Abstract
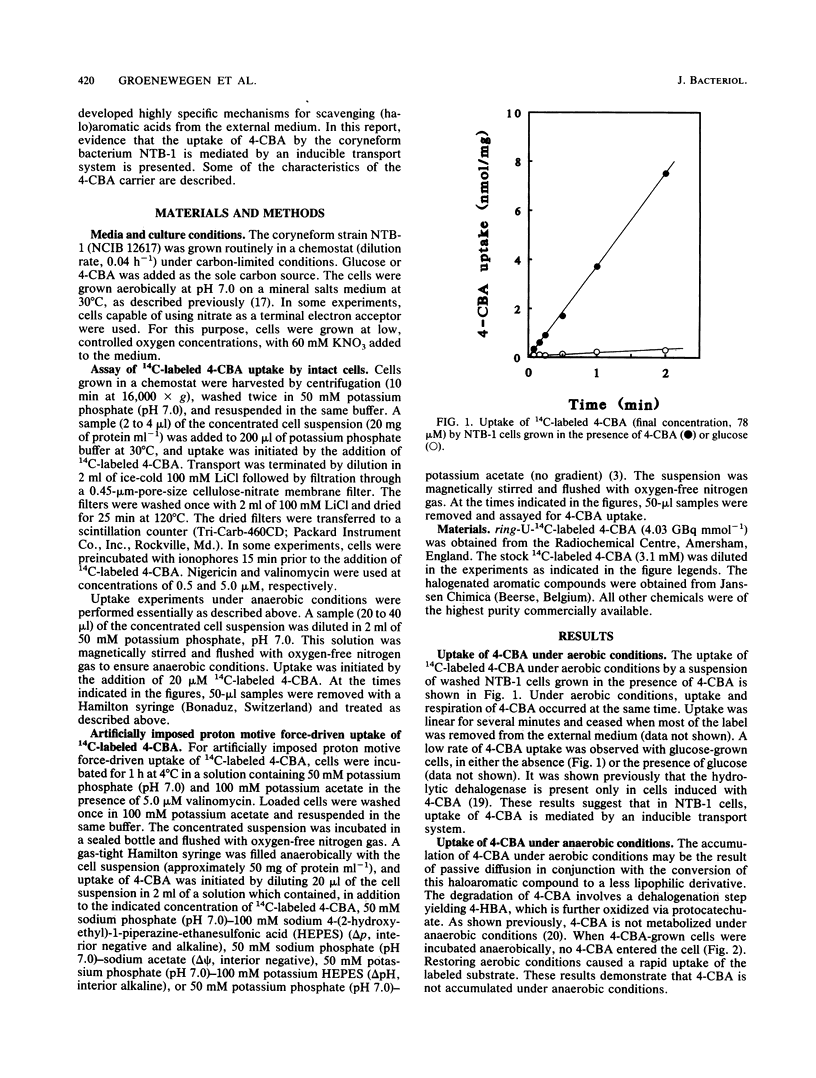
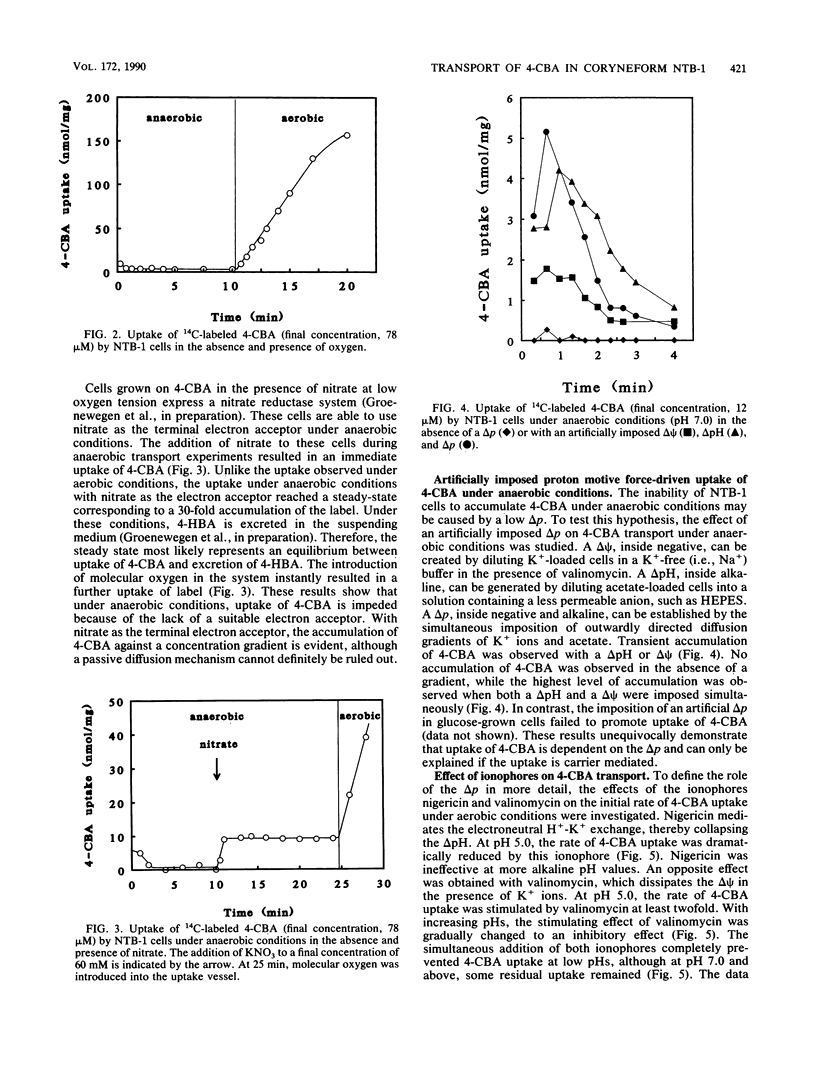
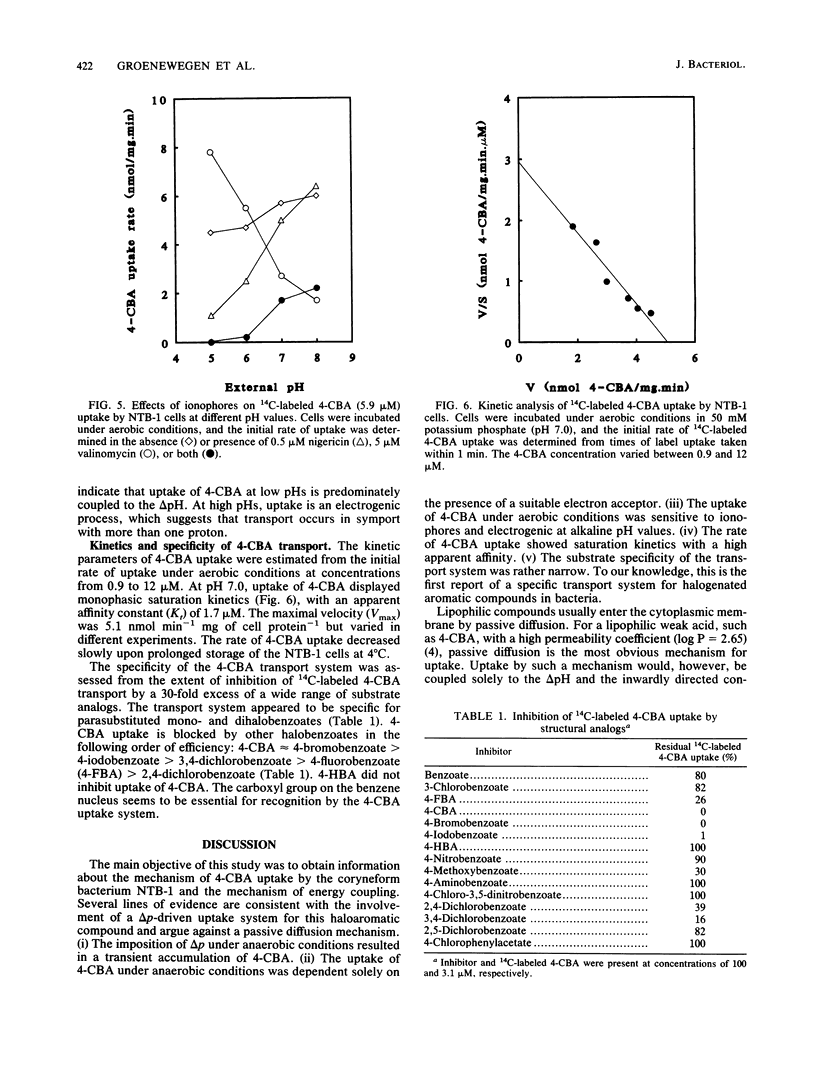
The uptake of 4-chlorobenzoate (4-CBA) in intact cells of the coryneform bacterium NTB-1 was investigated. Uptake and metabolism of 4-CBA were observed in cells grown in 4-CBA but not in glucose-grown cells. Under aerobic conditions, uptake of 4-CBA occurred with a high apparent affinity (apparent Kt, 1.7 microM) and a maximal velocity (Vmax) of 5.1 nmol min-1 mg of protein-1. At pH values below 7, the rate of 4-CBA uptake was greatly reduced by nigericin, an ionophore which dissipates the pH gradient across the membrane (delta pH). At higher pH values, inhibition was observed only with valinomycin, an ionophore which collapses the electrical potential across the membrane (delta psi). Under anaerobic conditions, no uptake of 4-CBA was observed unless an alternative electron acceptor was present. With nitrate as the terminal electron acceptor, 4-CBA was rapidly accumulated by the cells to a steady-state level, at which uptake of 4-CBA was balanced by excretion of 4-hydroxybenzoate. The mechanism of energy coupling to 4-CBA transport under anaerobic conditions was further examined by the imposition of an artificial delta psi, delta pH, or both. Uptake of 4-CBA was shown to be coupled to the proton motive force, suggesting a proton symport mechanism. Competition studies with various substrate analogs revealed a very narrow specificity of the 4-CBA uptake system. This is the first report of carrier-mediated transport of halogenated aromatic compounds in bacteria.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apajalahti J. H., Salkinoja-Salonen M. S. Dechlorination and para-hydroxylation of polychlorinated phenols by Rhodococcus chlorophenolicus. J Bacteriol. 1987 Feb;169(2):675–681. doi: 10.1128/jb.169.2.675-681.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Fewson C. A. Evidence for specific transport mechanisms for aromatic compounds in bacterium N.C.I.B. 8250. Biochim Biophys Acta. 1972 Dec 1;290(1):384–388. doi: 10.1016/0005-2736(72)90081-8. [DOI] [PubMed] [Google Scholar]
- Driessen A. J., Kodde J., de Jong S., Konings W. N. Neutral amino acid transport by membrane vesicles of Streptococcus cremoris is subject to regulation by internal pH. J Bacteriol. 1987 Jun;169(6):2748–2754. doi: 10.1128/jb.169.6.2748-2754.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler J. F., Harwood C. S., Gibson J. Purification and properties of benzoate-coenzyme A ligase, a Rhodopseudomonas palustris enzyme involved in the anaerobic degradation of benzoate. J Bacteriol. 1988 Apr;170(4):1709–1714. doi: 10.1128/jb.170.4.1709-1714.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood C. S., Gibson J. Uptake of benzoate by Rhodopseudomonas palustris grown anaerobically in light. J Bacteriol. 1986 Feb;165(2):504–509. doi: 10.1128/jb.165.2.504-509.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. II. Isolation and properties of blocked mutants. J Bacteriol. 1966 Mar;91(3):1155–1160. doi: 10.1128/jb.91.3.1155-1160.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins S. J., Mandelstam J. Evidence for induced synthesis of an active transport factor for mandelate in Pseudomonas putida. Biochem J. 1972 Feb;126(4):917–922. doi: 10.1042/bj1260917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara M., Macnab R. M. Cytoplasmic pH mediates pH taxis and weak-acid repellent taxis of bacteria. J Bacteriol. 1981 Mar;145(3):1209–1221. doi: 10.1128/jb.145.3.1209-1221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks T. S., Wait R., Smith A. R., Quirk A. V. The origin of the oxygen incorporated during the dehalogenation/hydroxylation of 4-chlorobenzoate by an Arthrobacter sp. Biochem Biophys Res Commun. 1984 Oct 30;124(2):669–674. doi: 10.1016/0006-291x(84)91607-3. [DOI] [PubMed] [Google Scholar]
- Meagher R. B., McCorkle G. M., Ornston M. K., Ornston L. N. Inducible uptake system for -carboxy-cis, cis-muconate in a permeability mutant of Pseudomonas putida. J Bacteriol. 1972 Aug;111(2):465–473. doi: 10.1128/jb.111.2.465-473.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkel S. M., Eberhard A. E., Gibson J., Harwood C. S. Involvement of coenzyme A thioesters in anaerobic metabolism of 4-hydroxybenzoate by Rhodopseudomonas palustris. J Bacteriol. 1989 Jan;171(1):1–7. doi: 10.1128/jb.171.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R., Thiele J., Klages U., Lingens F. Incorporation of [18O]water into 4-hydroxybenzoic acid in the reaction of 4-chlorobenzoate dehalogenase from pseudomonas spec. CBS 3. Biochem Biophys Res Commun. 1984 Oct 15;124(1):178–182. doi: 10.1016/0006-291x(84)90933-1. [DOI] [PubMed] [Google Scholar]
- Ornston L. N., Parke D. Properties of an inducible uptake system for beta-ketoadipate in Pseudomonas putida. J Bacteriol. 1976 Feb;125(2):475–488. doi: 10.1128/jb.125.2.475-488.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poolman B., Driessen A. J., Konings W. N. Regulation of solute transport in streptococci by external and internal pH values. Microbiol Rev. 1987 Dec;51(4):498–508. doi: 10.1128/mr.51.4.498-508.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimao M., Onishi S., Mizumori S., Kato N., Sakazawa C. Degradation of 4-Chlorobenzoate by Facultatively Alkalophilic Arthrobacter sp. Strain SB8. Appl Environ Microbiol. 1989 Feb;55(2):478–482. doi: 10.1128/aem.55.2.478-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer J. R., Wheelis M. L. Active transport of benzoate in Pseudomonas putida. J Gen Microbiol. 1982 Aug;128(8):1749–1753. doi: 10.1099/00221287-128-8-1749. [DOI] [PubMed] [Google Scholar]
- Thayer J. R., Wheelis M. L. Characterization of a benzoate permease mutant of Pseudomonas putida. Arch Microbiol. 1976 Oct 11;110(1):37–42. doi: 10.1007/BF00416966. [DOI] [PubMed] [Google Scholar]
- Wedemeyer G. Uptake of 2,4-dichlorophenoxyacetic acid by Pseudomonas fluorescens. Appl Microbiol. 1966 Jul;14(4):486–491. doi: 10.1128/am.14.4.486-491.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Tweel W. J., Kok J. B., de Bont J. A. Reductive dechlorination of 2,4-dichlorobenzoate to 4-chlorobenzoate and hydrolytic dehalogenation of 4-chloro-, 4-bromo-, and 4-iodobenzoate by Alcaligenes denitrificans NTB-1. Appl Environ Microbiol. 1987 Apr;53(4):810–815. doi: 10.1128/aem.53.4.810-815.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



