Abstract
The cloned sfu region of the Serratia marcescens chromosome confers the ability to grow on iron-limited media to an Escherichia coli K-12 strain that is unable to synthesize a siderophore. This DNA fragment was sequenced and found to contain three genes termed sfuA, sfuB, and sfuC, arranged and transcribed in that order. The sfuA gene encoded a periplasmic polypeptide with calculated molecular weights of 36,154 for the precursor and 33,490 for the mature protein. The sfuB gene product was a very hydrophobic protein with a molecular weight of 56,589. The sfuC gene was found to encode a rather polar but membrane-bound protein with a molecular weight of 36,671 which exhibited strong homology to consensus sequences of nucleotide-binding proteins. The number, structural characteristics, and locations of the SfuABC proteins were typical of a periplasmic-binding-protein-dependent transport mechanism. How Fe3+ is solubilized and taken up across the outer membrane remains an enigma.
Full text
PDF



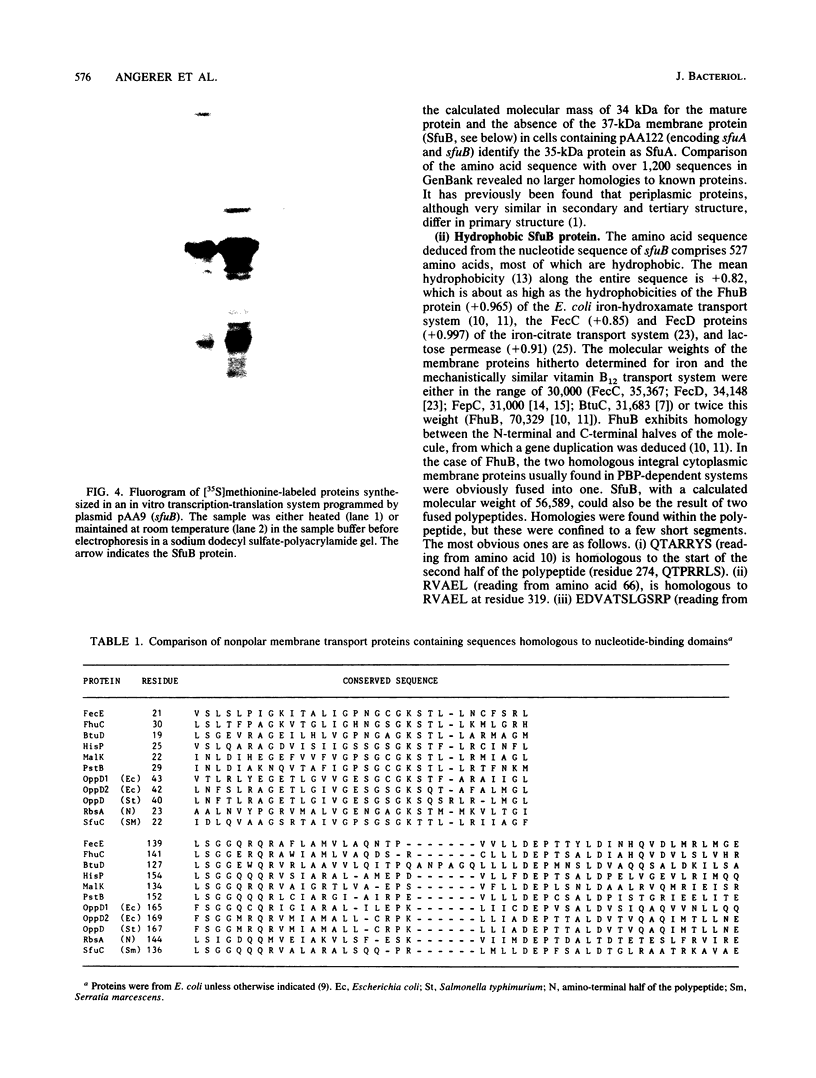


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F. Bacterial periplasmic transport systems: structure, mechanism, and evolution. Annu Rev Biochem. 1986;55:397–425. doi: 10.1146/annurev.bi.55.070186.002145. [DOI] [PubMed] [Google Scholar]
- Burkhardt R., Braun V. Nucleotide sequence of the fhuC and fhuD genes involved in iron (III) hydroxamate transport: domains in FhuC homologous to ATP-binding proteins. Mol Gen Genet. 1987 Aug;209(1):49–55. doi: 10.1007/BF00329835. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulton J. W., Mason P., Allatt D. D. fhuC and fhuD genes for iron (III)-ferrichrome transport into Escherichia coli K-12. J Bacteriol. 1987 Aug;169(8):3844–3849. doi: 10.1128/jb.169.8.3844-3849.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eick-Helmerich K., Braun V. Import of biopolymers into Escherichia coli: nucleotide sequences of the exbB and exbD genes are homologous to those of the tolQ and tolR genes, respectively. J Bacteriol. 1989 Sep;171(9):5117–5126. doi: 10.1128/jb.171.9.5117-5126.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich M. J., de Veaux L. C., Kadner R. J. Nucleotide sequence of the btuCED genes involved in vitamin B12 transport in Escherichia coli and homology with components of periplasmic-binding-protein-dependent transport systems. J Bacteriol. 1986 Sep;167(3):928–934. doi: 10.1128/jb.167.3.928-934.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Gallagher M. P., Mimmack M. L., Pearce S. R. A family of closely related ATP-binding subunits from prokaryotic and eukaryotic cells. Bioessays. 1988 Apr;8(4):111–116. doi: 10.1002/bies.950080406. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron hydroxamate transport of Escherichia coli: nucleotide sequence of the fhuB gene and identification of the protein. Mol Gen Genet. 1986 Sep;204(3):435–442. doi: 10.1007/BF00331021. [DOI] [PubMed] [Google Scholar]
- Köster W., Braun V. Iron-hydroxamate transport into Escherichia coli K12: localization of FhuD in the periplasm and of FhuB in the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):233–239. doi: 10.1007/BF02464886. [DOI] [PubMed] [Google Scholar]
- Ozenberger B. A., Nahlik M. S., McIntosh M. A. Genetic organization of multiple fep genes encoding ferric enterobactin transport functions in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3638–3646. doi: 10.1128/jb.169.8.3638-3646.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. R., Earhart C. F. Escherichia coli K-12 envelope proteins specifically required for ferrienterobactin uptake. J Bacteriol. 1986 Jun;166(3):930–936. doi: 10.1128/jb.166.3.930-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Braun V. Iron regulation of Serratia marcescens hemolysin gene expression. Infect Immun. 1988 Nov;56(11):2967–2971. doi: 10.1128/iai.56.11.2967-2971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole K., Schiebel E., Braun V. Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol. 1988 Jul;170(7):3177–3188. doi: 10.1128/jb.170.7.3177-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffler H., Braun V. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989 Jun;217(2-3):378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenmaier H., Van Hove B., Yaraghi Z., Braun V. Nucleotide sequences of the fecBCDE genes and locations of the proteins suggest a periplasmic-binding-protein-dependent transport mechanism for iron(III) dicitrate in Escherichia coli. J Bacteriol. 1989 May;171(5):2626–2633. doi: 10.1128/jb.171.5.2626-2633.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zimmermann L., Angerer A., Braun V. Mechanistically novel iron(III) transport system in Serratia marcescens. J Bacteriol. 1989 Jan;171(1):238–243. doi: 10.1128/jb.171.1.238-243.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorenzo V., Giovannini F., Herrero M., Neilands J. B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988 Oct 20;203(4):875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]