Abstract
Exocrine cells have an essential function of sorting secreted proteins into the correct secretory pathway. A clear understanding of sorting in salivary glands would contribute to the correct targeting of therapeutic transgenes. The present work investigated whether there is a change in the relative proportions of basic PRP and acidic PRPs in secretory granules in response to chronic isoproterenol treatment, and whether this alters the sorting of endogenous cargo proteins. Immunoblotting of secretory granules from rat parotids found a large increase of basic PRP over acidic PRPs in response to chronic isoproterenol treatment. Pulse chase experiments demonstrated that isoproterenol also decreased regulated secretion of newly synthesized secretory proteins, including PRPs, amylase and parotid secretory protein. This decreased efficiency of the apical regulated pathway may be mediated by alkalization of the secretory granules since it was reversed by treatment with mild acid. We also investigated changes in secretion through the basolateral (endocrine) pathways. A significant increase in parotid secretory protein and salivary amylase was detected in sera of isoproterenol-treated animals suggesting increased routing of the regulated secretory proteins to the basolateral pathway. These studies demonstrate that shifts of endogenous proteins can modulate regulated secretion and sorting of cargo proteins.
Keywords: amylase, parotid secretory protein, polarized secretion, parotid gland, sorting
INTRODUCTION
The parotid salivary gland contains acinar cells which are effective factories for regulated secretion of proteins, such as amylase and parotid secretory protein (PSP), which contribute to digestion and anti-bacterial activities of saliva. Secreted proteins are predominantly stored at high concentrations in dense core secretory granules of both endocrine and exocrine glands (15, 16, 34, 40). Multiple post-Golgi pathways exist for the intracellular trafficking of these secretory proteins. The major pathway is the regulated secretory pathway wherein the cargo proteins (e.g., hormones or digestive enzymes) are secreted in response to external stimuli. Alternatively, the constitutive pathway, which is common to all eukaryotic cells, secretes proteins in the absence of any stimulation (34). Both pathways originate in the trans-Golgi network. In addition, a minor-regulated pathway and a constitutive-like pathway, originating from the maturing secretory granules, are also present in the exocrine salivary acinar cells (2, 4, 7, 17). In the parotid gland, regulated secretion targets the cargo proteins to the apical cell surface and into the oral cavity while the constitutive secretory pathways allow secretion from both the apical (into the oral cavity) and basolateral (into the circulation) cell surfaces (25).
Delivery of the cargo proteins to specific post-Golgi vesicles involves processes collectively termed protein sorting (2, 34, 44). Sorting most likely involves either the selective entry (9) or retention (24, 25) of regulated cargo proteins into the nascent secretory granule. Several sorting signals and other mechanisms appear to be involved for different cargo proteins and cell types (23, 24). While calcium induced aggregation is a common theme for protein storage in granules of endocrine and exocrine cells (22, 35, 45, 46), such a mechanism is lacking in the storage of regulated secretory proteins of the parotid gland (47). However, pH has been shown to play a role in regulated secretion of proteins from the parotid gland (48, 49). Indeed, mild alkalization by addition of weak bases reduces the regulated secretion of the cargo proteins (38, 49).
The parotid and submandibular glands have been suggested to be opportune sites for production of therapeutic transgenic peptides (6, 20, 30, 33, 51). Several regulated and constitutive proteins such as human growth hormone, human α1 anti-trypsin, kallikrein, and human erythropoietin have been expressed in salivary glands (5, 20, 27, 29, 33, 50). However, proteins that are normally secreted in an endocrine fashion, when placed into the salivary gland enter the regulated secretory pathway into the oral cavity, which is not therapeutically useful (5, 51). Hence, therapeutic peptides from foreign genes would need to be routed to the correct secretory pathway (i.e., basal secretion to the blood, or apical regulated secretion to the oral cavity). Therefore, it is important to define the molecular mechanisms regulating sorting of secreted proteins in the parotid.
We have shown that a decrease of proteoglycans decreases storage of multiple cargo proteins in the parotid granule (48). This may be related to analogous mechanisms in which heparin proteoglycan is necessary for gastric mast cell secretion (18, 31). The core proteins of the sulfated proteoglycans are acidic proline rich proteins (PRPs; PRPg1 (GI:27544948) and PRPg2 (GI:25453417)) (14). In contrast to these, basic PRP (GI:206393) is not sulfated, but is predominantly present as a glycosylated form, in addition to a minor non-glycosylated form (13). Transfected basic PRPs enhance glycosylation of secreted proteoglycans, such as acidic PRPs. Furthermore, the anionic glycosaminoglycan (GAG) side chains of PRPs are important for normal regulated secretion of that protein (13), as well as other cargo proteins (amylase and PSP) in the parotid (48). It has been suggested that the anionic sulfated proteoglycans might serve to balance the charges of cationic cargo proteins (8, 13, 14), possibly by modulation of granule pH rather than a direct interaction with other granule components (48).
We hypothesize that the relative expression of PRPs (i.e., the ratio of basic PRP to acidic PRPs) may play an important role in the storage and regulated secretion of other proteins. PRP expression increases with chronic treatment of rats with the β-adrenergic agonist isoproterenol (Ipr) (32, 39, 42), however, the relative changes of basic and acidic PRPs have not been defined. Further, we have used this model system to investigate the effects of increased PRP on the processes of regulated secretion and sorting. We find that increased basic PRP expression decreases the efficiency of stimulated secretion from the parotid through a pH-sensitive mechanism, and increases cargo protein (amylase and PSP) secretion in the basolateral pathway.
MATERIALS AND METHODS
Isoproterenol treatment of rats and parotid tissue culture
Animal use was approved by the Institutional Animal Care and Use Committee at the University of Louisville. Male Sprague-Dawley rats (280–300 g) were treated daily with intraperitoneal injections of dl-isoproterenol (0.02 mg/g body weight) in 0.3 ml of saline for 10 days. This produces a short exposure to isoproterenol which is rapidly cleared from the body (28). Control rats received the same amount of saline alone. Control and Ipr-treated rats were euthanized by carbon-dioxide asphyxiation 24 h after the last injection. The parotid glands were surgically removed, cleaned of fat and connective tissue and placed in Krebs Ringer HEPES buffer (KRH; 10 mM HEPES, 129 mM NaCl, 5 mM Na2CO3, 4.8 mM KCl, 1.2 mM K2HPO4, 1.2 mM MgCl2, 1 mM CaCl2 and 2.8 mM glucose, pH 7.4) under constant oxygenation. The tissue was sliced into 0.5 mm slices using a Stadie-Riggs tissue slicer and the weight noted.
[3H]proline PRP pulse-chase radiolabeling
For [3H]proline radiolabeling of proline rich proteins (PRPs), parotid tissue slices from control and Ipr-treated rats were washed for 30 min with KRH and pulse-labeled for 2 h in 1 ml of KRH containing 200 μCi [3H]proline/ml at 37 C in a shaker water bath (48). All incubation media were oxygenated every 10 min. The pulse labeling medium was replaced with Dulbecco’s modified Eagles medium (DMEM) and the tissue washed for 30 min. Chase incubation in DMEM containing 1 mM cold proline was continued for 1 h, after which basal and stimulated secretion were measured sequentially in each culture. In order to measure secretion in the absence of any secretagogue (basal secretion), the tissue was incubated for 30 min in DMEM alone and the medium collected. For all in vitro secretion experiments, “basal secretion” is operationally defined as secretion in the absence of a secretagogue, and therefore does not refer to a particular secretion pathway since it includes several constitutive pathways (25). Stimulated secretion was measured in DMEM with 30 μM of the β-adrenergic agonist isoproterenol (Sigma, St. Louis) and the medium collected after 30 min. The basal and stimulated media were centrifuged at 16,000 × g for 15 min and the supernatants analyzed by SDS-PAGE. The entire lane was cut into 2 mm slices, and radioactivity was quantified across each lane by scintillation counting of all gel slices and summing the points included in the PRP peak, as previously described (21). For calculating the % PRP secreted, the total counts included the cpm in the PRP peak from basal media plus stimulated media plus in the tissue homogenate.
[3H]leucine pulse-chase radiolabeling
Newly synthesized proteins were labeled with [3H]leucine as described (48). Both PSP and amylase contain abundant leucine residues. Briefly, parotid tissue slices from either control or Ipr-treated rats was incubated in 1 ml of KRH with 100 μCi [3H]leucine for 2 h at 37 C in a shaker water bath. All incubation media were oxygenated every 10 min throughout the experiment. In order to directly compare the effects of isoproterenol treatment on secretion of newly synthesized proteins with that of the inhibition of proteoglycan synthesis, control tissue was also labeled in the presence of the proteoglycan synthesis inhibitor, p-nitrophenyl xylopyranoside (pNPX), as described (48). The tissue was washed for 30 min with DMEM and then chase incubated for 1 h in DMEM with 1 mM cold leucine. Following the chase incubation, the tissue was incubated for 30 min in DMEM with cold 1 mM leucine and the basal secretion medium collected. Subsequently, stimulated secretion was collected in medium containing 30 μM isoproterenol for 30 min. The secretion media along with the tissue homogenates were analyzed by SDS-PAGE as above. Secretion of either amylase or PSP was quantified as the radioactivity included within the peak for that protein (48). In experiments testing the effects of pH, 10 mM acetic acid was included in the [3H]leucine-labeling and all subsequent incubations, and compared to concurrent parallel incubations without acetic acid. For calculating the % secreted, the total counts included the cpm in a specific peak (amylase or PSP) in basal and stimulated media plus in the tissue homogenate.
Isolation of parotid and pancreatic secretory granules
Secretory granules were purified from the parotid glands of control and Ipr-treated rats by homogenization and differential centrifugation in sucrose solutions, as described (48). In addition to parotid granules, secretory granules were also purified from the pancreas of control rats (47). The purified granules were lysed by freeze/thaw in water, and the soluble contents (cargo proteins) separated from the membrane by centrifugation at 16,000 × g for 15 min. The pH of granules from control and Ipr-treated tissue, lysed in deionized water, was determined with a microelectrode. The protein content in the soluble protein fraction was determined by the Bradford-based Protein assay Kit (Pierce, Rockford IL) and equal amounts of protein loaded on SDS-PAGE gels. Western blot analysis was performed using antibodies to acidic or basic PRPs.
Gel electrophoresis and western blotting
Protein samples were separated by SDS-PAGE on 4–20% gradient gels (Life Gels, Clarkston, GA). The protein bands were visualized by staining with Gelcode Blue (Pierce, Rockford, IL) for 1 h followed by destaining in water. For immunoblotting, the unstained protein bands were transferred to polyvinylidene diflouride membranes (Bio-Rad, Hercules, CA). Membranes were blocked with 2% Tween 20 in TBST (Tris buffered saline, pH7.4 containing 0.05% Tween 20) for 10 min. They were then probed with either anti-rat basic PRP (1:1000) or anti-acidic PRP (1:5000) polyclonal antibodies (gifts from Drs. Anna and David Castle, UVA) or anti-rat PSP antibody (1:10,000; a gift from Dr. William Ball, Howard University) for 1 h. The membrane was washed with TBST and incubated in horseradish peroxidase-conjugated goat anti-rabbit IgG (1:5000; Chemicon) for 1 h. Immunoblots were processed using Supersignal West Pico-substrate (Pierce) and the signal visualized on a Kodak Imagestation. The bands were quantified using the Kodak 1D image analysis software. The antibody to basic PRP is specific, whereas the acidic PRP antibody can cross-react with the basic protein (8, 11), therefore, only appropriate sized bands (>33 kDa) were included when quantifying acidic PRPs on the western blots.
Collection and analysis of rat serum
Control and isoproterenol treated rats were euthanized and blood collected from the jugular vein. Blood was allowed to clot for 4 h at room temperature and overnight at 4 °C. The serum was separated by centrifugation at 500 × g. Total amylase activity in the serum was analyzed by the Phadebas amylase test kit (Pharmacia). Protein content in the serum was determined and equal amount of protein for the control and Ipr-treated sera was loaded for SDS-PAGE on 4–20% gels and probed for PSP by western blot analysis.
Separation of serum amylase isoenzymes
Serum samples were analyzed by agarose gel electrophoresis to separate amylase isoforms (36). Parotid and pancreatic granule extracts from control rats were run simultaneously to define the migration of the major isoforms. Electrophoresis was performed with 1% agarose gels in 50 mM Tris, 25 mM borate buffer, pH 8.7. Samples were loaded in wells at the center of the gel. Following electrophoresis, the gels were blotted for western analysis with anti-amylase antibody.
RESULTS
We sought to determine whether changes in storage within the regulated secretion pathway alter the efficiency of regulated secretion. We used a standard model for induction of PRPs in the parotid gland by the β-adrenergic agonist isoproterenol (8, 39). Daily Ipr treatment is expected to cause hypertrophy, which we confirmed histologically in these animals. The average length and width of the acini increased 103% and 73% respectively in response to treatment with Ipr. Hence, treatment of rats with isoproterenol for 10 days resulted in a significant (p<0.05) hypertrophy of the parotid glands, as expected (32, 42).
Western blot analysis was used to quantify the changes of both basic and acidic PRPs in response to Ipr. Secretory granules were isolated from parotid glands of control and Ipr-treated rats, and soluble cargo proteins were used for western blots probed with antibodies to acidic and basic PRP. Ipr caused a dramatic 18.6-fold increase in basic PRP stored in the granules (Fig. 1A). In contrast, the acidic PRP increased only 2.25-fold. This indicates not only a large increase of the basic PRP, but also a strong shift in the relative amounts of basic:acidic PRPs. This strong expression of basic PRP would be expected to contribute to the previously described increased pH of secretory granules from Ipr-treated rats (3). Consistent with this, we observed a significant (p<0.007) increase of pH in granules of Ipr-treated rats (pH 7.23) as compared to control rats (pH 6.67).
Figure 1.
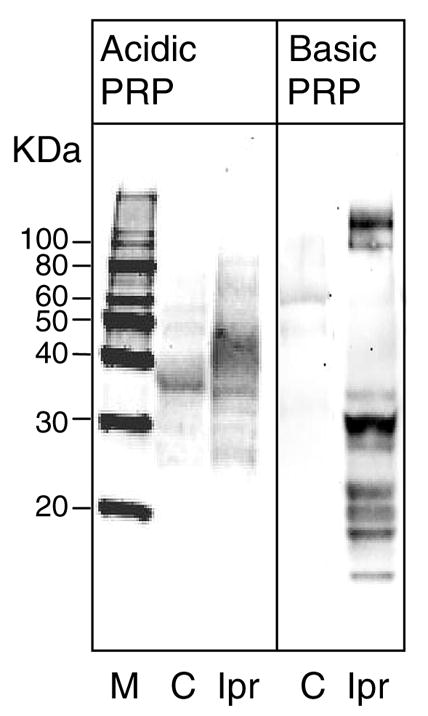

Isoproterenol treatment increases the (A) storage and (B) secretion of proline rich proteins in secretory granules. A. Parotid glands from control (C) and isoproterenol-treated (Ipr) rats were collected and secretory granules isolated. The granules were lysed and total protein in the extract determined. SDS-PAGE was run with 10 μg of protein, and proline rich proteins detected with either anti-acidic PRP (1:1000) or anti-basic PRP (1:10,000) by immunoblotting. Molecular size markers (M) are indicated on the left.
B. Parotid tissue from control and isoproterenol (Ipr) treated rats was collected and labeled with [3H]proline. The basal secretion medium was collected at the end of the chase incubation and the tissues then stimulated with isoproterenol to obtain the stimulated secretion medium. The basal (B) and stimulated (S) media along with the tissue homogenates were analyzed by SDS-PAGE followed by scintillation counting of the gel slices to determine the radioactivity incorporated into PRPs. The total cpm in the area under the peak representing newly synthesized PRPs was determined and the % PRP secreted was calculated ( (PRP cpm in basal or stimulated medium / total PRP cpm in basal media plus stimulated media plus tissue homogenate)×100). Data are the range of duplicates, and are representative of two independent experiments.
The increase in the synthesis of PRPs upon treatment with Ipr was also reflected in the overall secretion data. Secretion medium from [3H]proline labeling of treated and control parotid tissue, showed a clear increase of % PRPs in the basal as well as stimulated media (Fig 1B). Chronically Ipr-treated tissue (compared to control tissue) had a 4-fold increase of proline rich proteins in the basal media, and 3.2-fold increase in the stimulated secretion media. The efficiency of the regulated secretory pathway can be defined as the ‘fold stimulation,’ calculated as the % stimulated secretion over % basal secretion (48). The fold stimulation of PRP secretion in Ipr-treated cells (1.7) was decreased relative to control cells (2.04). The decreased regulated secretion may be caused by an over-abundance of the PRP cargo, since entry into the regulated secretion pathway may be saturable (29, 37). Alternatively, decreased regulated secretion may be due to alkalization of the granule by the increased ratio of basic:acidic PRPs. Mild alkalization of secretory granules has been shown to inhibit the regulated secretory pathway (29). Both these possible causes are addressed below.
Unlike the PRPs, the cargo proteins amylase and PSP decrease in concentration in chronically Ipr-stimulated parotid glands (3, 8, 32, 42, 43, 52). In agreement, we also observed a small decrease in total synthesis of labeled amylase (30% decrease) and PSP (44.8% decrease) after chronic Ipr treatment. Since they are not overexpressed in response to Ipr, they were used to test whether the increase in PRPs affects the trafficking of other cargo proteins. [3H]leucine pulse-chase experiments were performed with control and chronically Ipr-treated tissue (Fig. 2). The CPM for stimulated secretion of labeled PSP or amylase decreased in Ipr-treated cells, whereas the basal secretion increased (Fig. 2A). The normalized results from pooled experiments clearly show a significant shift in the fold stimulation (Fig. 2B). Secretion of amylase (expressed as the ratio of % stimulated to % basal) showed 2.5-fold stimulation for control parotid tissue, and 0.81-fold for Ipr-treated parotids (Fig. 2B). Similarly, secretion of PSP was 4.5-fold for control and 0.76 for Ipr-treated tissue (Fig. 2B). A fold-stimulation value of 1 or less indicates no secretion through the regulated pathway. Hence, chronic Ipr treatment dramatically and significantly decreased regulated secretion of both amylase and PSP. This occurred despite the fact that they are not overexpressed, indicating that chronic Ipr treatment can disrupt regulated secretion independent of overexpression of a cargo protein. As discussed below, this may represent increased routing of PSP and amylase into other pathways such as the constitutive basolateral pathways.
Figure 2.


Ipr treatment decreases in vitro regulated secretion of amylase or PSP. A. Newly synthesized secretory granule proteins in parotid tissue from control and chronically Ipr-treated rats was labeled with [3H]leucine. Proteins were also labeled in control tissue incubated in the presence of pNPX. The basal (B) and stimulated (S) media were collected and radioactivity (CPM) in secreted amylase and PSP determined by SDS-PAGE followed by scintillation counting of the gel slices. Data presented are mean ± SD from duplicate samples of a typical experiment. B. Parotid tissue from control (−) and Ipr-treated (+) animals was incubated with [3H]leucine to label newly synthesized cargo proteins. Control tissue was also incubated in the presence of pNPX (+). The media and cells were analyzed on SDS-PAGE followed by scintillation counting of the gel slices to determine radioactivity incorporated into peaks representing newly synthesized amylase or PSP. Data presented are mean ± SEM from three independent experiments performed in duplicate. The % stimulated secretion (for amylase) was calculated as the cpm in amylase in the stimulated medium divided by total counts (which included the cpm in basal medium plus stimulated medium plus in the tissue homogenate), as described in the legend to Figure 1B for PRP. A similar calculation was done for the % basal secretion, or for PSP. Fold stimulation = (% stimulated secretion / % basal secretion). *P<0.05 compared to control.
We compared the Ipr-response to treatment of parotid tissue with pNPX (Fig. 2). The glycoside pNPX blocks addition of the sulfated sugars to acidic PRP, thereby shifting the balance between acidic and basic forms of PRPs without overexpression of basic PRP. We find that the loss of regulated secretion is similar to that seen in response to chronic Ipr-treatment of the rats, for both amylase and PSP (Fig. 2). pNPX-treated tissue showed no increased secretion when stimulated. The similar decrease of regulated secretion of cargo proteins is consistent with the possibility that alkalization of the granule is an important mechanism in both cases, as previously described for pNPX treatment (48).
Next, we directly tested the hypothesis that the observed decreased efficiency of regulated secretion was mediated by alkalization of the secretory pathway. We conducted [3H]leucine pulse-chase secretion experiments with control and Ipr-treated tissue with, or without, 10 mM acetic acid, as previously described (48). Addition of acid to the incubation medium lowers the pH from 7.4 to 6.4. This is analogous to earlier work which examined the effects of alkalization by addition of mild bases (hydroxychloroquine, NH4Cl) (29, 49). Figure 3 shows that acetic acid treatment of control tissue had no significant effect on regulated amylase secretion. A similar lack of effect was observed for PSP. Hence, mild acid treatment does not affect sorting in control tissue. Ipr-treated tissue exhibited decreased regulated secretion of both amylase and PSP, consistent with the results shown in Figure 2. However, under mildly acidifying conditions stimulated secretion of amylase and PSP increased, only in Ipr-treated tissue. Hence, the effect of chronic Ipr treatment was largely reversed by acid treatment, consistent with the idea that alkalization of the granule is a part of the mechanism by which Ipr treatment decreases regulated secretion.
Figure 3.
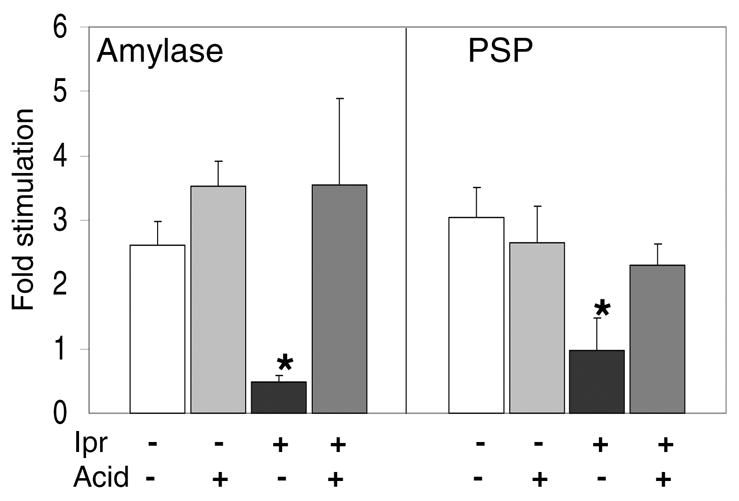
Weak acid partially reverses the decreased stimulated secretion of amylase and PSP in Ipr-treated tissue. Rat parotid tissue from control (−) and Ipr-treated (+) animals was labeled with [3H]leucine in the presence (+) or absence (−) of 10 mM acetic acid. Following chase-incubation, the basal media was collected and the tissue stimulated with isoproterenol. The media and tissue homogenates were analyzed by SDS-PAGE and scintillation counting of gel slices. Fold stimulation was calculated as described in Fig. 2. Data presented are the mean ± SEM (n= 4 – 5). *P<0.05, different from untreated control.
We next addressed the question of whether inhibition of the regulated secretion pathway correlated with a change in the sorting of cargo proteins into a different pathway. To determine if proteins were re-routed in vivo to the basolateral (circulatory) pathway, we analyzed serum samples collected from control and chronically Ipr-treated rats for the presence of endogenous PSP and salivary amylase. Serum samples were analyzed by SDS-PAGE followed by western analysis for PSP. No PSP immunoreactivity was detectable in the sera of 19 out of 20 control rats, even with long exposures (Fig. 4). In contrast, PSP is strongly expressed in serum from 12 of 14 Ipr-treated rats. In the single control rat which showed serum PSP, the concentration was 12.8-fold lower than the Ipr-treated samples. Hence, in the Ipr-treated rats, PSP was present in the serum. This suggests that there is enhanced endocrine (basolateral) secretion in the Ipr-treated rats. Since we have found a decrease of the regulated secretion pathway (Fig. 2), and we and others have found decreased synthesis of PSP in response to chronic Ipr, this increased endocrine secretion indicates a change of the sorting of PSP.
Figure 4.

Chronic isoproterenol treatment causes re-routing of PSP to endocrine secretion in vivo. Blood was collected from control (−) and isoproterenol-treated (+) rats and the serum fraction collected. The protein concentration in the serum was determined, and rat serum (250 μg; about 8 μL) were loaded on SDS-PAGE gels and probed with the anti-rat PSP antibody by western blotting. Similar results were observed with 20 control rats and 14 Ipr-treated rats. Molecular size markers are indicated.
To determine whether there was a shift in the sorting of another cargo protein, amylase activity was measured directly in serum samples. Despite the consistent presence of amylase activity in control rat sera, a small significant (P<0.05) increase in amylase activity was found in Ipr-treated rat serum (Fig. 5). Pancreatic amylase (Genbank # NM031502 ) is closely related to salivary amylase (Genbank # NM001010970) and can be present in serum as a marker of pancreatic cancer. To identify the source of amylase in the serum of Ipr-treated rats, we separated the isoforms of the enzyme on non-denaturing agarose gels (36), followed by western blots with anti-amylase. Fig. 6 shows that pancreatic and parotid amylase move in opposite directions when separated on agarose gels at pH 8.7 (36). Sera from all control rats show a band corresponding to salivary amylase, indicating that under normal conditions some salivary amylase is routed through a basolateral pathway. Sera collected from Ipr-treated rats show an increase in the salivary isoform of amylase (Fig. 6). These results demonstrate an increase of PSP in the basolateral secretion pathways in response to chronic Ipr treatment, as well as a small but significant increase of salivary amylase.
Figure 5.

Isoproterenol treatment increases amylase activity in blood. Blood was collected from the jugular vein of control (−) and isoproterenol treated (Ipr-treated, +) rats and the serum isolated. Amylase activity in the serum samples was determined by the Phadebas assay. Data presented are mean ± SEM from experiments with 21 control and 16 Ipr-treated rats. *P<0.01.
Figure 6.

Isoproterenol-treatment increases the parotid amylase in serum. A: Parotid (Par) and pancreatic (Pan) granule extracts, and serum samples from control (C) and Ipr-treated (Ipr) rats were separated on borate agarose gels and probed with antibody to amylase by western blotting. Pancreatic amylase shows a cathodic movement while parotid amylase moves towards the anode. Sera from Ipr-treated rats contain immunoreactive amylase that co-migrates with parotid salivary amylase. Ipr-treated rat serum demonstrates an increase of amylase immunoreactivity. The figure is representative of 4 independent experiments. The cathode (−) is towards the top of the figure. The arrow (O, origin) indicates the position of the wells.
DISCUSSION
PRPs make up about 15% of protein in normal saliva, and over 60% in saliva of rats chronically treated with the β-adrenergic agonist isoproterenol (32, 39). Parotid glands from these rats exhibit more translucent granules suggesting that an excess of PRPs correlates with less granule condensation (10). Initial reports demonstrated an increase of basic PRPs in response to chronic Ipr treatment (32, 39, 42). Subsequently, acidic PRP was cloned, and both mRNA and protein were shown to increase in parotid glands from chronically Ipr-treated rats or mice (1, 8, 54). However, the relative changes of acidic to basic PRPs have not been characterized. This is important since the ratio of acidic/basic PRPs apparently influences the internal environment of the granule (3). For example, Castle demonstrated that transfection of the basic PRP cDNA into AtT-20 cells causes enhanced glycosylation of acidic PRPs (13), which in turn are required for efficient storage and stimulated secretion of cargo proteins in the parotid (48). Therefore, we postulated that the relative changes of acidic and basic PRPs in response to Ipr may influence changes in regulated secretion.
Consistent with previous reports (32, 39, 42), both acidic and basic forms of PRP increase in response to chronic Ipr treatment, however, we found a dramatic shift towards relative expression of basic PRP. The relative increase of basic PRP was accompanied by alkalinization of the granules from pH 6.67 to 7.23. The increase in granule pH may be due to the abundance of basic PRP, as well as other basic proteins, as previously suggested (3).
We directly measured the efficiency of regulated secretion after these changes. This is possible because injected Ipr has a short serum half-life (<10 min) and is rapidly converted to the methylated glucuronide conjugate in tissue and cleared from the body (26, 28). Hence, injected Ipr is cleared well before the time of sacrifice, and standard protocols can be used to measure in vitro Ipr-stimulated secretion. Analysis of secretion in vitro found that chronic Ipr treatment caused a decreased activity of the regulated secretion pathway for each of the three cargo proteins (amylase, PRP and PSP)(Fig. 1B, 2B). Regulated secretion was essentially abolished for amylase and PSP in these experiments. Loss of the regulated pathway was due to an increase of constitutive secretion pathways and decrease of stimulated secretion for both amylase and PSP, suggesting a shift of cargo protein sorting. Similarly, increasing the amount of basic PRP by transfection decreased the stimulated secretion of a cargo protein (PRPg2) (13).
We have previously used a different method for shifting the ratio of acidic to basic charges, by blocking glycosylation of acidic PRP (48). Incubation of parotid tissue invitro with paranitrophenyl xyloside (pNPX) blocks addition of the negatively charged chondroitin sulfate to acidic PRPs thereby decreasing anionic charges in the granule. This occurs without the increased expression of PRP proteins seen with chronic Ipr treatment. However, the net effect is the same; inhibition of the regulated secretion pathway (Fig. 2A). This similarity is consistent with the idea that the shift towards cationic (basic) charges, not the amount of PRP, influences regulated secretion in the Ipr-treated parotid.
A more direct test of this idea shows that the decreased regulated secretion is dependant on alkalization of the granules (Fig. 3). Under normal conditions, the parotid secretory granule is a well buffered organelle, however, the internal pH of granules from Ipr-treated rats is higher than the pH of normal granules (3, present study). Several studies have shown that sorting of the regulated secretory proteins can be perturbed by mild alkalization of the secretory granule by treating either animals or cell cultures with chloroquine, hydroxychloroquine, or dilute ammonium chloride (12, 29, 38, 49). To test whether alkalization of the granules by chronic Ipr-treatment is required for the observed decreased efficiency of the regulated secretion pathway (Fig. 2A), we did the opposite experiment by treating the cells with a mild acid. Regulated secretion of newly formed cargo proteins in pulse-chase experiments with control parotid tissue was not altered by treatment with mild acid. However, acid reversed the loss of regulated secretion seen with parotid tissue from Ipr-treated rats (Fig. 3). This was true for both amylase and PSP cargo proteins. These results indicate that alkalization is required as part of the mechanism by which Ipr alters the efficiency of regulated secretion. While the effect of acetic acid is necessarily an interaction with changes caused by chronic Ipr treatment, we can not rule out the possibility that acetic acid repairs a cytosolic defect. These studies reinforce the importance of the pH of the parotid granule in regulated secretion, which is distinct from the mechanism of calcium-induced aggregation observed in endocrine regulated secretion (47).
The presence of distinct secretory pathways requires that acinar cells be capable of sorting different cargo proteins into discrete secretory vesicles. Therefore, we investigated whether Ipr-treatment ultimately caused a change in the sorting of cargo proteins into a basolateral pathway, in vivo. Serum levels of PSP have not been investigated prior to this study and we found that PSP was not detectable or was very low in most control rats. Hence, under normal conditions little or no PSP is secreted via a basolateral pathway into circulation. However, Ipr-treated rats consistently contained significant amounts of PSP in serum suggesting that an actual change in sorting of the protein from the apical pathway to a basolateral pathway had occurred. PSP is most strongly expressed in parotid glands, which are likely sources of the serum PSP. However, PSP mRNA is also expressed in trachea, uterus and testis (19), none of which are known to sort PSP into an endocrine secretory pathway. A change in sorting in any of these tissues would contribute to the observed serum PSP.
We also observed a small but significant increase in amylase activity in the serum. Typically, in rats, the major source of serum amylase is from the salivary glands with little or no contribution from the pancreas or liver (41). Despite the high level of amylase in controls, we observed a significant increase in amylase activity in serum from Ipr-treated rats, and demonstrated that this was an increase of the salivary isoform. These findings support our model that Ipr treatment increased synthesis of basic PRP, contributing to alkalization of the secretory granules, thereby decreasing efficiency of the apical regulated pathway and routing the apically secreted proteins to the basolateral side.
The use of parotid glands as a target for integration and expression of therapeutic transgenes requires that the therapeutic protein is secreted appropriately (6, 30, 51). Several attempts have been made to redirect transgenic human growth hormone (GH) to the basolateral pathway (30, 53). This change in sorting has been demonstrated either by mutation of the GH transgene, or by directly treating the rat with a weak amine (HCQ), neither of which has strong potential as a therapeutic approach (53). An alternative approach could be the co-expression of basic proteins along with the transgene. Our results suggest that future work in this area needs to closely consider the effects of transgenic proteins on granule pH, and subsequent effects on sorting efficiency.
Acknowledgments
The authors thank Drs. Anna and David Castle, and Dr. William Ball for kindly providing antibodies used in this work. We thank Dr. B. Diane Hopkins for critically reading this manuscript.
Footnotes
Grants
This work was supported by the National Institute of Dental and Craniofacial Research grant R01-DE-012215.
References
- 1.Ann DK, Clements S, Johnstone EM, Carlson DM. Induction of tissue-specific proline-rich protein multigene families in rat and mouse parotid glands by isoproterenol. Unusual strain differences of proline-rich protein mRNAs. J Biol Chem. 1987;262:899–904. [PubMed] [Google Scholar]
- 2.Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvan P, Castle JD. Isolated secretion granules from parotid glands of chronically stimulated rats possess an alkaline internal pH and inward-directed H+ pump activity. J Cell Biol. 1986;103:1257–1267. doi: 10.1083/jcb.103.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arvan P, Kuliawat R, Prabakaran D, Zavacki AM, Elahi D, Wang S, Pilkey D. Protein discharge from immature secretory granules displays both regulated and constitutive characteristics. J Biol Chem. 1991;266:14171–14174. [PubMed] [Google Scholar]
- 5.Baum BJ, Berkman ME, Marmary Y, Goldsmith CM, Baccaglini L, Wang S, Wellner RB, Hoque AT, Atkinson JC, Yamagishi H, Kagami H, Parlow AF, Chao J. Polarized secretion of transgene products from salivary glands in vivo. Hum Gene Ther. 1999;10:2789–2797. doi: 10.1089/10430349950016528. [DOI] [PubMed] [Google Scholar]
- 6.Baum BJ, Voutetakis A, Wang J. Salivary glands: novel target sites for gene therapeutics. Trends Mol Med. 2004;10:585–590. doi: 10.1016/j.molmed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Beaudoin AR, Grondin G. Zymogen granules of the pancreas and the parotid gland and their role in cell secretion. Int Rev Cytol. 1992;132:177–222. doi: 10.1016/s0074-7696(08)62456-0. [DOI] [PubMed] [Google Scholar]
- 8.Blair EA, Castle AM, Castle JD. Proteoglycan sulfation and storage parallels storage of basic secretory proteins in exocrine cells. Am J Physiol. 1991;261:C897–905. doi: 10.1152/ajpcell.1991.261.5.C897. [DOI] [PubMed] [Google Scholar]
- 9.Blazquez M, Thiele C, Huttner WB, Docherty K, Shennan KI. Involvement of the membrane lipid bilayer in sorting prohormone convertase 2 into the regulated secretory pathway. Biochem J 349 Pt. 2000;3:843–852. doi: 10.1042/bj3490843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloom GD, Carlsoo B, Danielsson A, Gustafsson H, Henriksson R. Quantitative structural analysis and the secretory behaviour of the rat parotid gland after long and short term isoprenaline treatment. Med Biol. 1979;57:224–233. [PubMed] [Google Scholar]
- 11.Cameron RS, Cameron PL, Castle JD. A common spectrum of polypeptides occurs in secretion granule membranes of different exocrine glands. J Cell Biol. 1986;103:1299–1313. doi: 10.1083/jcb.103.4.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplan MJ, Stow JL, Newman AP, Madri J, Anderson HC, Farquhar MG, Palade GE, Jamieson JD. Dependence on pH of polarized sorting of secreted proteins. Nature. 1987;329:632–635. doi: 10.1038/329632a0. [DOI] [PubMed] [Google Scholar]
- 13.Castle AM, Castle JD. Enhanced glycosylation and sulfation of secretory proteoglycans is coupled to the expression of a basic secretory protein. Mol Biol Cell. 1998;9:575–583. doi: 10.1091/mbc.9.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castle AM, Castle JD. Novel secretory proline-rich proteoglycans from rat parotid. Cloning and characterization by expression in AtT-20 cells. J Biol Chem. 1993;268:20490–20496. [PubMed] [Google Scholar]
- 15.Castle JD. Sorting and secretory pathways in exocrine cells. Am J Respir Cell Mol Biol. 1990;2:119–126. doi: 10.1165/ajrcmb/2.2.119. [DOI] [PubMed] [Google Scholar]
- 16.Castle JD, Castle AM. Sorting and secretion of salivary proteins. Crit Rev Oral Biol Med. 1993;4:393–398. doi: 10.1177/10454411930040031901. [DOI] [PubMed] [Google Scholar]
- 17.Castle JD, Castle AM. Two regulated secretory pathways for newly synthesized parotid salivary proteins are distinguished by doses of secretagogues. J Cell Sci. 1996;109 ( Pt 10):2591–2599. doi: 10.1242/jcs.109.10.2591. [DOI] [PubMed] [Google Scholar]
- 18.Forsberg E, Pejler G, Ringvall M, Lunderius C, Tomasini-Johansson B, Kusche-Gullberg M, Eriksson I, Ledin J, Hellman L, Kjellen L. Abnormal mast cells in mice deficient in a heparin-synthesizing enzyme. Nature. 1999;400:773–776. doi: 10.1038/23488. [DOI] [PubMed] [Google Scholar]
- 19.Geetha C, Venkatesh SG, Bingle L, Bingle CD, Gorr SU. Design and validation of anti-inflammatory peptides from human parotid secretory protein. J Dent Res. 2005;84:149–153. doi: 10.1177/154405910508400208. [DOI] [PubMed] [Google Scholar]
- 20.Goldfine ID, German MS, Tseng HC, Wang J, Bolaffi JL, Chen JW, Olson DC, Rothman SS. The endocrine secretion of human insulin and growth hormone by exocrine glands of the gastrointestinal tract. Nat Biotechnol. 1997;15:1378–1382. doi: 10.1038/nbt1297-1378. [DOI] [PubMed] [Google Scholar]
- 21.Gorr SU, Cohn DV. Secretion of sulfated and nonsulfated forms of parathyroid chromogranin A (secretory protein-I) J Biol Chem. 1990;265:3012–3016. [PubMed] [Google Scholar]
- 22.Gorr SU, Dean WL, Radley TL, Cohn DV. Calcium-binding and aggregation properties of parathyroid secretory protein-I (chromogranin A) Bone Miner. 1988;4:17–25. [PubMed] [Google Scholar]
- 23.Gorr SU, Jain RK, Kuehn U, Joyce PB, Cowley DJ. Comparative sorting of neuroendocrine secretory proteins: a search for common ground in a mosaic of sorting models and mechanisms. Mol Cell Endocrinol. 2001;172:1–6. doi: 10.1016/s0303-7207(00)00342-7. [DOI] [PubMed] [Google Scholar]
- 24.Gorr SU, Moore YR. Sorting of a constitutive secretory protein to the regulated secretory pathway of exocrine cells. Biochem Biophys Res Commun. 1999;257:545–548. doi: 10.1006/bbrc.1999.0504. [DOI] [PubMed] [Google Scholar]
- 25.Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. 2005;84:500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadwiger ME, Park S, Torchia SR, Lunte CE. Simultaneous determination of the elimination profiles of the individual enantiomers of racemic isoproterenol using capillary electrophoresis and microdialysis sampling. J Pharm Biomed Anal. 1997;15:621–629. doi: 10.1016/s0731-7085(96)01896-1. [DOI] [PubMed] [Google Scholar]
- 27.He X, Goldsmith CM, Marmary Y, Wellner RB, Parlow AF, Nieman LK, Baum BJ. Systemic action of human growth hormone following adenovirus-mediated gene transfer to rat submandibular glands. Gene Ther. 1998;5:537–541. doi: 10.1038/sj.gt.3300622. [DOI] [PubMed] [Google Scholar]
- 28.Hertting G. The Fate of 3H-Iso-Proterenol in the Rat. Biochem Pharmacol. 1964;13:1119–1128. doi: 10.1016/0006-2952(64)90112-1. [DOI] [PubMed] [Google Scholar]
- 29.Hoque AT, Baccaglini L, Baum BJ. Hydroxychloroquine enhances the endocrine secretion of adenovirus-directed growth hormone from rat submandibular glands in vivo. Hum Gene Ther. 2001;12:1333–1341. doi: 10.1089/104303401750270986. [DOI] [PubMed] [Google Scholar]
- 30.Hoque AT, Yamano S, Baccaglini L, Baum BJ. Using salivary glands as a tissue target for gene therapeutics. J Drug Target. 2001;9:485–494. doi: 10.3109/10611860108998782. [DOI] [PubMed] [Google Scholar]
- 31.Humphries DE, Wong GW, Friend DS, Gurish MF, Qiu WT, Huang C, Sharpe AH, Stevens RL. Heparin is essential for the storage of specific granule proteases in mast cells. Nature. 1999;400:769–772. doi: 10.1038/23481. [DOI] [PubMed] [Google Scholar]
- 32.Johnson DA, Cortez JE. Chronic treatment with beta adrenergic agonists and antagonists alters the composition of proteins in rat parotid saliva. J Dent Res. 1988;67:1103–1108. doi: 10.1177/00220345880670080801. [DOI] [PubMed] [Google Scholar]
- 33.Kagami H, O’Connell BC, Baum BJ. Evidence for the systemic delivery of a transgene product from salivary glands. Hum Gene Ther. 1996;7:2177–2184. doi: 10.1089/hum.1996.7.17-2177. [DOI] [PubMed] [Google Scholar]
- 34.Kelly RB. Pathways of protein secretion in eukaryotes. Science. 1985;230:25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- 35.Lebel D, Grondin G, Paquette J. In vitro stability of pancreatic zymogen granules: roles of pH and calcium. Biol Cell. 1988;63:343–353. [PubMed] [Google Scholar]
- 36.Leclerc P, Forest JC. Electrophoretic determination of isoamylases in serum with commercially available reagents. Clin Chem. 1982;28:37–40. [PubMed] [Google Scholar]
- 37.Marmorstein AD, Csaky KG, Baffi J, Lam L, Rahaal F, Rodriguez-Boulan E. Saturation of, and competition for entry into, the apical secretory pathway. Proc Natl Acad Sci U S A. 2000;97:3248–3253. doi: 10.1073/pnas.070049497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore HP, Gumbiner B, Kelly RB. Chloroquine diverts ACTH from a regulated to a constitutive secretory pathway in AtT-20 cells. Nature. 1983;302:434–436. doi: 10.1038/302434a0. [DOI] [PubMed] [Google Scholar]
- 39.Muenzer J, Bildstein C, Gleason M, Carlson DM. Properties of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979;254:5629–5634. [PubMed] [Google Scholar]
- 40.Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975;189:347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- 41.Proctor GB, Asking B, Garrett JR. Serum amylase of non-parotid and non-pancreatic origin increases on feeding in rats and may originate from the liver. Comp Biochem Physiol B. 1991;98:631–635. doi: 10.1016/0305-0491(91)90267-h. [DOI] [PubMed] [Google Scholar]
- 42.Robinovitch MR, Keller PJ, Johnson DA, Iversen JM, Kauffman DL. Changes in rat parotid salivary proteins induced by chronic isoproterenol administration. J Dent Res. 1977;56:290–303. doi: 10.1177/00220345770560031501. [DOI] [PubMed] [Google Scholar]
- 43.Ryberg M, Johansson I, Ericson T, Mornstad H, Henriksson R, Jonsson G, Sundstrom S. Effects of chronic stimulation of salivary gland beta-adrenoceptors on saliva composition and caries development in the rat. J Oral Pathol Med. 1989;18:529–532. doi: 10.1111/j.1600-0714.1989.tb01357.x. [DOI] [PubMed] [Google Scholar]
- 44.Shennan KI. Intracellular targeting of secretory proteins in neuroendocrine cells. Biochem Soc Trans. 1996;24:535–539. doi: 10.1042/bst0240535. [DOI] [PubMed] [Google Scholar]
- 45.Shennan KI, Taylor NA, Docherty K. Calcium- and pH-dependent aggregation and membrane association of the precursor of the prohormone convertase PC2. J Biol Chem. 1994;269:18646–18650. [PubMed] [Google Scholar]
- 46.Tooze J, Kern HF, Fuller SD, Howell KE. Condensation-sorting events in the rough endoplasmic reticulum of exocrine pancreatic cells. J Cell Biol. 1989;109:35–50. doi: 10.1083/jcb.109.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venkatesh SG, Cowley DJ, Gorr SU. Differential aggregation properties of secretory proteins that are stored in exocrine secretory granules of the pancreas and parotid glands. Am J Physiol Cell Physiol. 2004;286:C365–371. doi: 10.1152/ajpcell.00338.2003. [DOI] [PubMed] [Google Scholar]
- 48.Venkatesh SG, Gorr SU. A sulfated proteoglycan is necessary for storage of exocrine secretory proteins in the rat parotid gland. Am J Physiol Cell Physiol. 2002;283:C438–445. doi: 10.1152/ajpcell.00552.2001. [DOI] [PubMed] [Google Scholar]
- 49.von Zastrow M, Castle AM, Castle JD. Ammonium chloride alters secretory protein sorting within the maturing exocrine storage compartment. J Biol Chem. 1989;264:6566–6571. [PubMed] [Google Scholar]
- 50.Voutetakis A, Bossis I, Kok MR, Zhang W, Wang J, Cotrim AP, Zheng C, Chiorini JA, Nieman LK, Baum BJ. Salivary glands as a potential gene transfer target for gene therapeutics of some monogenetic endocrine disorders. J Endocrinol. 2005;185:363–372. doi: 10.1677/joe.1.06171. [DOI] [PubMed] [Google Scholar]
- 51.Voutetakis A, Wang J, Baum BJ. Utilizing endocrine secretory pathways in salivary glands for systemic gene therapeutics. J Cell Physiol. 2004;199:1–7. doi: 10.1002/jcp.10429. [DOI] [PubMed] [Google Scholar]
- 52.Vugman I, Hand AR. Quantitative immunocytochemical study of secretory protein expression in parotid glands of rats chronically treated with isoproterenol. Microsc Res Tech. 1995;31:106–117. doi: 10.1002/jemt.1070310203. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Cawley NX, Voutetakis A, Rodriguez YM, Goldsmith CM, Nieman LK, Hoque AT, Frank SJ, Snell CR, Loh YP, Baum BJ. Partial redirection of transgenic human growth hormone secretion from rat salivary glands. Hum Gene Ther. 2005;16:571–583. doi: 10.1089/hum.2005.16.571. [DOI] [PubMed] [Google Scholar]
- 54.Ziemer MA, Swain WF, Rutter WJ, Clements S, Ann DK, Carlson DM. Nucleotide sequence analysis of a proline-rich protein cDNA and peptide homologies of rat and human proline-rich proteins. J Biol Chem. 1984;259:10475–10480. [PubMed] [Google Scholar]


