Abstract
We constructed a system in which wild-type RepA or RepAcop1 protein was supplied in trans in various amounts to coexisting mini-Rts1 plasmids by clones of the repA or repAcop1 gene under the control of the native promoter with or without its operator sequence. RepAcop1 protein which contains a single amino acid substitution (Arg-142 to Lys) within its 288 amino acids could initiate the replication of the mini-Rts1 plasmid efficiently at both 37 and 42 degrees C even if it was supplied in excess. In contrast, excess wild-type RepA inhibited plasmid replication at 37 degrees C but supported replication at 42 degrees C. Therefore, it appears that the initiator activity of RepA is not related to the incompatibility phenotype associated with an excess of RepA protein. An immunoblot analysis revealed that neither RepA nor RepAcop1 synthesis was temperature sensitive and that both were autogenously regulated to a similar extent because of the presence of an operator located immediately upstream of the promoter. Two mutant RepA proteins, each of which contains a 4-amino-acid insertion in the middle of the protein, maintained the autorepressor and incompatibility activities but lost the ori(Rts1)-activating function.
Full text
PDF
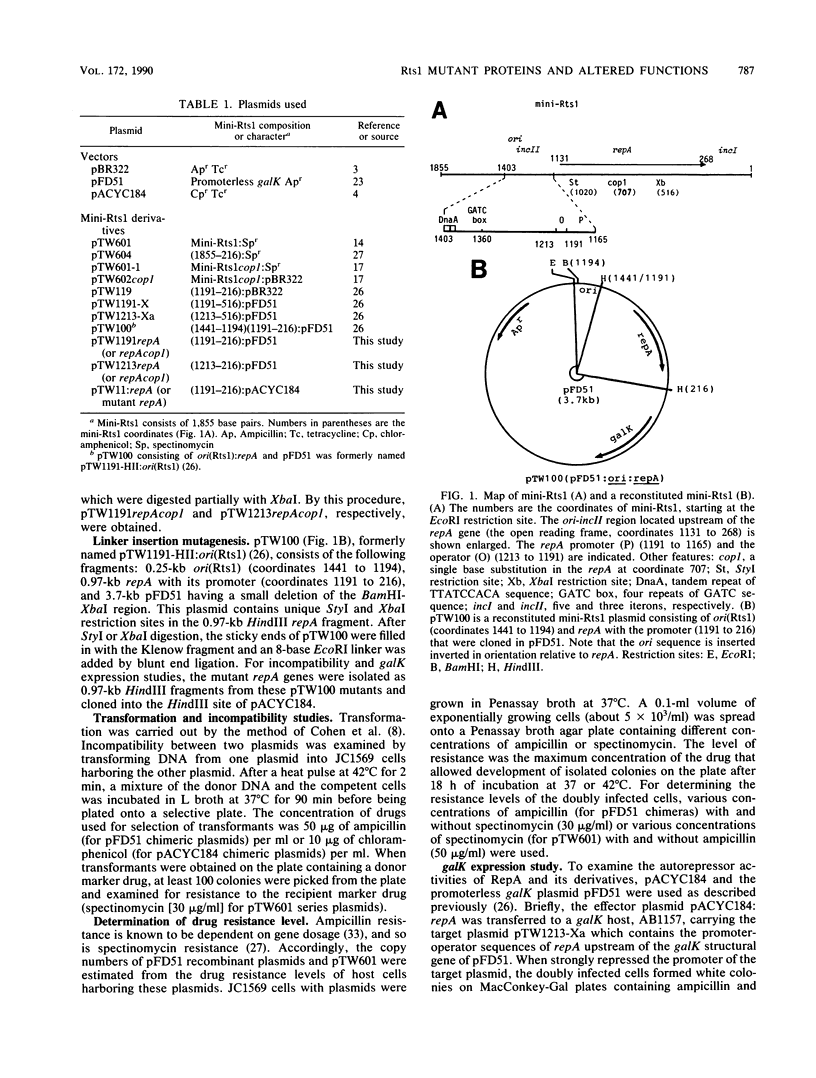





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles A. L., Austin S. J. P1 plasmid replication requires methylated DNA. EMBO J. 1987 Oct;6(10):3185–3189. doi: 10.1002/j.1460-2075.1987.tb02630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles A. L., Snyder K. M., Chattoraj D. K. P1 plasmid replication: replicon structure. J Mol Biol. 1984 Mar 5;173(3):307–324. doi: 10.1016/0022-2836(84)90123-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj D. K., Snyder K. M., Abeles A. L. P1 plasmid replication: multiple functions of RepA protein at the origin. Proc Natl Acad Sci U S A. 1985 May;82(9):2588–2592. doi: 10.1073/pnas.82.9.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filutowicz M., McEachern M. J., Helinski D. R. Positive and negative roles of an initiator protein at an origin of replication. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9645–9649. doi: 10.1073/pnas.83.24.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. A LOCUS THAT CONTROLS FILAMENT FORMATION AND SENSITIVITY TO RADIATION IN ESCHERICHIA COLI K-12. Genetics. 1964 Feb;49:237–246. doi: 10.1093/genetics/49.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inuzuka M., Wada Y. A single amino acid alteration in the initiation protein is responsible for the DNA overproduction phenotype of copy number mutants of plasmid R6K. EMBO J. 1985 Sep;4(9):2301–2307. doi: 10.1002/j.1460-2075.1985.tb03930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishaq M., Kaji A. Mechanism of T4 phage restriction by plasmid Rts 1. Cleavage of T4 phage DNA by Rts 1-specific enzyme. J Biol Chem. 1980 May 10;255(9):4040–4047. [PubMed] [Google Scholar]
- Itoh Y., Kamio Y., Furuta Y., Terawaki Y. Cloning of the replication and incompatibility regions of a plasmid derived from Rts1. Plasmid. 1982 Nov;8(3):232–243. doi: 10.1016/0147-619x(82)90061-0. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Kamio Y., Terawaki Y. Essential DNA sequence for the replication of Rts1. J Bacteriol. 1987 Mar;169(3):1153–1160. doi: 10.1128/jb.169.3.1153-1160.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Itoh Y., Terawaki Y. Purification of Rts1 RepA protein and binding of the protein to mini-Rts1 DNA. J Bacteriol. 1988 Sep;170(9):4411–4414. doi: 10.1128/jb.170.9.4411-4414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamio Y., Tabuchi A., Itoh Y., Katagiri H., Terawaki Y. Complete nucleotide sequence of mini-Rts1 and its copy mutant. J Bacteriol. 1984 Apr;158(1):307–312. doi: 10.1128/jb.158.1.307-312.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller J., Manis J., Kline B., Bishop A. Nonintegrated plasmid-folded chromosome complexes: genetic studies on formation and possible relationship to plasmid replication. Plasmid. 1978 Jun;1(3):273–283. doi: 10.1016/0147-619x(78)90045-8. [DOI] [PubMed] [Google Scholar]
- Murotsu T., Matsubara K., Sugisaki H., Takanami M. Nine unique repeating sequences in a region essential for replication and incompatibility of the mini-F plasmid. Gene. 1981 Nov;15(2-3):257–271. doi: 10.1016/0378-1119(81)90135-9. [DOI] [PubMed] [Google Scholar]
- Okawa N., Tanaka M., Finver S., Kaji A. Identification of the Rts 1 DNA fragment responsible for temperature sensitive growth of host cells harboring a drug resistant factor Rts 1. Biochem Biophys Res Commun. 1987 Feb 13;142(3):1084–1088. doi: 10.1016/0006-291x(87)91526-9. [DOI] [PubMed] [Google Scholar]
- Rak B., von Reutern M. Insertion element IS5 contains a third gene. EMBO J. 1984 Apr;3(4):807–811. doi: 10.1002/j.1460-2075.1984.tb01889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. R. Regulation of plasmid replication. Microbiol Rev. 1984 Mar;48(1):1–23. doi: 10.1016/b978-0-12-048850-6.50006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalker D. M., Kolter R., Helinski D. R. Nucleotide sequence of the region of an origin of replication of the antibiotic resistance plasmid R6K. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1150–1154. doi: 10.1073/pnas.76.3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Hong Z., Itoh Y., Kamio Y. Importance of the C terminus of plasmid Rts1 RepA protein for replication and incompatibility of the plasmid. J Bacteriol. 1988 Mar;170(3):1261–1267. doi: 10.1128/jb.170.3.1261-1267.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Itoh Y. Copy mutant of mini-Rts1: lowered binding affinity of mutated RepA protein to direct repeats. J Bacteriol. 1985 Apr;162(1):72–77. doi: 10.1128/jb.162.1.72-77.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Kakizawa Y., Takayasu H., Yoshikawa M. Temperature sensitivity of cell growth in Escherichia coli associated with the temperature sensitive R(KM) factor. Nature. 1968 Jul 20;219(5151):284–285. doi: 10.1038/219284a0. [DOI] [PubMed] [Google Scholar]
- Terawaki Y., Rownd R. Replication of the R factor Rts1 in Proteus mirabilis. J Bacteriol. 1972 Feb;109(2):492–498. doi: 10.1128/jb.109.2.492-498.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokino T., Murotsu T., Matsubara K. Purification and properties of the mini-F plasmid-encoded E protein needed for autonomous replication control of the plasmid. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4109–4113. doi: 10.1073/pnas.83.12.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomizawa J., Sakakibara Y., Kakefuda T. Replication of colicin E1 plasmid DNA in cell extracts. Origin and direction of replication. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2260–2264. doi: 10.1073/pnas.71.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlin B. E., Nordström K. R plasmid gene dosage effects in Escherichia coli K-12: copy mutants of the R plasmic R1drd-19. Plasmid. 1977 Nov;1(1):1–7. doi: 10.1016/0147-619x(77)90003-8. [DOI] [PubMed] [Google Scholar]
- Vocke C., Bastia D. Primary structure of the essential replicon of the plasmid pSC101. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6557–6561. doi: 10.1073/pnas.80.21.6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada C., Imai M., Yura T. Host control of plasmid replication: requirement for the sigma factor sigma 32 in transcription of mini-F replication initiator gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8849–8853. doi: 10.1073/pnas.84.24.8849. [DOI] [PMC free article] [PubMed] [Google Scholar]




