NPY−/− mice in the EDWD significantly different from all other groups (P < 0.05).
4. Discussion
Here we show that a lack of normal NPY production predisposes 129/SvEv mice to increased anxiety-like behavior stemming from exposure to and/or withdrawal from ethanol. Thus, ethanol-withdrawn NPY−/− mice showed significantly less open arm time and total proportion of time spent in the open arm of the EPM relative to ethanol-withdrawn NPY+/+ mice, and when compared with NPY−/− and NPY+/+ mice that had access to the CD. On the other hand, ethanol-withdrawn NPY+/+ mice did not show altered EPM behavior relative to controls. Further, the altered EPM activity resulting from ethanol withdrawal in NPY−/− mice was not related to changes in locomotor activity as there were no group differences in total arm entries. Thus, these preliminary observations indicate that NPY−/− mice are more sensitive to the anxiety-like behavior associated with exposure to and/or withdrawal from ethanol.
In the present study, groups that received continuous access to ethanol diet up to the EPM test were not employed. It is therefore possible that elevated anxiety-like behavior by the NPY−/− mice in the ED-WD condition resulted from exposure to ethanol, rather than ethanol withdrawal per se. While we did not assess blood ethanol concentrations (BECs), a previous report found that 129/SvJ mice metabolize ethanol at a rate of 1.1 mg/dl/min (Homanics et al., 1998). At this rate, after 6-hours mice in the present experiment could have metabolized up to 396 mg/dl, a BEC they were unlikely to have exceeded at the time ethanol was removed. Thus, it is unlikely that increased anxiety-like behavior by the NPY−/− mice was related to the presence of ethanol in the system at the time of EPM testing. Nonetheless, in the absence of BEC data and continuous ethanol access groups, a more conservative conclusion for the present work is that NPY−/− mice show increased anxiety-like behavior stemming from ethanol exposure, with the possibility that ethanol withdrawal contributes to this response.
Here, wild-type 129/SvEv mice did not display increased anxiety-like behavior 6-hours following the removal of ethanol. It is possible that EPM testing at time points greater than 6-hours following ethanol withdrawal, more days with access to ethanol diet, and/or multiple cycles of ethanol access and withdrawal (Breese et al., 2004; Knapp et al., 2004; Overstreet et al., 2002, 2004) may augment anxiety-like behavior in the wild-type mice. Such manipulations will be the subject of future studies. We predict that NPY−/− mice will continue to show increased anxiety-like behavior in all cases. Additionally, contrary to a previous observation (Palmiter et al., 1998), NPY−/− mice in the CD condition did not show increased anxiety-like behavior relative to NPY+/+ mice. Although the reason for this discrepancy is unclear, different genetic background of NPY−/− mice in the present study (129/SvEv) and the previous work (C57BL/6J × 129/SvEv) may account for such differences. In fact, genetic background effects on ethanol-associated phenotypes in NPY−/− mice have previously been reported (Thiele et al., 2000).
Because NPY−/− mice lack NPY throughout the central nervous system, we can only speculate on the brain region(s) in which NPY modulates anxiety-like behavior in NPY−/− mice of the present study. One candidate region is the amygdala. Infusion of NPY into the amygdala reduces anxiety-like behavior in rodents (Heilig et al., 1993; Sajdyk et al., 1999). Furthermore, NPY expression is blunted in the central and medial nuclei the amygdala of rats following ethanol withdrawal (Roy and Pandey, 2002), and infusion of a protein kinase A (PKA) activator into the central nucleus of the amygdala, a treatment that causes increases of amygdalar NPY levels (Pandey et al., 2005), protects against withdrawal-induced anxiety in rats (Pandey et al., 2003b). These observations provide convincing evidence that low NPY signaling in the amygdala can modulate withdrawal-induced anxiety-like behavior, and suggest the possibility that a lack of NPY signaling in the amygdala of NPY−/− mice predisposes these animals to increased anxiety-like behavior following ethanol exposure and withdrawal.
In conclusion, the present investigation reveals that NPY−/− mice show enhanced anxiety-like behavior stemming from ethanol exposure and/or withdrawal form ethanol, indicating that NPY−/− mice are a useful model for studying the role of NPY in modulating ethanol-associated anxiety-like responses. The present and past (Pandey et al., 2003b; Roy and Pandey, 2002) observations suggest that targets aimed at NPY receptors may be useful compounds for treating anxiety associated with ethanol exposure and withdrawal, and thus may be useful for preventing relapse that is triggered by withdrawal-induced anxiety or anxiety stemming from general life stressors (Breese et al., 2005). Because quantitative trait locus (QTL) analyses suggest that there are multiple candidate genes for the modulation of anxiety-like behavior, each revealed with different testing procedures (e.g., elevated plus maze, open field activity, etc.) (Henderson et al., 2004; Turri et al., 2001), additional studies are required for a more complete characterization of withdrawal-induced anxiety-like behavior in NPY−/− mice. Important next steps also include a characterization of the time course of withdrawal-induced anxiety-like behavior, identifying the NPY receptors that are involved, determining sensitivity of the present phenotype to the genetic background of the NPY−/− mice, and identifying the brain regions in which NPY modulates withdrawal responses in these mice.
ACKNOWLEDGEMENTS
This work was supported by NIH grants AA013573, AA015148, AA015875, AA015878, AA011605, AA014949, and the Department of Defense grant PR054214. We thank Richard Palmiter for supplying the NPY−/− mice.
REFERENCES
- Badia-Elder NE, Stewart RB, Powrozek TA, Roy KF, Murphy JM, Li TK. Effect of neuropeptide Y (NPY) on oral ethanol intake in Wistar, alcohol-preferring (P), and -nonpreferring (NP) rats. Alcohol Clin. Exp. Res. 2001;25:386–390. [PubMed] [Google Scholar]
- Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp. Biol. Med. 2003;228:217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- Bibb JL, Chambless DL. Alcohol use and abuse among diagnosed agoraphobics. Behav. Res. Ther. 1986;24:49–58. doi: 10.1016/0005-7967(86)90149-x. [DOI] [PubMed] [Google Scholar]
- Breese GR, Knapp DJ, Overstreet DH. Stress sensitization of ethanol withdrawal-induced reduction in social interaction: inhibition by CRF-1 and benzodiazepine receptor antagonists and a 5-HT1A-receptor agonist. Neuropsychopharmacology. 2004;29:470–482. doi: 10.1038/sj.npp.1300282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism--a “kindling”/stress hypothesis. Psychopharmacology. 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappell H, Herman CP. Alcohol and tension reduction. A review. Q. J. Stud. Alcohol. 1972;33:33–64. [PubMed] [Google Scholar]
- Colmer WF, Wahlestedt C. The biology of neuropeptide Y and related peptides. Humana Press; Totowa, NJ: 1993. [Google Scholar]
- Cornelius JR, Bukstein O, Salloum I, Clark D. Alcohol and psychiatric comorbidity. Recent Dev. Alcohol. 2003;16:361–374. doi: 10.1007/0-306-47939-7_24. [DOI] [PubMed] [Google Scholar]
- Dumont Y, Martel JC, Fournier A, St-Pierre S, Quirion R. Neuropeptide Y and neuropeptide Y receptor subtypes in brain and peripheral tissues. Prog. Neurobiol. 1992;38:125–167. doi: 10.1016/0301-0082(92)90038-g. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Clegg KE, Palmiter RD. Sensitivity to leptin and susceptibility to seizures of mice lacking neuropeptide Y. Nature. 1996;381:415–418. doi: 10.1038/381415a0. [DOI] [PubMed] [Google Scholar]
- Gray TS, Morley JE. Neuropeptide Y: anatomical distribution and possible function in mammalian nervous system. Life Sci. 1986;38:389–401. doi: 10.1016/0024-3205(86)90061-5. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heilig M, Soderpalm B, Engel JA, Widerlov E. Centrally administered neuropeptide Y (NPY) produces anxiolytic- like effects in animal anxiety models. Psychopharmacology. 1989;98:524–529. doi: 10.1007/BF00441953. [DOI] [PubMed] [Google Scholar]
- Henderson ND, Turri MG, DeFries JC, Flint J. QTL analysis of multiple behavioral measures of anxiety in mice. Behav. Genet. 2004;34:267–293. doi: 10.1023/B:BEGE.0000017872.25069.44. [DOI] [PubMed] [Google Scholar]
- Homanics GE, Le NQ, Kist F, Mihalek R, Hart AR, Quinlan JJ. Ethanol tolerance and withdrawal responses in GABA(A) receptor alpha 6 subunit null allele mice and in inbred C57BL/6J and strain 129/SvJ mice. Alcohol Clin. Exp. Res. 1998;22:259–265. [PubMed] [Google Scholar]
- Knapp DJ, Overstreet DH, Moy SS, Breese GR. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004;32:101–111. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Criswell HE, Breese GR. Flumazenil blockade of anxiety following ethanol withdrawal in rats. Psychopharmacology. 1997;131:354–360. doi: 10.1007/s002130050303. [DOI] [PubMed] [Google Scholar]
- Moy SS, Knapp DJ, Duncan GE, Breese GR. Enhanced ultrasonic vocalization and Fos protein expression following withdrawal: Effects of flumazenil. Psychopharmacology. 2000;152:208–215. doi: 10.1007/s002130000507. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Accentuated decrease in social interaction in rats subjected to repeated ethanol withdrawals. Alcohol Clin. Exp. Res. 2002;26:1259–1268. doi: 10.1097/01.ALC.0000023983.10615.D7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol. Biochem. Behav. 2004;77:405–413. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD, Erickson JC, Hollopeter G, Baraban SC, Schwartz MW. Life without neuropeptide Y. Recent Prog. Horm. Res. 1998;53:163–199. [PubMed] [Google Scholar]
- Pandey SC, Carr LG, Heilig M, Ilveskoski E, Thiele TE. Neuropeptide Y and alcoholism: genetic, molecular, and pharmacological evidence. Alcohol Clin. Exp. Res. 2003a;27:149–154. doi: 10.1097/01.ALC.0000052706.21367.0E. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Roy A, Zhang H. The decreased phosphorylation of cyclic adenosine monophosphate (cAMP) response element binding (CREB) protein in the central amygdala acts as a molecular substrate for anxiety related to ethanol withdrawal in rats. Alcohol Clin. Exp. Res. 2003b;27:396–409. doi: 10.1097/01.ALC.0000056616.81971.49. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Zhang H, Roy A, Xu T. Deficits in amygdaloid cAMP-responsive elements-binding protein signaling play a role in genetic predisposition to anxiety and alcoholism. J. Clin. Invest. 2005;115:2697–2699. doi: 10.1172/JCI24381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Pandey SC. The decreased cellular expression of neuropeptide Y protein in rat brain structures during ethanol withdrawal after chronic ethanol exposure. Alcohol Clin. Exp. Res. 2002;26:796–803. [PubMed] [Google Scholar]
- Sajdyk TJ, Vandergriff MG, Gehlert DR. Amygdalar neuropeptide Y Y-1 receptors mediate the anxiolytic-like actions of neuropeptide Y in the social interaction test. Eur. J. Pharmacol. 1999;368:143–147. doi: 10.1016/s0014-2999(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Hesselbrock V. Alcohol dependence and anxiety disorders: what is the relationship? Am. J. Psychiatry. 1994;151:1723–1734. doi: 10.1176/ajp.151.12.1723. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Koh MT, Pedrazzini T. Voluntary alcohol consumption is controlled via the neuropeptide Y Y1 receptor. J. Neurosci. 2002;22:RC208. doi: 10.1523/JNEUROSCI.22-03-j0006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Marsh DJ, Ste. Marie L, Bernstein IL, Palmiter RD. Ethanol consumption and resistance are inversely related to neuropeptide Y levels. Nature. 1998;396:366–369. doi: 10.1038/24614. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Miura GI, Marsh DJ, Bernstein IL, Palmiter RD. Neurobiological responses to ethanol in mutant mice lacking neuropeptide Y or the Y5 receptor. Pharmacol. Biochem. Behav. 2000;67:683–691. doi: 10.1016/s0091-3057(00)00413-5. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Sparta DR, Hayes DM, Fee JR. A role for neuropeptide Y in neurobiological responses to ethanol and drugs of abuse. Neuropeptides. 2004;38(4):235–243. doi: 10.1016/j.npep.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Turri MG, Datta SR, DeFries J, Henderson ND, Flint J. QTL analysis identifies multiple behavioral dimensions in ethological tests of anxiety in laboratory mice. Curr. Biol. 2001;11:725–734. doi: 10.1016/s0960-9822(01)00206-8. [DOI] [PubMed] [Google Scholar]
- Winer BJ, Brown DR, Michels KM. Statistical Principles in Experimental Design. McGraw-Hill, Inc.; New York: 1991. [Google Scholar]