Abstract
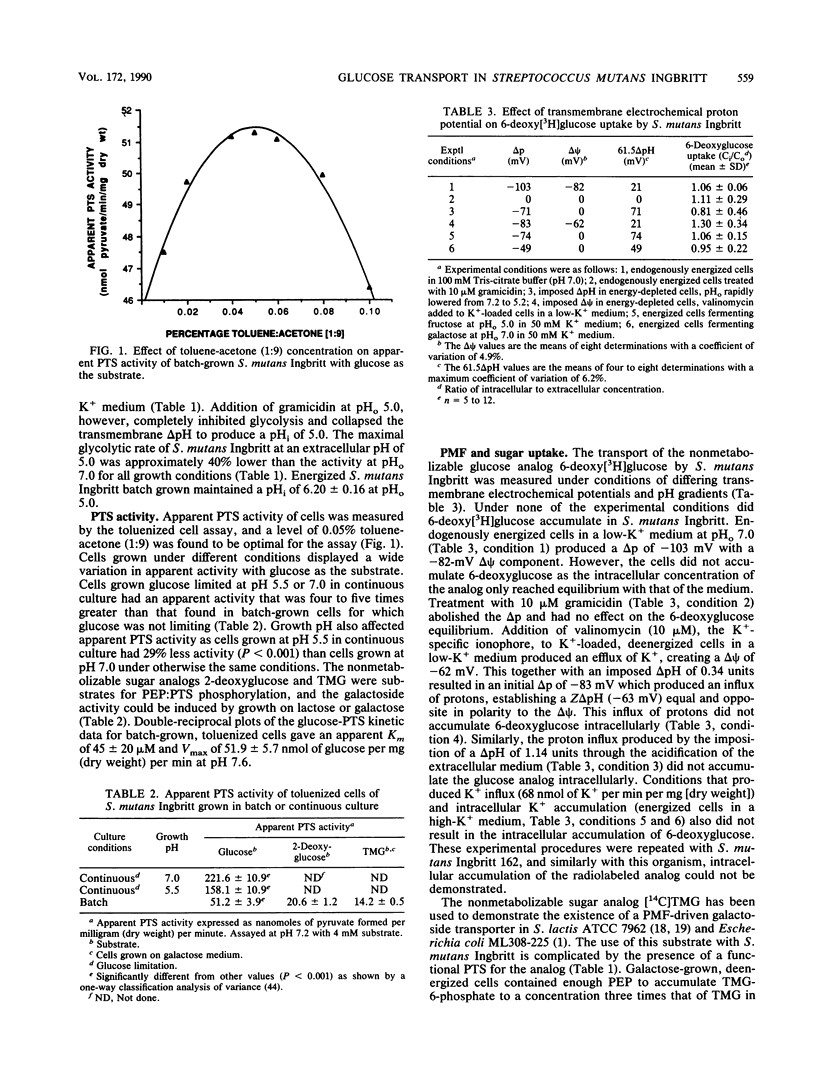
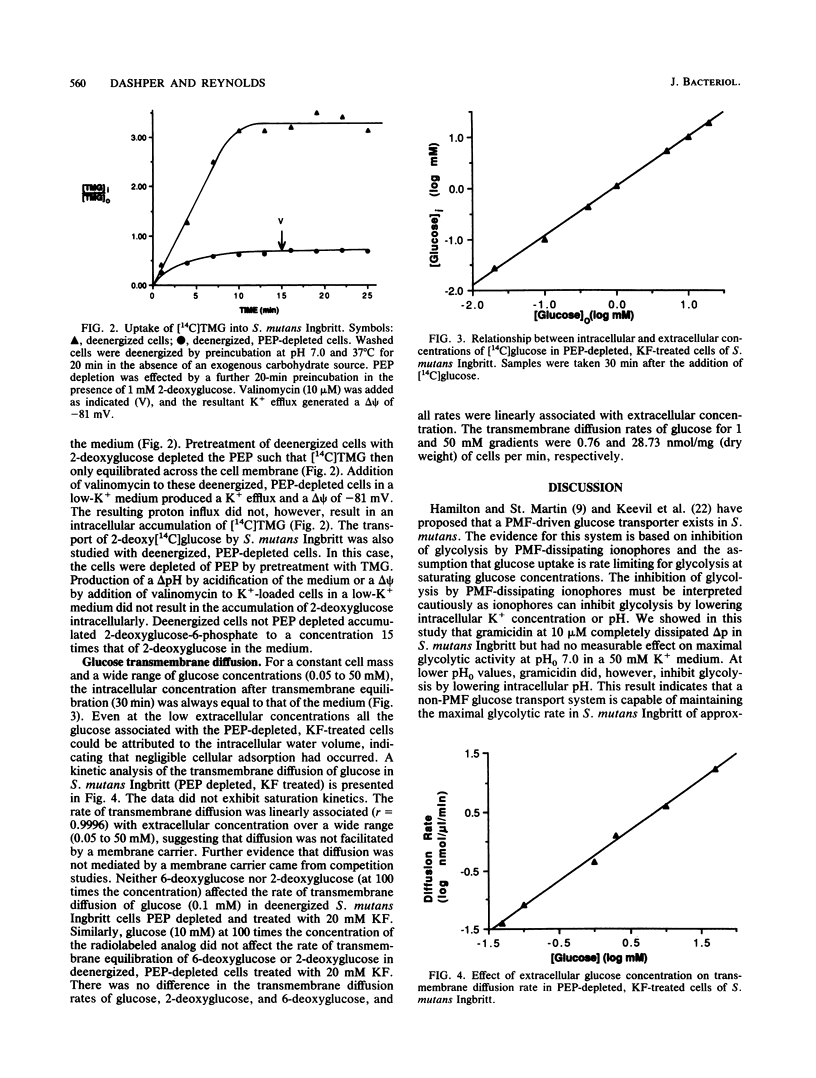
The transmembrane movement of radiolabeled, nonmetabolizable glucose analogs in Streptococcus mutants Ingbritt was studied under conditions of differing transmembrane electrochemical potentials (delta psi) and pH gradients (delta pH). The delta pH and delta psi were determined from the transmembrane equilibration of radiolabeled benzoate and tetraphenylphosphonium ions, respectively. Growth conditions of S. mutants Ingbritt were chosen so that the cells had a low apparent phosphoenolpyruvate (PEP)-dependent glucose:phosphotransferase activity. Cells energized under different conditions produced transmembrane proton potentials ranging from -49 to -103 mV but did not accumulate 6-deoxyglucose intracellularly. An artificial transmembrane proton potential was generated in deenergized cells by creating a delta psi with a valinomycin-induced K+ diffusion potential and a delta pH by rapid acidification of the medium. Artificial transmembrane proton potentials up to -83 mV, although producing proton influx, could not accumulate 6-deoxyglucose in deenergized cells or 2-deoxyglucose or thiomethylgalactoside in deenergized, PEP-depleted cells. The transmembrane diffusion of glucose in PEP-depleted, KF-treated cells did not exhibit saturation kinetics or competitive inhibition by 6-deoxyglucose or 2-deoxyglucose, indicating that diffusion was not facilitated by a membrane carrier. As proton-linked membrane carriers have been shown to facilitate diffusion in the absence of a transmembrane proton potential, the results therefore are not consistent with a proton-linked glucose carrier in S. mutans Ingbritt. This together with the lack of proton-linked transport of the glucose analogs suggests that glucose transmembrane movement in S. mutans Ingbritt is not linked to the transmembrane proton potential.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed S., Booth I. R. Quantitative measurements of the proton-motive force and its relation to steady state lactose accumulation in Escherichia coli. Biochem J. 1981 Dec 15;200(3):573–581. doi: 10.1042/bj2000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Harold F. M. Energy coupling to potassium transport in Streptococcus faecalis. Interplay of ATP and the protonmotive force. J Biol Chem. 1980 Jan 25;255(2):433–440. [PubMed] [Google Scholar]
- Crowley P. J., Fischlschweiger W., Coleman S. E., Bleiweis A. S. Intergeneric bacterial coaggregations involving mutans streptococci and oral actinomyces. Infect Immun. 1987 Nov;55(11):2695–2700. doi: 10.1128/iai.55.11.2695-2700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J., Pevec B., Beyreuther K., Kiltz H. H., Hengstenberg W. Streptococcal phosphoenolpyruvate-sugar phosphotransferase system: amino acid sequence and site of ATP-dependent phosphorylation of HPr. Biochemistry. 1986 Oct 21;25(21):6543–6551. doi: 10.1021/bi00369a031. [DOI] [PubMed] [Google Scholar]
- Ellwood D. C., Phipps P. J., Hamilton I. R. Effect of growth rate and glucose concentration on the activity of the phosphoenolpyruvate phosphotransferase system in Streptococcus mutans Ingbritt grown in continuous culture. Infect Immun. 1979 Feb;23(2):224–231. doi: 10.1128/iai.23.2.224-231.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gachelin G. Studies on the alpha-methylglucoside permease of Escherichia coli. A two-step mechanism for the accumulation of alpha-methylglucoside 6-phosphate. Eur J Biochem. 1970 Oct;16(2):342–357. doi: 10.1111/j.1432-1033.1970.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Hamada S., Slade H. D. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., St Martin E. J. Evidence for the involvement of proton motive force in the transport of glucose by a mutant of Streptococcus mutans strain DR0001 defective in glucose-phosphoenolpyruvate phosphotransferase activity. Infect Immun. 1982 May;36(2):567–575. doi: 10.1128/iai.36.2.567-575.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwami Y., Yamada T. Rate-limiting steps of the glycolytic pathway in the oral bacteria Streptococcus mutans and Streptococcus sanguis and the influence of acidic pH on the glucose metabolism. Arch Oral Biol. 1980;25(3):163–169. doi: 10.1016/0003-9969(80)90015-1. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Mimura C. S., Scott P. J., Thompson P. W. Identification and properties of distinct sucrose and glucose phosphotransferase enzyme II activities in Streptococcus mutans 6715g. Infect Immun. 1984 Dec;46(3):854–856. doi: 10.1128/iai.46.3.854-856.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Site-directed mutagenesis and ion-gradient driven active transport: on the path of the proton. Annu Rev Physiol. 1988;50:243–256. doi: 10.1146/annurev.ph.50.030188.001331. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Barker S. L. Effects of potassium ions on the electrical and pH gradients across the membrane of Streptococcus lactis cells. J Bacteriol. 1977 Jun;130(3):1017–1023. doi: 10.1128/jb.130.3.1017-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Blanchard A. G., Metzger W. C. Proton motive force during growth of Streptococcus lactis cells. J Bacteriol. 1980 Jul;143(1):128–134. doi: 10.1128/jb.143.1.128-134.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R. The proton motive force in bacteria: a critical assessment of methods. Annu Rev Microbiol. 1985;39:219–242. doi: 10.1146/annurev.mi.39.100185.001251. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Proton-coupled accumulation of galactoside in Streptococcus lactis 7962. Proc Natl Acad Sci U S A. 1973 Oct;70(10):2866–2869. doi: 10.1073/pnas.70.10.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Protonmotive force in fermenting Streptococcus lactis 7962 in relation to sugar accumulation. Biochem Biophys Res Commun. 1974 Aug 5;59(3):879–886. doi: 10.1016/s0006-291x(74)80061-6. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Role of metabolic energy in the transport of -galactosides by Streptococcus lactis. J Bacteriol. 1972 Feb;109(2):784–789. doi: 10.1128/jb.109.2.784-789.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil C. W., Marsh P. D., Ellwood D. C. Regulation of glucose metabolism in oral streptococci through independent pathways of glucose 6-phosphate and glucose 1-phosphate formation. J Bacteriol. 1984 Feb;157(2):560–567. doi: 10.1128/jb.157.2.560-567.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil C. W., McDermid A. S., Marsh P. D., Ellwood D. C. Protonmotive force driven 6-deoxyglucose uptake by the oral pathogen, Streptococcus mutans Ingbritt. Arch Microbiol. 1986 Nov;146(2):118–124. doi: 10.1007/BF00402337. [DOI] [PubMed] [Google Scholar]
- Keevil C. W., Williamson M. I., Marsh P. D., Ellwood D. C. Evidence that glucose and sucrose uptake in oral streptococcal bacteria involves independent phosphotransferase and proton-motive force-mediated mechanisms. Arch Oral Biol. 1984;29(11):871–878. doi: 10.1016/0003-9969(84)90085-2. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L., Reeves R. E. Inducible phosphoenolpyruvate-dependent hexose phosphotransferase activities in Escherichia coli. Biochem J. 1972 Aug;128(5):1339–1344. doi: 10.1042/bj1281339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasse B. Human streptococci and experimental caries in hamsters. Arch Oral Biol. 1966 Apr;11(4):429–436. doi: 10.1016/0003-9969(66)90107-5. [DOI] [PubMed] [Google Scholar]
- Liberman E. S., Bleiweis A. S. Transport of glucose and mannose by a common phosphoenolpyruvate-dependent phosphotransferase system in Streptococcus mutans GS5. Infect Immun. 1984 Mar;43(3):1106–1109. doi: 10.1128/iai.43.3.1106-1109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986 Dec;50(4):353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C. Obligatory coupling between proton entry and the synthesis of adenosine 5'-triphosphate in Streptococcus lactis. J Bacteriol. 1977 Nov;132(2):564–575. doi: 10.1128/jb.132.2.564-575.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. ATP synthesis driven by a protonmotive force in Streptococcus lactis. J Membr Biol. 1975;25(3-4):285–310. doi: 10.1007/BF01868580. [DOI] [PubMed] [Google Scholar]
- Maloney P. C., Wilson T. H. Quantitative aspects of active transport by the lactose transport system of Escherichia coli. Biochim Biophys Acta. 1973 Dec 13;330(2):196–205. doi: 10.1016/0005-2736(73)90225-3. [DOI] [PubMed] [Google Scholar]
- Marsh P. D., Williamson M. I., Keevil C. W., McDermid A. S., Ellwood D. C. Influence of sodium and potassium ions on acid production by washed cells of Streptococcus mutans ingbritt and Streptococcus sanguis NCTC 7865 grown in a chemostat. Infect Immun. 1982 May;36(2):476–483. doi: 10.1128/iai.36.2.476-483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noji S., Sato Y., Suzuki R., Taniguchi S. Effect of intracellular pH and potassium ions on a primary transport system for glutamate/aspartate in Streptococcus mutans. Eur J Biochem. 1988 Aug 15;175(3):491–495. doi: 10.1111/j.1432-1033.1988.tb14221.x. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W. Phosphoenolpyruvate:carbohydrate phosphotransferase system of bacteria. Microbiol Rev. 1985 Sep;49(3):232–269. doi: 10.1128/mr.49.3.232-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Stock J. B. Enzymes II of the phosphotransferase system do not catalyze sugar transport in the absence of phosphorylation. J Bacteriol. 1980 Feb;141(2):476–484. doi: 10.1128/jb.141.2.476-484.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds E. C., Black C. L. Confectionery composition and rat caries. Caries Res. 1987;21(6):538–545. doi: 10.1159/000261064. [DOI] [PubMed] [Google Scholar]
- Reynolds E. C., Riley P. F. Kinetics of hydroxyapatite dissolution during incubation with sucrose and Streptococcus mutans and its application to cariogenicity testing. Caries Res. 1981;15(6):501–507. doi: 10.1159/000260558. [DOI] [PubMed] [Google Scholar]
- Reynolds E. C., Riley P. F., Storey E. Phosphoprotein inhibition of hydroxyapatite dissolution. Calcif Tissue Int. 1982;34 (Suppl 2):S52–S56. [PubMed] [Google Scholar]
- Robillard G. T., Lolkema J. S. Enzymes II of the phosphoenolpyruvate-dependent sugar transport systems: a review of their structure and mechanism of sugar transport. Biochim Biophys Acta. 1988 Oct 11;947(3):493–519. doi: 10.1016/0304-4157(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Mayo J. A. Phosphoenolpyruvate-dependent glucose transport in oral streptococci. J Dent Res. 1973 Nov-Dec;52(6):1209–1215. doi: 10.1177/00220345730520060801. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Vadeboncoeur C., Mayrand D., Trahan L. A comparative study of enzymes involved in glucose phosphorylation in oral streptococci. J Dent Res. 1982 Jan;61(1):60–65. doi: 10.1177/00220345820610011401. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H., Wilson T. H. The role of energy coupling in the transport of beta-galactosides by Escherichia coli. J Biol Chem. 1966 May 25;241(10):2200–2211. [PubMed] [Google Scholar]



