Synopsis
Over the past 30 years there has been an increase in the prevalence of obesity and diabetes, both of which can have serious consequences for longevity and quality of life. Sleep durations may have also decreased over this time period. This chapter reviews laboratory and epidemiologic evidence for an association between sleep loss and impairments in glucose metabolism and appetite regulation, which could increase the risk of diabetes or weight gain.
Keywords: sleep, appetite, diabetes, obesity, ghrelin, leptin
Introduction
Diabetes and obesity are two debilitating chronic diseases that are increasing at an alarming rate worldwide [1, 2]. Voluntary sleep restriction may play a role in the rapid increase in the prevalence of diabetes and obesity, and this chapter will review the evidence for such a link. Sleep restriction or impaired sleep may be more common in modern society than in past decades [3, 4]. A survey study from 1960 found modal sleep duration to be 8.0 to 8.9 hours [5], while another survey study in 1995 observed a modal category of only 7 hours [6]. Recent national data also indicate that a greater percentage of adult Americans report sleeping 6 hours or less in 2004 than in 1985 [7]. Thus, the increase in the prevalence of obesity and diabetes appears to be mirrored by a decrease in average sleep duration in the U.S.
In simplistic terms, weight gain occurs when there is positive energy balance, that is, energy intake is greater than energy expenditure (see Figure 1). Sleep restriction could affect endogenous processes related to energy balance, such as impairments in glucose metabolism and an upregulation of appetite. Sleep restriction could also affect exogenous factors such as food choice and increased time available to eat. Sleep loss could also lead to reductions in physical activity or energy expenditure, but evidence in support of this hypothesis is lacking. Both impaired glucose metabolism and excess weight can increase the risk of developing type 2 diabetes. Thus, this chapter will first review laboratory studies that examine the effects of sleep loss on glucose metabolism and appetite regulation. The chapter will then review the epidemiological evidence for an association between sleep restriction and diabetes risk, increased body mass index and risk of obesity.
Figure 1.
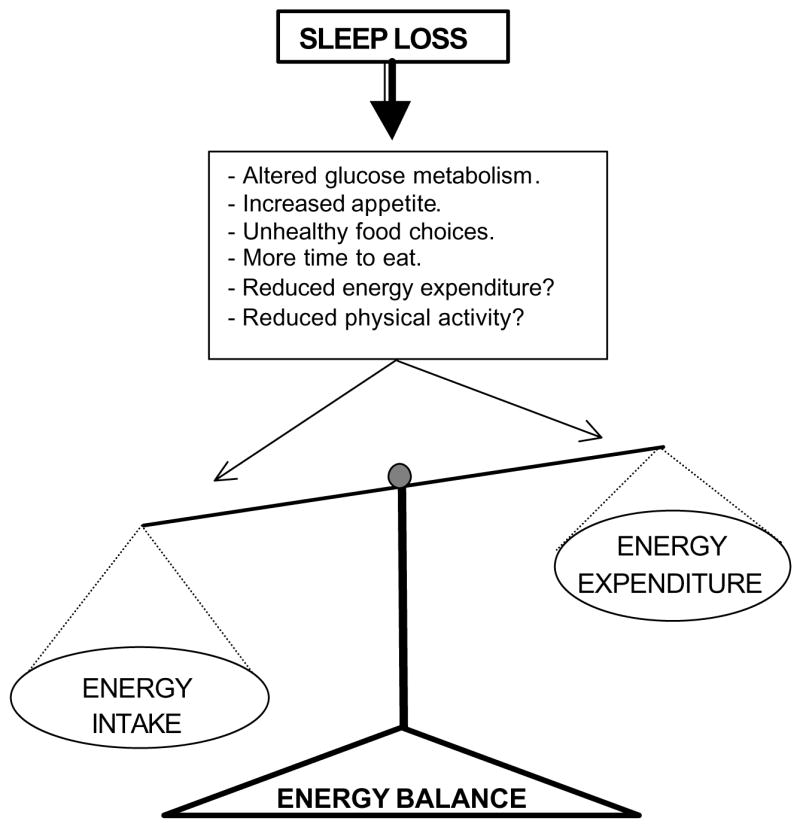
Schematic representation of potential pathways through which sleep loss may lead to a positive energy balance in which energy intake is greater than energy expenditure.
Sleep disordered breathing is a common sleep disorder that can lead to sleep loss, however it is also associated with sleep fragmentation and hypoxia. Therefore, it is a much more complex condition than behavioral sleep restriction. Chapters 11–13 of this volume discuss the effects of sleep apnea and sleep disordered breathing on health.
Sleep & Glucose Metabolism
Normal Conditions
Under normal conditions, glucose homeostasis results from a tightly controlled balance between glucose production (from the liver in the post-absorptive state and from the gut in the post-prandial state) and glucose utilization. Insulin plays a key role in this process by inhibiting hepatic glucose production and by stimulating glucose uptake by insulin-sensitive tissues. Glucose tolerance refers to the ability of the body to metabolize exogenous glucose and return to a baseline level of blood glucose. Glucose tolerance may be assessed in response to oral glucose administration, intravenous glucose administration or ingestion of meals containing carbohydrates. Insulin resistance (or reduced insulin sensitivity) indicates that greater amounts of insulin are required to metabolize the same amount of glucose. To study variations in glucose tolerance across a 24-hour cycle, a constant glucose challenge, such as identical meals or snacks, identical loads of oral glucose, constant glucose infusion or continuous enteral nutrition, must be used. Constant glucose infusion and continouous enteral nutrition further allow for the assessment of nocturnal glucose tolerance during sleep. Studies using these procedures have shown that, in normal, lean individuals, glucose tolerance varies across the day. Glucose tolerance typically is optimal in the morning and reaches its minimum in the middle of the night [8, 9]. This diurnal variation appears to be partly due to a reduction in insulin sensitivity that occurs simultaneously with a reduction in insulin secretion in response to elevated glucose levels [10–12]. A study that has attempted to isolate the intrinsic circadian component of this diurnal variation in glucose tolerance has indicated that the circadian acrophase for both glucose and insulin levels occurs around the usual time of awakening [13], and that the optimal glucose tolerance that normally characterizes the morning period is the result of a decline in glucose and insulin levels that only occurs during sleep.
Multiple studies have indeed shown that sleep plays a role in the 24-hour pattern of glucose concentrations. For example, an 8-hour period of fasting while awake is associated with a continuous decline in glucose levels, however, during sleep, which is also a fasting state, glucose levels remain fairly constant [14]. One study examined the association between sleep and glucose regulation during constant glucose infusion, a condition that inhibits endogenous glucose production and therefore reveals changes in glucose utilization [15]. This study observed that during the early part of nocturnal sleep, levels of glucose increased by an average of approximately 20 percent but returned to baseline levels in the morning [15], suggestive of reduced glucose utilization during sleep. Another study using continuous enteral nutrition also showed elevated glucose levels during sleep and further indicated that this impact of sleep in glucose homeostasis occurred even when sleep was shifted to the daytime [16]. In the study with constant glucose infusion overnight [15], non-REM sleep was associated with increases in glucose levels, while REM sleep was associated with stable levels of glucose [15]. Studies using positron emission tomography (PET) have indicated that whole-brain glucose metabolism declines by approximately 11% during non-REM sleep [17], and this may partly account for the increase in glucose levels during non-REM sleep. The increase in glucose during non-REM sleep may also be related to a reduction in peripheral glucose utilization [18, 19]. The return to baseline glucose levels in the morning appears related to the increase in wake and REM stages [15], which are associated with higher glucose utilization than non-REM stages [18–21]. Finally, daytime sleep was associated with marked elevations of glucose levels and insulin secretion, which indicates that sleep exerts modulatory influences on glucose regulation independently of time of day [22]. In summary, glucose utilization appears lowest during non-REM sleep and highest during wake, with intermediate levels during REM sleep [15].
Laboratory studies of sleep and glucose metabolism
Total Sleep Deprivation
A study comparing normal nocturnal sleep to total sleep deprivation in normal young adults receiving a constant glucose infusion observed that during total sleep deprivation plasma glucose levels increased much less during the first half of the night than during normal sleep (Figure 2) [15]. During the second half of the night, glucose levels decreased under both sleep conditions. This early morning decrease during total sleep deprivation was approximately twice as large as the initial nighttime increase, while during normal nocturnal sleep, the nighttime decrease was of similar magnitude as the initial nighttime increase [15]. Furthemore, the glucose profiles illustrated in Figure 2 indicate that, after sleep deprivation , plasma glucose levels were higher in the mid-morning to late afternoon despite similar insulin levels, suggesting decreased daytime insulin action. Thus, the pattern of glucose secretion during nocturnal sleep deprivation differs significantly from the pattern during nocturnal sleep, and it is likely due in part to the absence of slow wave sleep and growth hormone secretion in the beginning of the night.
Figure 2.
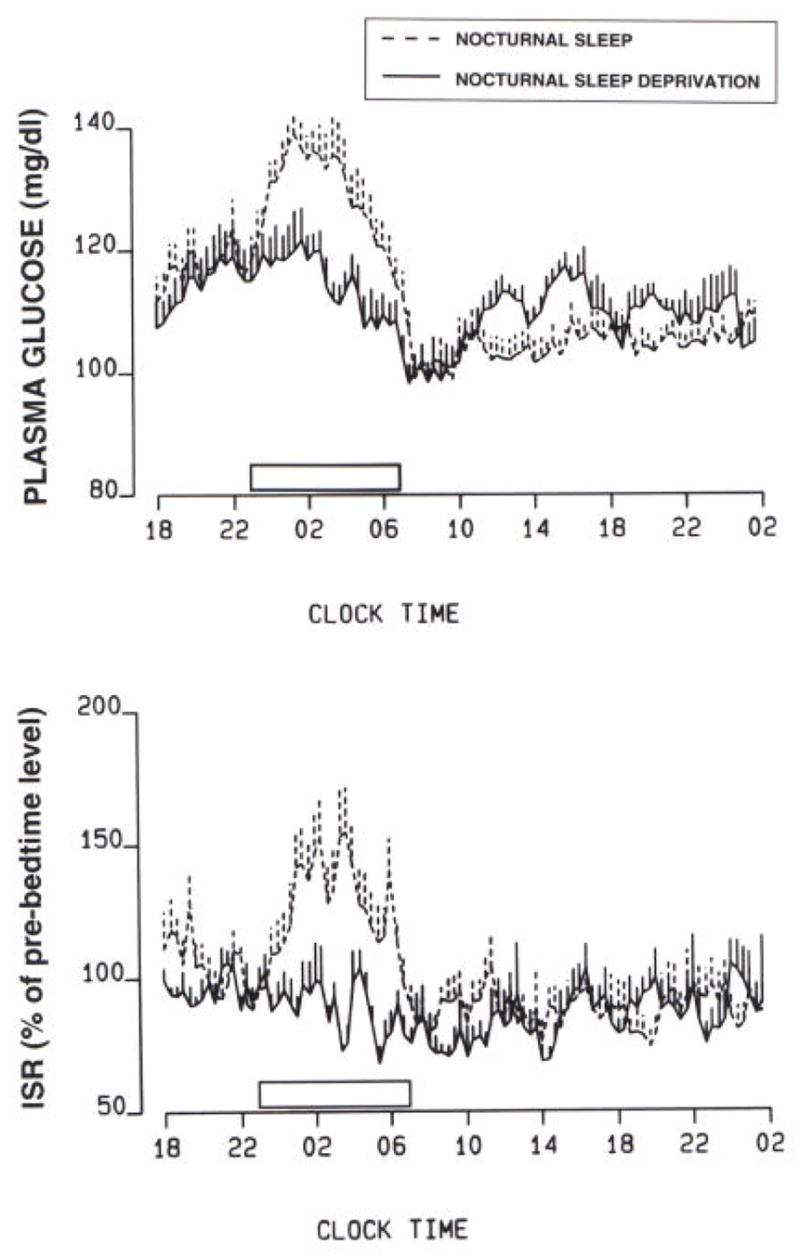
Plasma glucose levels and insulin secretion rate (ISR) under conditions of constant glucose infusion during both nocturnal sleep (dashed lines) and nocturnal sleep deprivation (solid lines).
Partial Sleep Deprivation
Total sleep deprivation cannot be maintained for more than a few days, and this paradigm does not reflect chronic real world behavior. On the other hand, partial sleep deprivation, or bedtime restriction, is commonly practiced. Laboratory studies have examined the effects of partial sleep deprivation on glucose metabolism. One study enrolled 11 healthy young men and subjected them to 6 nights of 4 hours in bed followed by 7 nights of 12 hours in bed [23]. The subjects ate 3 identical carbohydrate-rich meals and were at continuous bed rest on the last two days of each condition. On the fifth morning of each condition, subjects underwent an intravenous glucose tolerance test (ivGTT) and on the sixth day of each condition blood samples were collected every 10–30 minutes for 24 hours [23]. The results of the ivGTT during the sleep restriction condition demonstrated that the rate of glucose clearance was approximately 40% lower and the acute insulin response to glucose (AIRG) was 30% lower compared to the sleep extension condition [23]. The glucose clearance rates during sleep restriction were similar to those reported for older adults with impaired glucose tolerance [24], while the glucose clearance rates after sleep extension were typical of healthy young subjects [25]. In this study, glucose effectiveness, which is a measure of the ability of glucose to mediate its own disposal, was 30% lower and this is very similar to the difference reported between groups of patients with type-2 diabetes and healthy white men [26]. Decreased AIRG is an early marker in the development of diabetes and AIRG decrements similar those observed in this study have been described in aging [27] and gestational diabetes [28]. Finally, the glucose response to the breakfast meal was higher after the sleep restriction condition than after sleep extension despite similar insulin secretion, and this response would likely lead to a diagnosis of impaired glucose tolerance [23]. Thus, one week of restricted sleep produced physiological alterations that are consistent with the future development of type 2 diabetes.
These alterations in glucose metabolism may be associated with the hypothalamic-pituitary axis. Under normal conditions, glucose tolerance and insulin sensitivity begin to improve during the later part of the night, reflecting a delayed effect of low cortisol levels during the evening and early part of the night [29]. Disturbances in the secretory profiles of the counter-regulatory hormones, growth hormone (GH) and cortisol, may partially contribute to the alterations in glucose regulation observed during sleep loss. Previously it was reported that 6 days of sleep restriction were associated with an extended duration of elevated nighttime GH concentrations and with an increase in evening cortisol levels [23]. An extended exposure of peripheral tissues to higher GH levels may induce a rapid decrease in muscular glucose uptake adversely affecting glucose regulation. Also, elevated evening cortisol concentrations are likely to result in reduced insulin sensitivity on the following morning [30]. Finally, the reduction in AIRg may be attributed to measured changes in sympathovagal balance that indicated increased sympathetic nervous activity, which inhibits pancreatic function [23].
Another study recruited age- and weight-matched groups of healthy normal-weight habitual short sleepers (<6.5 hours per night) and normal sleepers (7.5-8.5 hours per night). During an ivGTT, the glucose tolerance was similar for the two groups, however the short sleepers secreted an average of 50% more insulin during both the first and second phases of response resulting in a 40% lower insulin sensitivity [31]. Thus, there does not appear to be a healthy adaptation to sleep loss in terms of carbohydrate metabolism since larger amounts of insulin were secreted in order to achieve the normal glucose tolerance.
These two studies of partial sleep deprivation suggest possible mechanisms by which sleep loss could lead to impaired glucose tolerance and eventually type 2 diabetes. After only a week of sleep restriction, subjects were unable to metabolize the glucose at rates observed in healthy young individuals. Subjects who report having been short sleepers for at least 6 months, on the other hand, had glucose clearance rates similar to healthy long sleepers, but they had to secrete more insulin to achieve this glucose profile. Increasing levels of insulin could lead to insulin resistance, a risk factor for type 2 diabetes. Thus, over long periods of time as short sleepers age, the risk of developing type 2 diabetes and obesity may increase.
Sleep & Appetite Regulation
Normal Conditions
Appetite is regulated by two opposing sets of neuronal circuitry, appetite simulating and appetite-inhibiting, in the arcuate nucleus of the hypothalamus, and several hormones have been identified that affect these neuronal regions [32]. Leptin is an appetite-inhibiting hormone that is primarily secreted by adipose tissue and promotes feelings of satiety [32]. Leptin has a circadian rhythm such that there is a peak in leptin levels in the early part of the sleep period (Figure 3) [33] Ghrelin is an appetite stimulating peptide secreted primarily from the stomach. In rodents, ghrelin administration increases food intake and reduces fat oxidation, which generates a positive energy balance and increased adiposity [34]. Studies in humans also indicate that ghrelin increases appetite and food intake [34]. Circulating concentrations of ghrelin peak exhibit a nocturnal maximum (Figure 3) [33]. Thus, leptin and ghrelin exert opposing effects on appetite but are both increased during sleep under normal circumstances. Sleep deprivation has been associated with alterations in both of these hormones.
Figure 3.
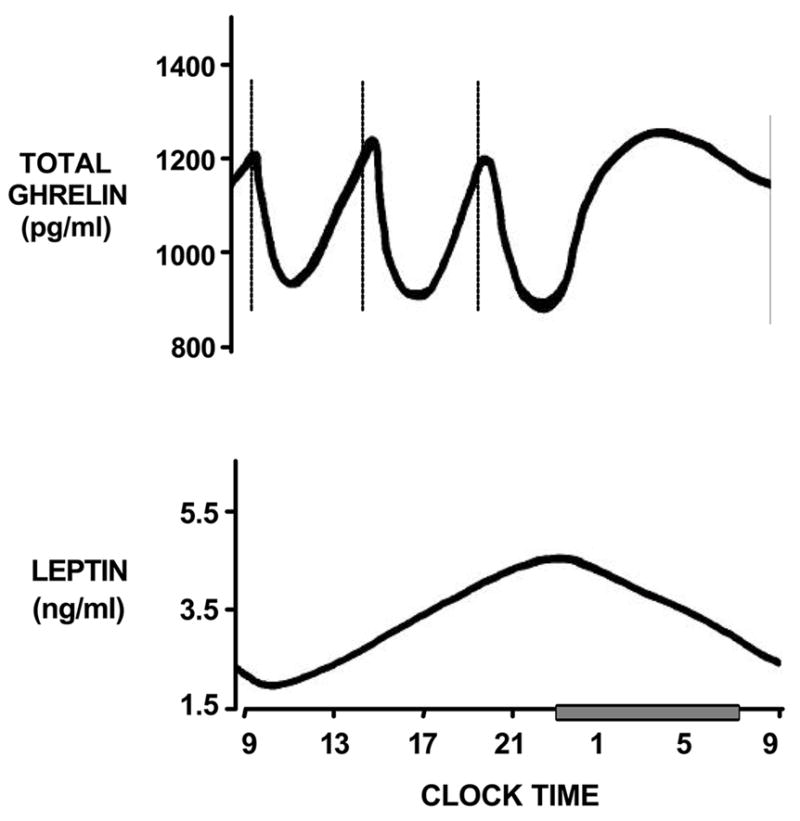
Stylized representation of total ghrelin and leptin levels across 24-hour period. Sleep period is identified by grey bar. Dashed lines represent timing of identical high-carbohydrate meals at 9:00, 14:00 and 19:00.
Another important system involved in sleep/wake and energy homeostasis is the orexin (or hypocretin) system [35]. Orexin is a hypothalamic peptide that was first associated with sleep when it was found that human narcolepsy patients have a deficiency in the orexin system. As such, the orexin system will be discussed in Chapter 15 of this volume.
Laboratory studies of sleep and appetite regulation
Total Sleep Deprivation
A few studies have been published that report on the effects of total sleep deprivation on leptin levels. One such study compared leptin levels in seven healthy young men receiving continuous enteral nutrition after a night of normal sleep to the levels after a night of total sleep deprivation followed by daytime recovery sleep [14]. During normal nocturnal sleep, leptin levels increased during the early part of the night reaching a maximum at the midpoint of sleep and then decreased until mid afternoon [14]. During nocturnal sleep deprivation, leptin levels also increased but the maximum level occurred later in the night. During daytime recovery sleep, leptin levels rose with a maximum level in the mid afternoon. Thus, this study indicated that, under carefully controlled conditions of caloric intake, leptin levels rise during sleep regardless of when sleep occurs.
A second study involved 88 hours of wakefulness and observed a reduction in the amplitude of the 24-hour variation of leptin levels across the sleep deprivation period [36]. Finally, another study that used a 38-hour constant routine protocol (continuous wakefulness in semi-recumbent posture with identical snacks at 2-h intervals) attempted to distinguish the effects of sleep deprivation from circadian effects [13] and observed a linear increase in leptin levels throughout the prolonged wakefulness period.
Thus, acute total sleep deprivation appears to be associated with an increase in leptin levels relative to daytime concentrations but more extended sleep deprivation seems to result in a decrease of leptin levels.
Partial Sleep Deprivation
In the laboratory study discussed above that compared 6 nights of 4-hour bedtimes to 6 nights of 12-hour recovery sleep, mean leptin levels were approximately 19% lower during sleep restriction [37]. The circadian rhythm of leptin was also affected by sleep restriction: the acrophase was 2 hours earlier and 26% lower and the amplitude of the diurnal variation was 20% lower [37]. These changes occurred despite no differences in caloric intake, physical activity or body mass index [37]. Maximal leptin levels differed on average by 1.7 ng/ml between the sleep restriction and sleep extension periods, and this is somewhat larger than the decrease in leptin reported after three days of dietary intake restricted to 70% of energy requirements [38, 39]. This study did not measure levels of ghrelin, appetite or hunger, however, a second laboratory study that used a randomized cross-over design did. This study involved 2 days of 4-hour bedtimes and 2 days of 10-hour bedtimes with constant glucose infusion, and levels of leptin, ghrelin, hunger and appetite were measured at the end of the 2 days of each condition [40]. Mean leptin levels were 18% lower and mean ghrelin levels were 28% higher in the sleep restriction condition relative to sleep extension [40]. Hunger and appetite scores measured using visual analog scales were also increased during the sleep restriction condition. Hunger ratings were approximately 24% higher and appetite ratings were 23% higher during sleep restriction, and the increase in appetite for carbohydrate rich foods (sweets, salty snacks, starchy foods) tended to be greater [40]. Finally, the change in the ratio of ghrelin-to-leptin between the two conditions was strongly correlated to the change in hunger ratings, suggesting that the changes observed in these appetite hormones was partially responsible for the increase in appetite and hunger. These observed changes would suggest that these subjects, if allowed ad libidum food, would have increased their energy intake.
A population-based study, the Wisconsin Sleep Cohort Study, observed an association between short sleep duration and decreased leptin and increased ghrelin levels [41]. This study involved one night of polysomnography (PSG) in the laboratory and they collected sleep diaries from which average nightly sleep was calculated. In the morning following the PSG, a single fasting blood sample was taken. Total sleep time from PSG was significantly negatively associated with ghrelin levels (beta coefficient = −0.69) and average nightly sleep was significantly positively associated with leptin levels (beta coefficient = 0.11) [41]. Thus, ghrelin, which may act more rapidly on appetite, is associated with an acute, short-term measure of sleep while leptin, which is often considered to be a long-term energy balance factor, is associated with the more chronic long term sleep measure [32]. Another large study of post-menopausal women observed no association between self-reported sleep and measures of ghrelin or leptin [42]. The association between sleep and these hormones may be modified by age and gender. Finally, a recent study in Canada observed that plasma leptin levels among short sleepers (5–6 hours per night) were significantly lower than what would be predicted based on the subject’s body fat mass [43]. Table 1 summarizes the results from three studies discussed above that, despite very different study designs, demonstrate very similar findings. The reduction in leptin levels associated with shorter bedtimes ranges from 15–18% in these studies.
Table 1.
The reduction in plasma leptin levels associated with short sleep durations from three different studies.
| Study | n | Design | % Change in Leptin |
|---|---|---|---|
| Laboratory Study [40] | 12 | Within-subject Comparison4-hr vs. 10-hr bedtimes | − 18% |
| Epidemiologic Study – Wisconsin Cohort Study [41] | 1024 | Between Subject Comparison5-hr vs. 8-hr habitual bedtimes. | − 16% |
| Epidemiologic Study – Quebec Family Study [43] | 740 | Comparison of measured vs. values predicted based on body fat mass for those sleeping 5–6 hours habitually. | −15% men
−17% women |
In summary, sleep duration seems to play an important role in the regulation of human leptin and ghrelin levels, hunger and appetite. Differences in energy balance between sleep restriction and sleep extension do not entirely explain the differences in appetite regulation since the laboratory studies kept energy intake and activity levels constant for both bedtime conditions. Sleep loss therefore may alter the ability of leptin and ghrelin to accurately signal energy requirements, which could lead to increased food intake, which is consistent with reports of increased food intake in human subjects and in laboratory rodents submitted to total sleep deprivation [44, 45]. Furthermore, the preference for high carbohydrate foods suggests sleep deprived individuals may make unhealthy food choices, and a study in Japan has found that self-reported short sleepers do in fact report less health eating behaviors, such as irregular meals, increased frequency of snacking and a preference for salty foods [46]. Future research is necessary to determine whether sleep restriction does in fact lead to increased energy intake and a positive energy balance.
Epidemiologic Studies of Sleep
Several large epidemiologic studies have examined sleep duration and its association with risk of diabetes, obesity or increased body mass index. Generally, these studies support the associations observed in the laboratory studies described above.
Diabetes Risk
The association between sleep duration or disturbance and the development of diabetes has been examined in several prospective epidemiological studies. A report from the Nurses Health Study, which recruited married female nurses aged 30–55 years in 1976, found a 15–30% increased risk of incident diabetes over 10 years among those reporting sleep durations of 6 hours or less and 9 or more hours relative to 7–8 hours, but when adjusting for BMI the association was no longer significant suggesting that BMI may mediate this association [47]. However, those reporting sleeping 5 hours or less remained at a 37% increased risk of developing symptomatic diabetes, which may reflect more severe disease, after adjustment for covariates including BMI [47]. A prospective study in Japan followed adult men for 8 years from 1984 to 1992, and subjects who reported either a high frequency of difficulty initiating sleep or difficulty maintaining sleep had 2 to 3 times the risk of developing type 2 diabetes than those with a low frequency of these sleep disturbances [48]. A prospective Swedish study examined men aged 35–51 years between 1974 and 1984 and again 7–22 years later and found a 50% increased risk of incident diabetes among those who reported difficulty falling asleep or use of sleeping pills after controlling for numerous covariates including age, BMI at baseline, physical activity, smoking, and family history of diabetes [49]. Another prospective study conducted in Sweden followed 1,187 men and women free of diabetes at baseline for 12-years [50]. After adjustment for covariates, men who reported difficulty maintaining sleep were at almost 5 times the risk of developing diabetes and those who reported sleep duration of 5 hours or less had almost 3 times the risk of developing diabetes [50]. Sleep duration or disturbances did not significantly predict incident diabetes in women in this sample after adjustment for covariates. A third prospective study from Sweden followed over 600 women for 32 years beginning in 1968–69, but the incidence of diabetes over a 32-year period was not associated with the self-reported sleep problems, sleep medication use or sleep duration at baseline [51]. A prospective study from Germany followed 8,269 non-diabetic men and women for an average of 7.5 years and observed a significant increased risk of incident type 2 diabetes for those who reported difficulty maintaining sleep at baseline, even after adjustment for numerous covariates (OR 1.60, 95%CI 1.05–2.45 for men; 1.98, 95%CI 1.20–3.29 for women) [52]. Finally, The Massachusetts Male Aging Study recruited men aged 40–70 years in 1987–89 and re-interviewed them in 1995–97 and in 2002–2004 [53]. Men who reported sleeping 6 hours or less per night were at almost twice the risk of incident diabetes after adjustment for covariates such as age, hypertension, smoking, self-rated health, waist circumference and education [53].
These studies, which varied greatly in subject populations, geographical locations and cultures, were remarkably consistent in indicating that short or poor sleep may increase the risk of developing type 2 diabetes. Although the majority of these epidemiologic studies suggest an association between sleep duration or disturbance and a risk of developing type 2 diabetes, which is consistent with the findings from the laboratory studies, they all relied on self-reported measures of sleep. Additional studies that use objective measures of sleep and preferably an interventional design are required to determine if sleep loss is on the causal pathway to the development of diabetes.
Body Mass Index and Obesity Risk
Several large epidemiological studies from different countries have observed an association between sleep duration and body mass index (BMI) in both adults and children. Short sleep durations have been associated with increased prevalence of obesity in Spain, Japan and the U.S. [54–57]. A French study of adults dichotomized sleep duration and found a slightly but significantly higher mean BMI among women reporting sleeping 6 hours less versus those sleeping more than six hours (24.4 vs. 23.4 kg/m2) after adjustment for age and area of residence, but this difference was not observed among men [58]. Several studies have observed a U-shaped association between sleep duration and body mass index (BMI) [41, 59, 60], which indicates that both short and long sleep is associated with higher BMI. For example, the Wisconsin Sleep Cohort Study observed the lowest BMI at an average bedtime of 7.7 hours per night. A recent study in the rural U.S. observed a significant negative association between sleep duration and BMI (beta coefficient = −0.42; 95% CI: −0.77 to −0.07) [61]. Thus, most studies have observed increased BMI or obesity prevalence with shorter sleep duration, and some have found higher BMI among those who report long sleep durations.
All of the studies discussed above were cross-sectional, however, three published studies in adults have employed a longitudinal design. First, researchers recently analyzed data from the first National Health and Nutrition Examination Survey (NHANES I), which measured weight and asked about usual sleep hours in 1982–84 and obtained self-reported weights in 1987 and 1992 [62]. Among the 32–49 age group, those reporting sleeping 2–4 h, 5 h or 6 h/night in 1982–84 had a higher mean BMI in 1982–84, 1987 and 1992 relative to those reporting 7 h/night in 1982–84 [62]. Those reporting more than 7 hours of sleep per night did not have a significantly higher or lower mean BMI relative to the 7-hour group. A second prospective study analyzed the association between sleep and BMI over a 13-year period [63]. Longitudinal analysis of the data indicated that the odds ratio for sleep duration predicting obesity was 0.50, which means that every hour increase in sleep duration was associated with a 50% reduction in risk of obesity [63]. Furthermore, the change in BMI per year appeared to be negatively associated with the average sleep duration from the 4 interviews [63]. Finally, results from the Nurses’ Health Study indicated that over 16 years women who reported sleeping 6 hours or less gained an average of 0.71 to 1.14 kg more than those who reported 7 hours of sleep [64]. One major limitation to all of these studies is that they did not use objective measures of sleep duration, and error in the subjective reporting of sleep may be associated with health, weight or other factors that are associated with BMI.
Several studies among children have also observed an association between sleep and body mass index. Risk of obesity was higher among short sleepers in children from France [65], Germany [66], Japan [67], and Canada [68]. A prospective study in the US followed children from birth to 9.5 years of age, and sleep duration was assessed annually between the ages of 2 to 5 years [69]. Average sleep duration between the ages 3 to 5 years was approximately 30 minutes less among children who were overweight at 9.5 years of age, however the difference in sleep between overweight and lean children was primarily due to daytime naps and not nocturnal sleep [69]. A study in the UK followed over 5,000 children from birth, collected sleep duration information at 38 months of age and examined obesity at age 7 years [70]. Sleep durations of <10.5 h and 10.5–11.4 h were associated with a 30–45% increased risk of obesity at age 7 relative to ≥ 12 h per night [70]. Finally, an analysis of the National Longitudinal Study of Adolescent Health in the US indicated that self–reported sleep duration was weakly associated with BMI z score and risk of overweight among male adolescents but not among females [71].
All of the studies in children discussed above used subjective measures of sleep duration reported either by the parent or by the child, however, one study in the US used a more objective measure of sleep, a 24-hour period of actigraphy recording in 383 adolescents aged 11–16 years [72]. This study defined obesity as a BMI above the 85th percentile for sex and age as well as having a percent body fat of 25% or above for males or 30% or above for females, as measured by bioelectrical impedance [72]. After adjusting for age, sex, sexual maturity, and ethnicity, total sleep time had an odds ratio of 0.20 (95% CI 0.11–0.34) predicting obesity, which indicates that every hour increase in sleep is associated with an 80% reduction in risk of being obese [72]. These studies indicate a possible association between sleep duration and risk of being overweight or obese in children. However, similar to the studies conducted in adults, the majority of these studies relied on subjective reports of sleep duration. Also, the majority of studies in both adults and children are cross-sectional in design, which means that the direction of causality cannot be inferred. Short sleep could lead to weight gain, but overweight or obesity could also lead to an inability to obtain sufficient amounts of sleep.
Conclusion
The research reviewed here suggests that sleep loss can lead to impairments in glucose metabolism and increases in insulin levels, which could increase the risk of the development of diabetes. Partial sleep deprivation is also associated with changes in the appetite regulating hormones, leptin and ghrelin, and these changes would indicate an increase in appetite, which may lead to increased food intake and weight gain. Epidemiological evidence, which have examined population-based samples, support the laboratory findings. These studies have observed associations between short sleep durations and increased risk of diabetes and obesity. Future research into the association between sleep loss and diabetes or obesity risk, however, need to incorporate objective measures of sleep and body size and to utilize longitudinal and intervention methods to better understand the mechanisms linking sleep loss to alterations in metabolism. For example, intervention studies could determine if increased sleep can improve glucose tolerance. Other studies could examine whether sleep loss does lead to increased food intake, reduction in energy expenditure or reductions in physical activity (Figure 1). Considering the morbidity and mortality risks associated with diabetes and obesity, such avenues of research are very important.
Acknowledgments
Funding support: Research was supported by P01 AG-11412, R01 HL-075079, RO1 HL–72694, University of Chicago Diabetes Research and Training Grant (NIH P60 DK–20595), and the University of Chicago General Clinical Research Center (NIH MO1–RR–00055).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mokdad A, Bowman B, Ford E, Vinicor F, Marks J, Koplan J. The Continuing Epidemics of Obesity and Diabetes in the United States. JAMA. 2001;286(10):1195–1200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Diabetes. Geneva, Switzerland: 2006. Office of Health Communications and Public Relations. [Google Scholar]
- 3.Jean-Louis G, Kripke D, Ancoli-Israel S, Klauber M, Sepulveda R. Sleep duration, illumination, and activity patterns in a population sample: effects of gender and ethnicity. Biological Psychiatry. 2000;47(10):921–927. doi: 10.1016/s0006-3223(99)00169-9. [DOI] [PubMed] [Google Scholar]
- 4.Bliwise DL. Historical change in the report of daytime fatigue. Sleep. 1996;19:462–464. doi: 10.1093/sleep/19.6.462. [DOI] [PubMed] [Google Scholar]
- 5.Kripke D, Simons R, Garfinkel L, Hammond E. Short and long sleep and sleeping pills. Is increased mortality associated? Archives of General Psychiatry. 1979;36(1):103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 6.Gallup Organization. Sleep in America. Princeton, NJ: Gallup Organization; 1995. [Google Scholar]
- 7.National Center for Health Statistics. QuickStats: Percentage of adults who reported an average of 6 hours of sleep per 24-hour period, by sex and age group - United States, 1985 and 2004. MMWR Morb Mortal Wkly Rep. 2005 [Google Scholar]
- 8.Shapiro ET, Polonsky KS, Copinschi G, et al. Nocturnal elevation of glucose levels during fasting in noninsulin-dependent diabetes. J Clin Endocrinol Metab. 1991;72:444–454. doi: 10.1210/jcem-72-2-444. [DOI] [PubMed] [Google Scholar]
- 9.Van Cauter E, Desir D, Decoster C, Féry F, Balasse EO. Nocturnal decrease in glucose tolerance during constant glucose infusion. J Clin Endocrinol Metab. 1989;69(3):604–611. doi: 10.1210/jcem-69-3-604. [DOI] [PubMed] [Google Scholar]
- 10.Jarrett RJ. Rhythms in insulin and glucose. In: Krieger D, editor. Endocrine Rhythms. Vol. 1. New York: Raven Press; 1979. pp. 247–258. [Google Scholar]
- 11.Verrillo A, De Teresa A, Martino C, et al. Differential roles of splanchnic and peripheral tissues in determining diurnal fluctuation of glucose tolerance. Am J Physiol. 1989;257:E459–E465. doi: 10.1152/ajpendo.1989.257.4.E459. [DOI] [PubMed] [Google Scholar]
- 12.Lee A, Ader M, Bray GA, Bergman RN. Diurnal variation in glucose tolerance: cyclic suppression of insulin action and insulin secretion in normal weight, but not obese, subjects. Diabetes. 1992;41:742–749. doi: 10.2337/diab.41.6.750. [DOI] [PubMed] [Google Scholar]
- 13.Shea SA, Hilton MF, Orlova C, Ayers RT, Mantzoros CS. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J Clin Endocrinol Metab. 2005 May;90(5):2537–2544. doi: 10.1210/jc.2004-2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon C, Gronfier C, Schlienger JL, Brandenberger G. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: Relationship to sleep and body temperature. J Clin Endocrinol Metab. 1998;83:1893–1899. doi: 10.1210/jcem.83.6.4864. [DOI] [PubMed] [Google Scholar]
- 15.Scheen AJ, Byrne MM, Plat L, Van Cauter E. Relationships between sleep quality and glucose regulation in normal humans. Am J Physiol. 1996;271:E261–E270. doi: 10.1152/ajpendo.1996.271.2.E261. [DOI] [PubMed] [Google Scholar]
- 16.Simon C. Ultradian pulsatility of plasma glucose and insulin secretion rate: circadian and sleep modulation. Horm Res. 1998;49(3–4):185–190. doi: 10.1159/000023169. [DOI] [PubMed] [Google Scholar]
- 17.Nofzinger EA, Buysse DJ, Miewald JM, et al. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain. 2002 May;125(Pt 5):1105–1115. doi: 10.1093/brain/awf103. [DOI] [PubMed] [Google Scholar]
- 18.Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during sleep-wake cycle in man determined by positron emission tomography and [18F]2-fluoro-2-deoxy-D-glucose method. Brain Res. 1990;513:136–143. doi: 10.1016/0006-8993(90)91099-3. [DOI] [PubMed] [Google Scholar]
- 19.Boyle PJ, Scott JC, Krentz AJ, Nagy RJ, Comstock E, Hoffman C. Diminished brain glucose metabolism is a significant determinant for falling rates of systemic glucose utilization during sleep in normal humans. J Clin Invest. 1994;93:529–535. doi: 10.1172/JCI117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchsbaum MS, Gillin JC, Wu J, et al. Regional cerebral glucose metabolic rate in human sleep assessed by positron emission tomography. Life Sci. 1989;45:1349–1356. doi: 10.1016/0024-3205(89)90021-0. [DOI] [PubMed] [Google Scholar]
- 21.Maquet P, Dive D, Salmon E, et al. Cerebral glucose utilization during stage 2 sleep in man. Brain Res. 1992;571:149–153. doi: 10.1016/0006-8993(92)90522-b. [DOI] [PubMed] [Google Scholar]
- 22.Van Cauter E, Blackman JD, Roland D, Spire JP, Refetoff S, Polonsky KS. Modulation of glucose regulation and insulin secretion by circadian rhythmicity and sleep. J Clin Invest. 1991;88:934–942. doi: 10.1172/JCI115396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 24.Garcia G, Freeman R, Supiano M, Smith M, Galecki A, Halter J. Glucose metabolism in older adults: a study including subjects more than 80 years of age. J Am Geriatr Soc. 1997;45:813–817. doi: 10.1111/j.1532-5415.1997.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 25.Prigeon RL, Kahn SE, Porte D., Jr Changes in insulin sensitivity, glucose effectiveness, and B-Cell function in regularly exercising subjects. Metabolism. 1995;44:1259–1263. doi: 10.1016/0026-0495(95)90026-8. [DOI] [PubMed] [Google Scholar]
- 26.Bergman RN. Toward physiological understanding of glucose tolerance. Minimal model approach. Diabetes. 1989;38:1512–1527. doi: 10.2337/diab.38.12.1512. [DOI] [PubMed] [Google Scholar]
- 27.Kahn S, Prigeon R, McCulloch D, et al. Quantification of the relationship between insulin sensitivity and B-cell function in human subjects: evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 28.Catalano P, Tyzbir E, Wolfe R, et al. Carbohydrate metabolism during pregnancy in control subjects and women with gestational diabetes. Am J Physiol (Endocrinol Metab 27) 1993;264(27):E60–E67. doi: 10.1152/ajpendo.1993.264.1.E60. [DOI] [PubMed] [Google Scholar]
- 29.Plat L, Byrne MM, Sturis J, et al. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol. 1996;270:E36–E42. doi: 10.1152/ajpendo.1996.270.1.E36. [DOI] [PubMed] [Google Scholar]
- 30.Plat L, Féry F, L'Hermite-Balériaux M, Mockel J, Van Cauter E. Metabolic effects of short-term physiological elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84:3082–3092. doi: 10.1210/jcem.84.9.5978. [DOI] [PubMed] [Google Scholar]
- 31.Mander BA, Colecchia E, Spiegel KS, Van Cauter E. Short Sleep: a risk factor for insulin resistance and obesity. Sleep. 2001;24(supplement):A74–75. [Google Scholar]
- 32.Gale SM, Castracane VD, Mantzoros CS. Energy homeostasis, obesity and eating disorders: recent advances in endocrinology. J Nutr. 2004 Feb;134(2):295–298. doi: 10.1093/jn/134.2.295. [DOI] [PubMed] [Google Scholar]
- 33.Yildiz BO, Suchard MA, Wong ML, McCann SM, Licinio J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc Natl Acad Sci U S A. 2004 Jul 13;101(28):10434–10439. doi: 10.1073/pnas.0403465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Lely A, Tschop M, Heiman M, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 35.Sakurai T. Roles of orexin/hypocretin in regulation of sleep/wakefulness and energy homeostasis. Sleep Med Rev. 2005 Aug;9(4):231–241. doi: 10.1016/j.smrv.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 36.Mullington JM, Chan JL, Van Dongen HP, et al. Sleep loss reduces diurnal rhythm amplitude of leptin in healthy men. J Neuroendocrinol. 2003;15(9):851–854. doi: 10.1046/j.1365-2826.2003.01069.x. [DOI] [PubMed] [Google Scholar]
- 37.Spiegel K, Leproult R, L'Hermite-Baleriaux M, Copinschi G, Penev P, Van Cauter E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 38.Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005 Nov;99(5):2008–2019. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 39.Chin-Chance C, Polonsky KS, Schoeller D. Twenty-four hour leptin levels respond to cumulative short-term energy imbalance and predict subsequent intake. J Clin Endocrinol Metab. 2000;85:2685–2691. doi: 10.1210/jcem.85.8.6755. [DOI] [PubMed] [Google Scholar]
- 40.Spiegel K, Tasali E, Penev P, Van Cauter E. Sleep curtailment in healthy young men is associated with decreased leptin levels, elevated ghrelin levels and increased hunger and appetite. Ann Intern Med. 2004;141(11):846–850. doi: 10.7326/0003-4819-141-11-200412070-00008. [DOI] [PubMed] [Google Scholar]
- 41.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Medicine. 2004;1(3):e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Littman AJ, Vitiello MV, Foster-Schubert K, et al. Sleep, ghrelin, leptin and changes in body weight during a 1-year moderate-intensity physical activity intervention. Int J Obes (Lond) 2006 Aug 15; doi: 10.1038/sj.ijo.0803438. [DOI] [PubMed] [Google Scholar]
- 43.Chaput JP, Despres JP, Bouchard C, Tremblay A. Short sleep duration is associated with reduced leptin levels and increased adiposity: Results from the Quebec family study. Obesity (Silver Spring) 2007 Jan;15(1):253–261. doi: 10.1038/oby.2007.512. [DOI] [PubMed] [Google Scholar]
- 44.Rechtschaffen A, Bergmann BM. Sleep deprivation in the rat by the disk-over-water method. Behav Brain Res. 1995;69(1–2):55–63. doi: 10.1016/0166-4328(95)00020-t. [DOI] [PubMed] [Google Scholar]
- 45.Dinges D, Chugh D. Physiological correlates of sleep deprivation. In: Kinney J, Tucker H, editors. Physiology, Stress, and Malnutrition: Functional Correlates, Nutritional Intervention. Philadelphia, PA: Lippincott Williams & Wilkins; 1997. p. 668. [Google Scholar]
- 46.Imaki M, Hatanaka Y, Ogawa Y, Yoshida Y, Tanada S. An epidemiological study on relationship between the hours of sleep and life style factors in Japanese factory workers. J Physiol Anthropol Appl Human Sci. 2002 Mar;21(2):115–120. doi: 10.2114/jpa.21.115. [DOI] [PubMed] [Google Scholar]
- 47.Ayas NT, White DP, Al-Delaimy WK, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003 Feb;26(2):380–384. doi: 10.2337/diacare.26.2.380. [DOI] [PubMed] [Google Scholar]
- 48.Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004 Jan;27(1):282–283. doi: 10.2337/diacare.27.1.282. [DOI] [PubMed] [Google Scholar]
- 49.Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004 Oct;27(10):2464–2469. doi: 10.2337/diacare.27.10.2464. [DOI] [PubMed] [Google Scholar]
- 50.Mallon L, Broman JE, Hetta J. High incidence of diabetes in men with sleep complaints or short sleep duration: a 12-year follow-up study of a middle-aged population. Diabetes Care. 2005 Nov;28(11):2762–2767. doi: 10.2337/diacare.28.11.2762. [DOI] [PubMed] [Google Scholar]
- 51.Bjorkelund C, Bondyr-Carlsson D, Lapidus L, et al. Sleep disturbances in midlife unrelated to 32-year diabetes incidence: the prospective population study of women in Gothenburg. Diabetes Care. 2005 Nov;28(11):2739–2744. doi: 10.2337/diacare.28.11.2739. [DOI] [PubMed] [Google Scholar]
- 52.Meisinger C, Heier M, Loewel H. Sleep disturbance as a predictor of type 2 diabetes mellitus in men and women from the general population. Diabetologia. 2005 Feb;48(2):235–241. doi: 10.1007/s00125-004-1634-x. [DOI] [PubMed] [Google Scholar]
- 53.Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006 Mar;29(3):657–661. doi: 10.2337/diacare.29.03.06.dc05-0879. [DOI] [PubMed] [Google Scholar]
- 54.Vioque J, Torres A, Quiles J. Time spent watching television, sleep duration and obesity in adults living in Valencia, Spain. Int J Obes Relat Metab Disord. 2000;24(12):1683–1688. doi: 10.1038/sj.ijo.0801434. [DOI] [PubMed] [Google Scholar]
- 55.Shigeta H, Shigeta M, Nakazawa A, Nakamura N, Yoshikawa T. Lifestyle, obesity, and insulin resistance. Diabetes Care. 2001 Mar;24(3):608. doi: 10.2337/diacare.24.3.608. [DOI] [PubMed] [Google Scholar]
- 56.Vorona R, Winn M, Babineau T, Eng B, Feldman H, Ware J. Overweight and Obese Patients in a Primary Care Population Report Less Sleep Than Patients With a Normal Body Mass Index. Arch Intern Med. 2005;165:25–30. doi: 10.1001/archinte.165.1.25. [DOI] [PubMed] [Google Scholar]
- 57.Singh M, Drake CL, Roehrs T, Hudgel DW, Roth T. The association between obesity and short sleep duration: A population-based study. Journal of Clinical Sleep Medicine. 2005;1(4):357–363. [PubMed] [Google Scholar]
- 58.Cournot M, Ruidavets JB, Marquie JC, Esquirol Y, Baracat B, Ferrieres J. Environmental factors associated with body mass index in a population of Southern France. Eur J Cardiovasc Prev Rehabil. 2004 Aug;11(4):291–297. doi: 10.1097/01.hjr.0000129738.22970.62. [DOI] [PubMed] [Google Scholar]
- 59.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 60.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27(3):440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 61.Kohatsu ND, Tsai R, Young T, et al. Sleep duration and body mass index in a rural population. Arch Intern Med. 2006 Sep 18;166(16):1701–1705. doi: 10.1001/archinte.166.16.1701. [DOI] [PubMed] [Google Scholar]
- 62.Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate Sleep as a Risk Factor for Obesity: Analyses of the NHANES I. Sleep. 2005;28(10):1289–1296. doi: 10.1093/sleep/28.10.1289. [DOI] [PubMed] [Google Scholar]
- 63.Hasler G, Buysse D, Klaghofer R, et al. The Association Between Short Sleep duration and Obesity in Young Adults: a 13-Year Prospective Study. Sleep. 2004;27(4):661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 64.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between Reduced Sleep and Weight Gain in Women. Am J Epidemiol. 2006 Nov 15;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Locard E, Mamelle N, Billette A, Miginiac M, Munoz F, Rey S. Risk factors of obesity in a five year old population. Parental versus environmental factors. International Journal of Obesity & Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1992;16(10):721–729. [PubMed] [Google Scholar]
- 66.von Kries R, Toschke AM, Wurmser H, Sauerwald T, Koletzko B. Reduced risk for overweight and obesity in 5– and 6-y-old children by duration of sleep--a cross-sectional study. Int J Obes Relat Metab Disord. 2002;26(5):710–716. doi: 10.1038/sj.ijo.0801980. [DOI] [PubMed] [Google Scholar]
- 67.Sekine M, Yamagami T, Handa K, et al. A dose-response relationship between short sleeping hours and childhood obesity: results of the Toyama Birth Cohort Study. Child Care, Health and Development. 2002;28(2):163–170. doi: 10.1046/j.1365-2214.2002.00260.x. [DOI] [PubMed] [Google Scholar]
- 68.Chaput JP, Brunet M, Tremblay A. Relationship between short sleeping hours and childhood overweight/obesity: results from the 'Quebec en Forme' Project. Int J Obes (Lond) 2006 Mar 14; doi: 10.1038/sj.ijo.0803291. [DOI] [PubMed] [Google Scholar]
- 69.Agras WS, Hammer LD, McNicholas F, Kraemer HC. Risk factors for childhood overweight: a prospective study from birth to 9.5 years. J Pediatr. 2004 Jul;145(1):20–25. doi: 10.1016/j.jpeds.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 70.Reilly J, Armstrong J, Dorosty A, et al. Early life risk factors for obesity in childhood: cohort study. Br Med J. 2005 doi: 10.1136/bmj.38470.670903.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr. 2005 Dec;147(6):830–834. doi: 10.1016/j.jpeds.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 72.Gupta NK, Mueller WH, Chan W, Meininger JC. Is obesity associated with poor sleep quality in adolescents? American Journal of Human Biology. 2002;14(6):762–768. doi: 10.1002/ajhb.10093. [DOI] [PubMed] [Google Scholar]


