Abstract
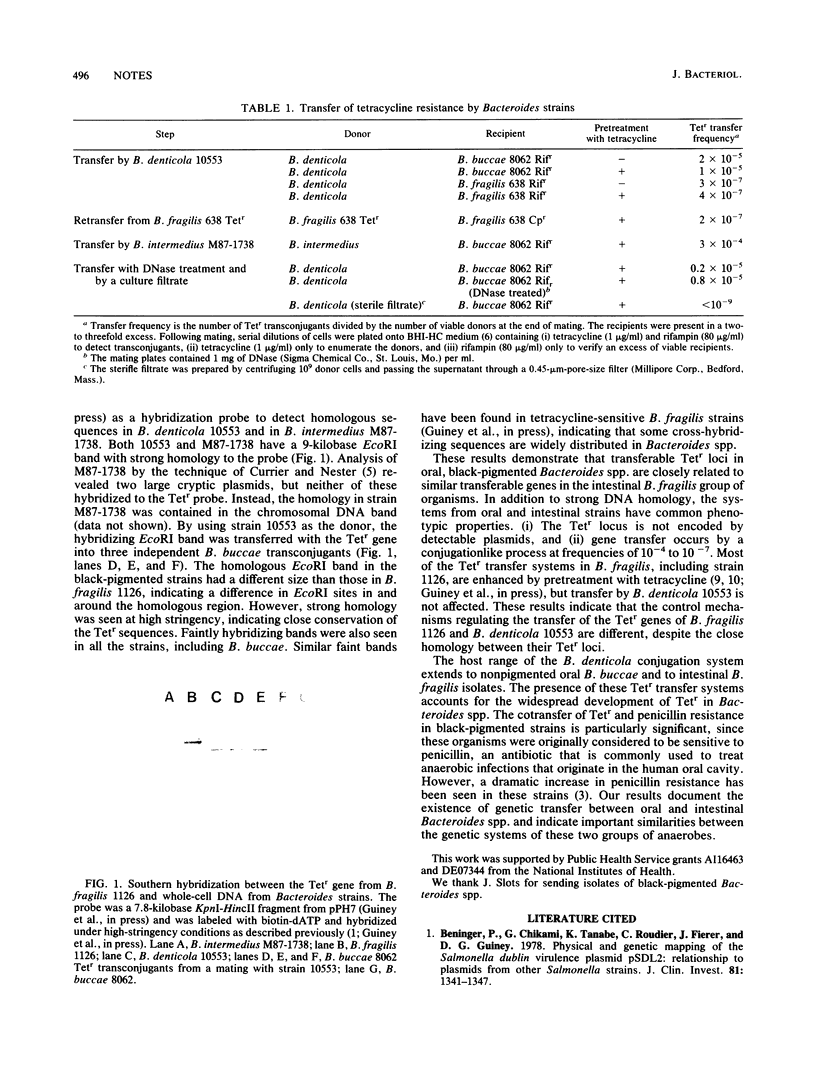
Oral, black-pigmented Bacteroides spp. are important pathogens in oral anaerobic infections and dental disease. We detected conjugation systems in isolates of Bacteroides denticola and Bacteroides intermedius that transferred tetracycline resistance (Tetr) and penicillin resistance to Bacteroides buccae and to Bacteroides fragilis, an intestinal Bacteroides species. A cloned Tetr gene from B. fragilis hybridized to the transferable Tetr locus in the oral strains, indicating that genetic exchange occurs between these two groups of anaerobes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beninger P. R., Chikami G., Tanabe K., Roudier C., Fierer J., Guiney D. G. Physical and genetic mapping of the Salmonella dublin virulence plasmid pSDL2. Relationship to plasmids from other Salmonella strains. J Clin Invest. 1988 May;81(5):1341–1347. doi: 10.1172/JCI113461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook I. Pathogenicity and therapy of anaerobic bacteria in upper respiratory tract infections. Pediatr Infect Dis J. 1987 Jan;6(1):131–136. doi: 10.1097/00006454-198701000-00054. [DOI] [PubMed] [Google Scholar]
- Brook I. Role of anaerobic beta-lactamase-producing bacteria in upper respiratory tract infections. Pediatr Infect Dis J. 1987 Mar;6(3):310–316. doi: 10.1097/00006454-198703000-00045. [DOI] [PubMed] [Google Scholar]
- Chow A. W., Roser S. M., Brady F. A. Orofacial odontogenic infections. Ann Intern Med. 1978 Mar;88(3):392–402. doi: 10.7326/0003-4819-88-3-392. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Guiney D. G., Jr, Hasegawa P., Stalker D., Davis C. E. Genetic analysis of clindamycin resistance in Bacteroides species. J Infect Dis. 1983 Mar;147(3):551–558. doi: 10.1093/infdis/147.3.551. [DOI] [PubMed] [Google Scholar]
- Loesche W. J., Syed S. A., Laughon B. E., Stoll J. The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol. 1982 Apr;53(4):223–230. doi: 10.1902/jop.1982.53.4.223. [DOI] [PubMed] [Google Scholar]
- Odelson D. A., Rasmussen J. L., Smith C. J., Macrina F. L. Extrachromosomal systems and gene transmission in anaerobic bacteria. Plasmid. 1987 Mar;17(2):87–109. doi: 10.1016/0147-619x(87)90016-3. [DOI] [PubMed] [Google Scholar]
- Privitera G., Fayolle F., Sebald M. Resistance to tetracycline, erythromycin, and clindamycin in the Bacteroides fragilis group: inducible versus constitutive tetracycline resistance. Antimicrob Agents Chemother. 1981 Sep;20(3):314–320. doi: 10.1128/aac.20.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privitera G., Sebald M., Fayolle F. Common regulatory mechanism of expression and conjugative ability of a tetracycline resistance plasmid in Bacteroides fragilis. Nature. 1979 Apr 12;278(5705):657–659. doi: 10.1038/278657a0. [DOI] [PubMed] [Google Scholar]
- Salyers A. A., Shoemaker N. B., Guthrie E. P. Recent advances in Bacteroides genetics. Crit Rev Microbiol. 1987;14(1):49–71. doi: 10.3109/10408418709104435. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Barber R. D., Salyers A. A. Cloning and characterization of a Bacteroides conjugal tetracycline-erythromycin resistance element by using a shuttle cosmid vector. J Bacteriol. 1989 Mar;171(3):1294–1302. doi: 10.1128/jb.171.3.1294-1302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]