Abstract
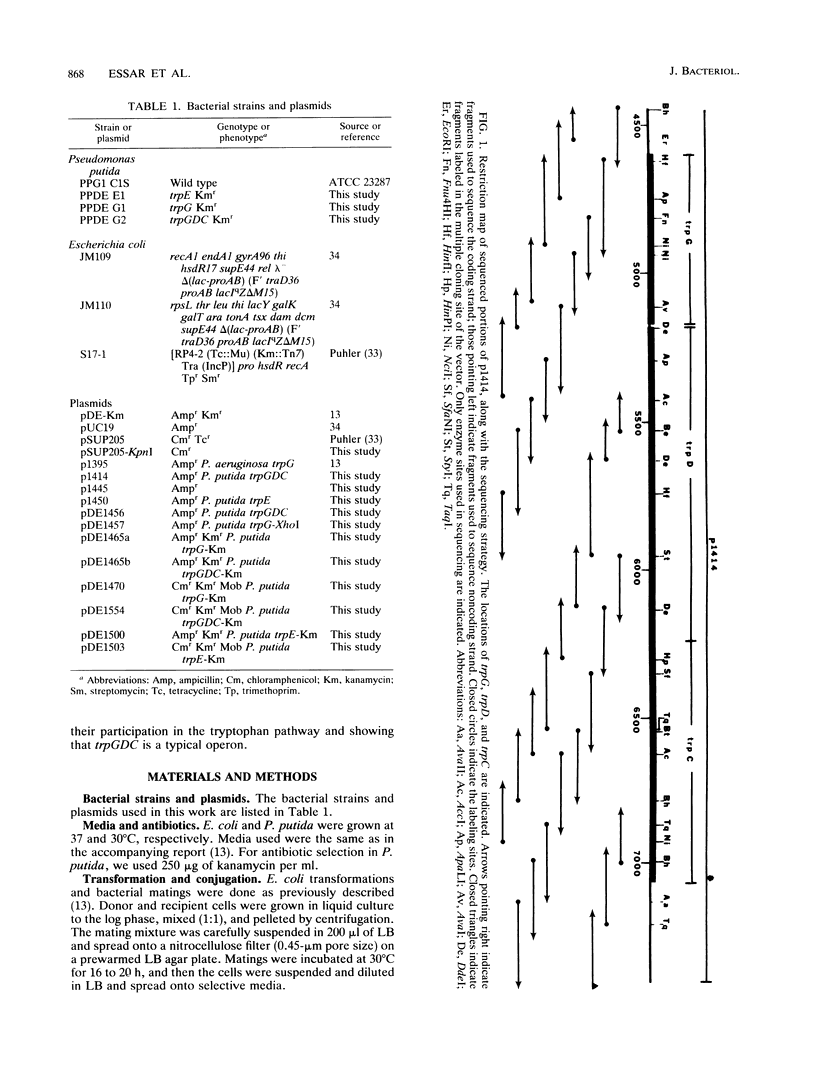
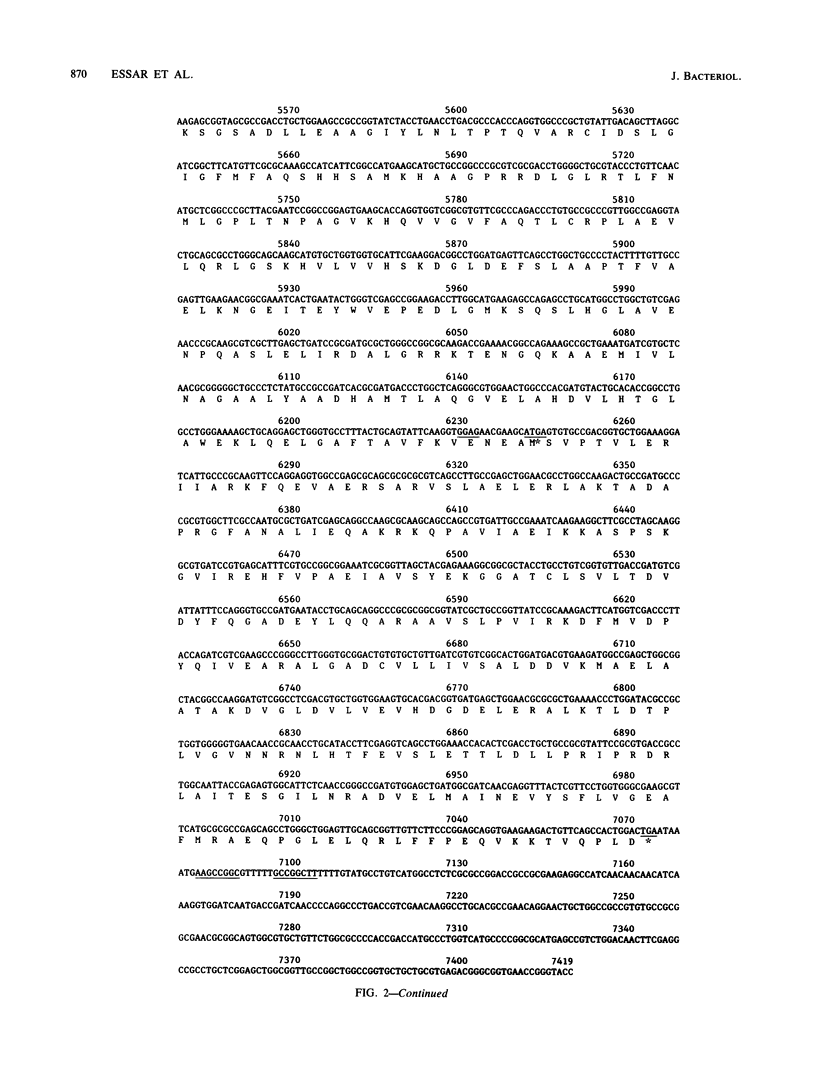
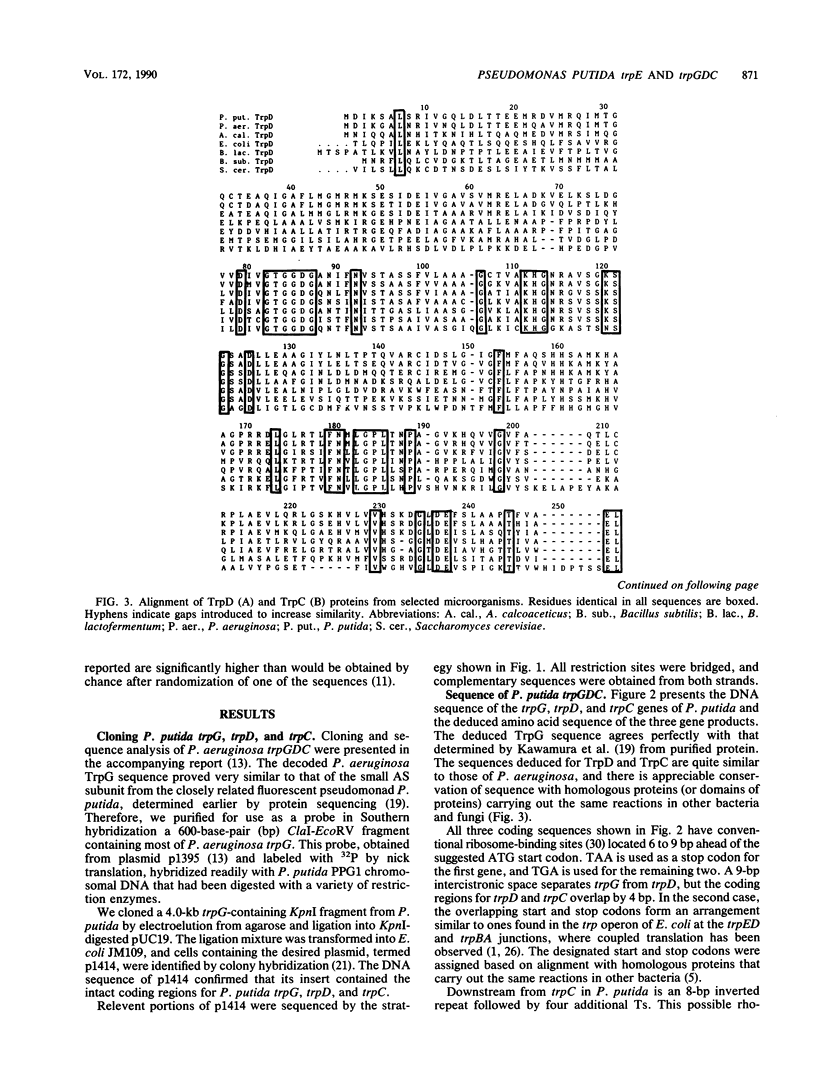
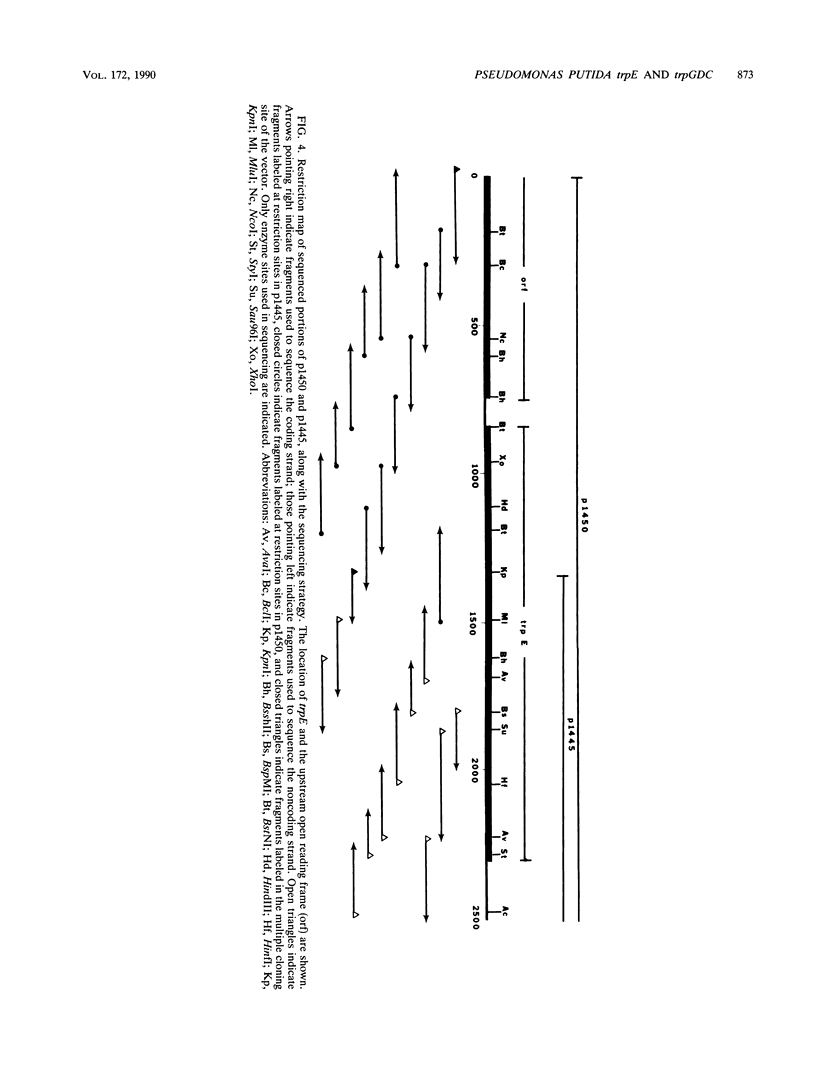
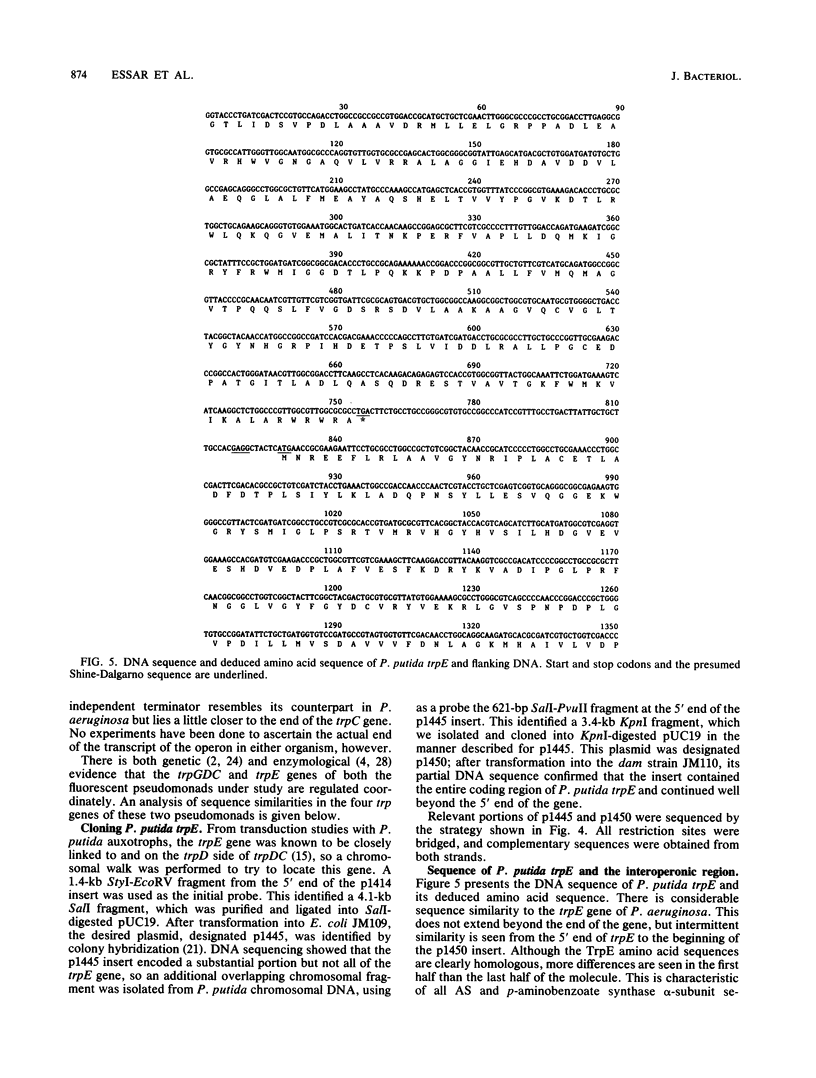
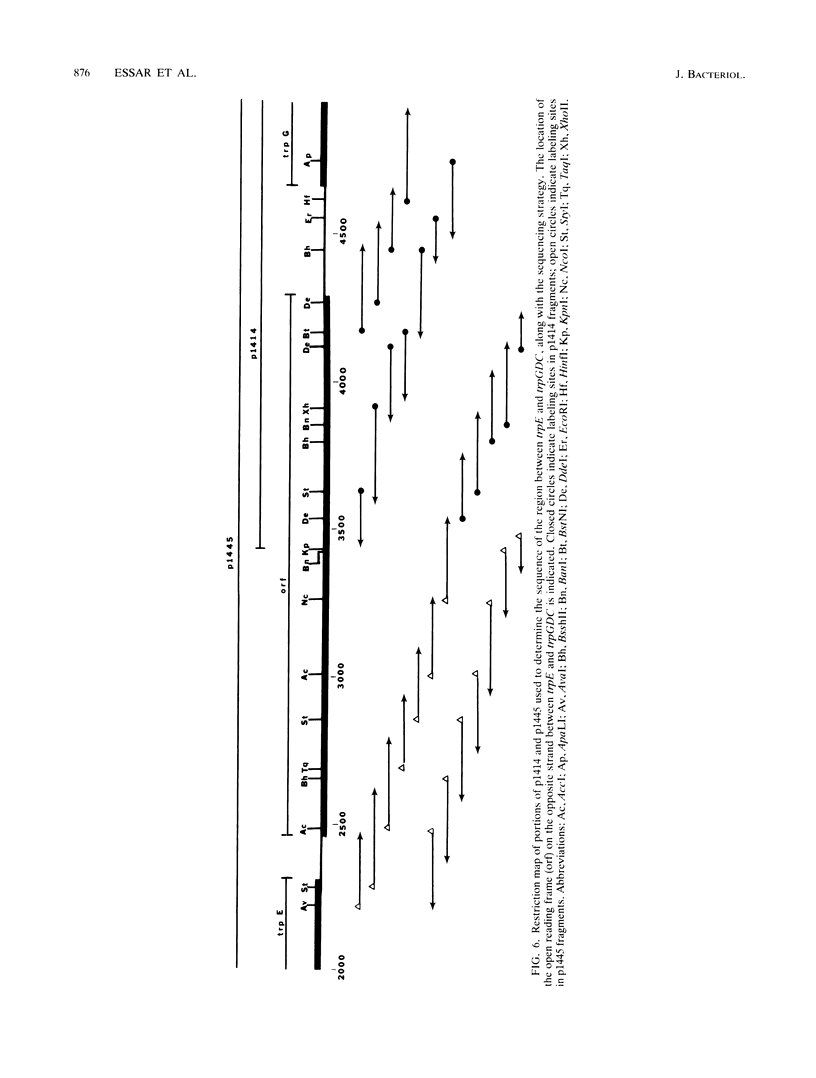
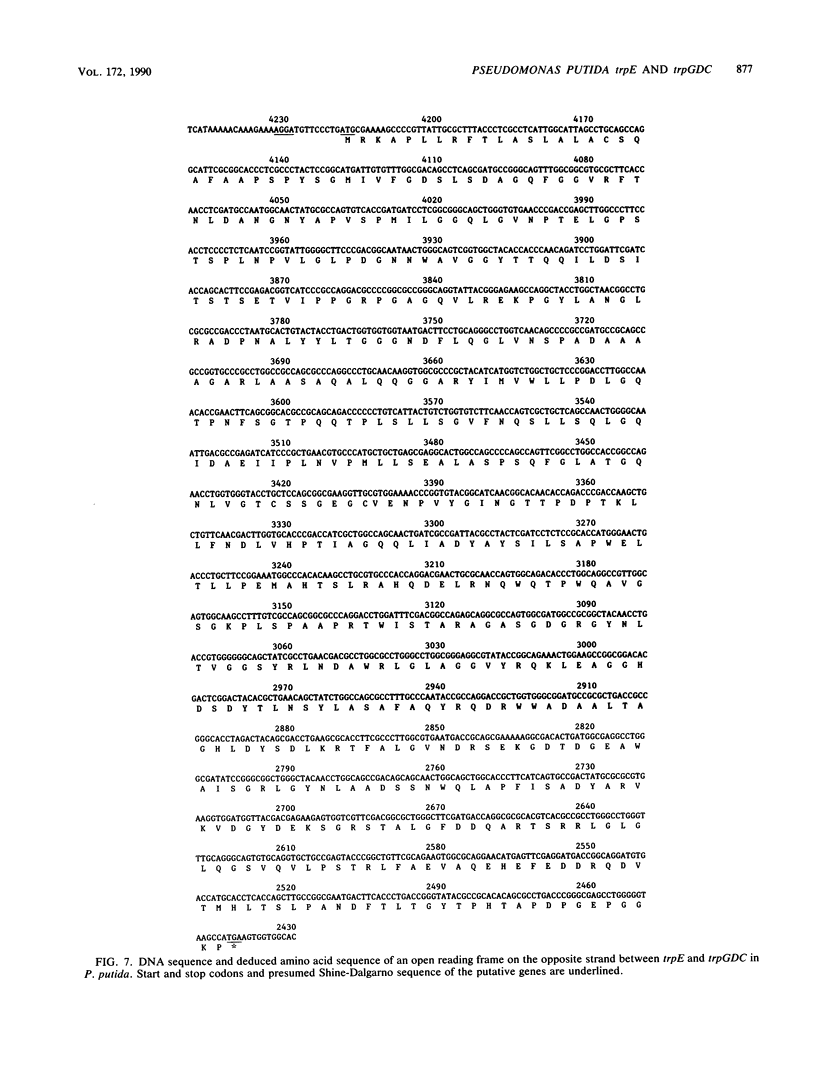
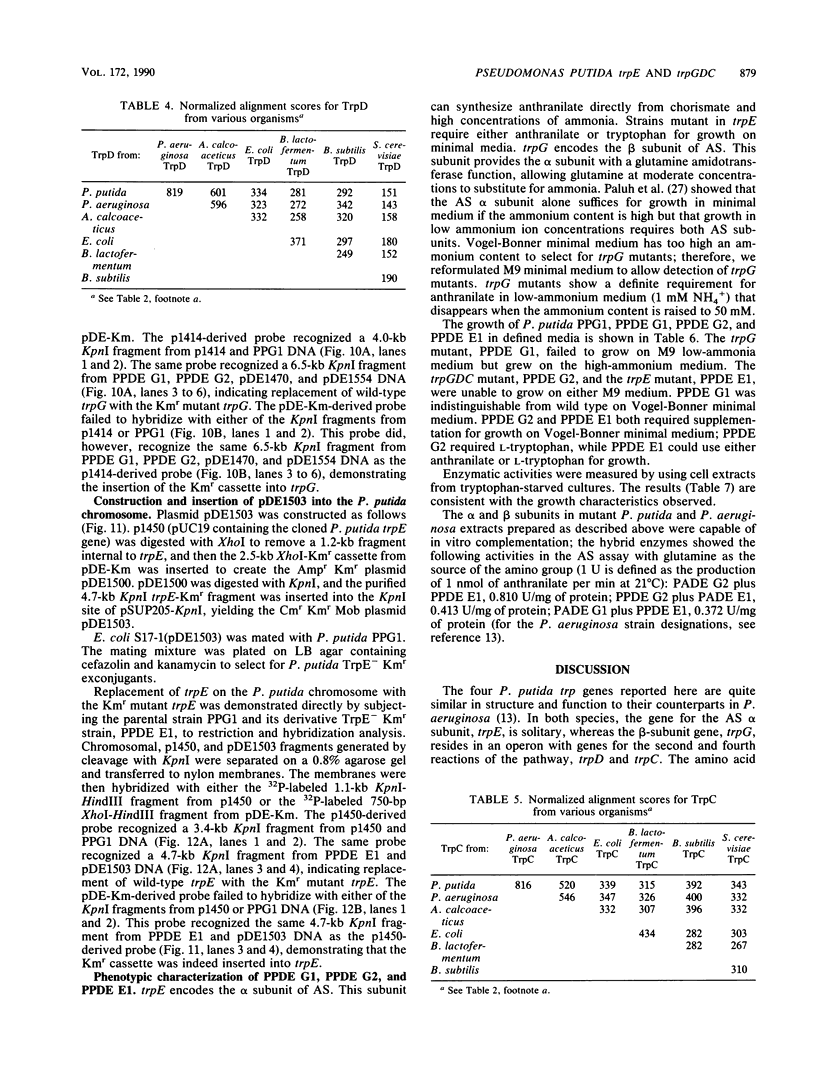
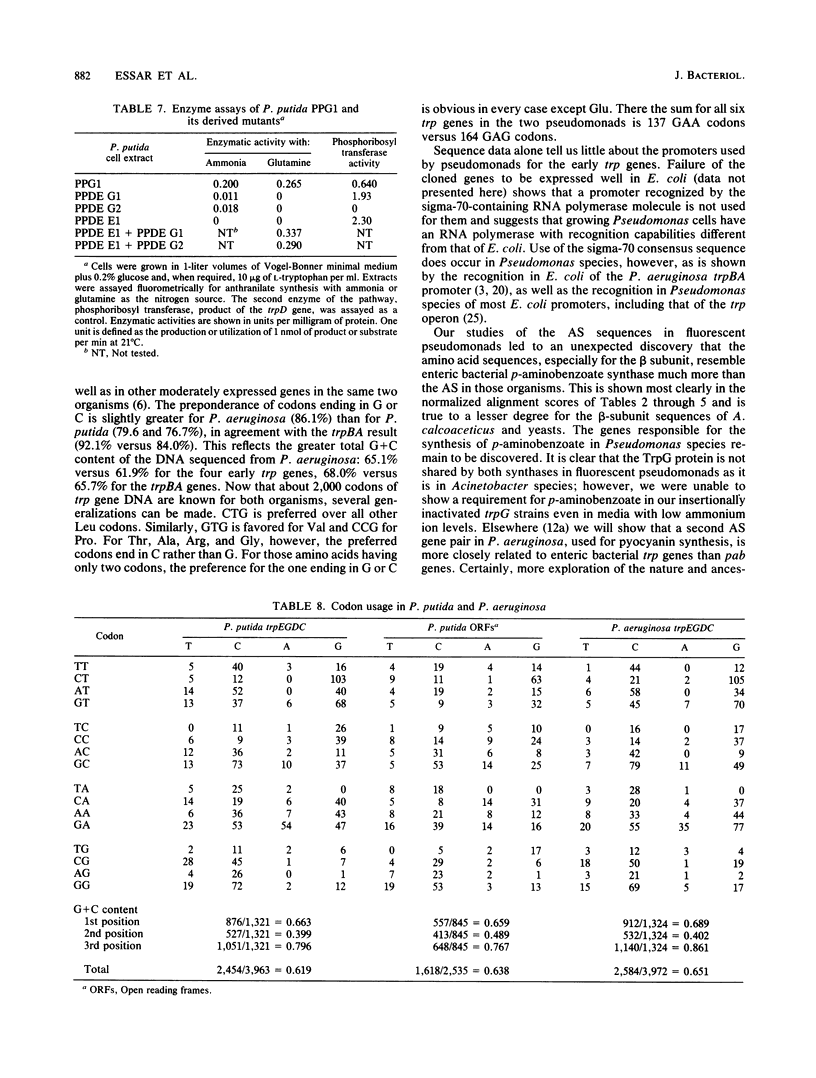
Pseudomonas putida possesses seven structural genes for enzymes of the tryptophan pathway. All but one, trpG, which encodes the small (beta) subunit of anthranilate synthase, have been mapped on the circular chromosome. This report describes the cloning and sequencing of P. putida trpE, trpG, trpD, and trpC. In P. putida and Pseudomonas aeruginosa, DNA sequence analysis as well as growth and enzyme assays of insertionally inactivated strains indicated that trpG is the first gene in a three-gene operon that also contains trpD and trpC. In P. putida, trpE is 2.2 kilobases upstream from the trpGDC cluster, whereas in P. aeruginosa, they are separated by at least 25 kilobases (T. Shinomiya, S. Shiga, and M. Kageyama, Mol. Gen. Genet., 189:382-389, 1983). The DNA sequence in P. putida shows an open reading frame on the opposite strand between trpE and trpGDC; this putative gene was not characterized. Evidence is also presented for sequence similarities in the 5' untranslated regions of trpE and trpGDC in both pseudomonads; the function of these regions is unknown, but it is possible that they play some role in regulation of these genes, since all the genes respond to repression by tryptophan. The sequences of the anthranilate synthase genes in the fluorescent pseudomonads resemble those of p-aminobenzoate synthase genes of the enteric bacteria more closely than the anthranilate synthase genes of those organisms; however, no requirement for p-aminobenzoate was found in the Pseudomonas mutants created in this study.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aksoy S., Squires C. L., Squires C. Translational coupling of the trpB and trpA genes in the Escherichia coli tryptophan operon. J Bacteriol. 1984 Feb;157(2):363–367. doi: 10.1128/jb.157.2.363-367.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun D. H., Pierson D. L., Jensen R. A. The regulation of tryptophan biosynthesis in Pseudomonas aeruginosa. Mol Gen Genet. 1973 Mar 1;121(2):117–132. doi: 10.1007/BF00277526. [DOI] [PubMed] [Google Scholar]
- Chang M., Hadero A., Crawford I. P. Sequence of the Pseudomonas aeruginosa trpI activator gene and relatedness of trpI to other procaryotic regulatory genes. J Bacteriol. 1989 Jan;171(1):172–183. doi: 10.1128/jb.171.1.172-183.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P. Anthranilate synthase from fluorescent Pseudomonads. Methods Enzymol. 1987;142:300–306. doi: 10.1016/s0076-6879(87)42040-5. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Eberly L. DNA sequence of the tryptophan synthase genes of Pseudomonas putida. Biochimie. 1989 Apr;71(4):521–531. doi: 10.1016/0300-9084(89)90183-1. [DOI] [PubMed] [Google Scholar]
- Crawford I. P. Evolution of a biosynthetic pathway: the tryptophan paradigm. Annu Rev Microbiol. 1989;43:567–600. doi: 10.1146/annurev.mi.43.100189.003031. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Wilde A., Yelverton E. M., Figurski D., Hedges R. W. Structure and regulation of the anthranilate synthase genes in Pseudomonas aeruginosa: II. Cloning and expression in Escherichia coli. Mol Biol Evol. 1986 Sep;3(5):449–458. doi: 10.1093/oxfordjournals.molbev.a040409. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Yanofsky C. Pseudomonas putida tryptophan synthetase: partial sequence of the subunit. J Bacteriol. 1971 Oct;108(1):248–253. doi: 10.1128/jb.108.1.248-253.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enatsu T., Crawford I. P. Pseudomonas putida tryptophan synthetase. J Bacteriol. 1971 Oct;108(1):431–438. doi: 10.1128/jb.108.1.431-438.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essar D. W., Eberly L., Hadero A., Crawford I. P. Identification and characterization of genes for a second anthranilate synthase in Pseudomonas aeruginosa: interchangeability of the two anthranilate synthases and evolutionary implications. J Bacteriol. 1990 Feb;172(2):884–900. doi: 10.1128/jb.172.2.884-900.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essar D. W., Eberly L., Han C. Y., Crawford I. P. DNA sequences and characterization of four early genes of the tryptophan pathway in Pseudomonas aeruginosa. J Bacteriol. 1990 Feb;172(2):853–866. doi: 10.1128/jb.172.2.853-866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharoff P., Nichols B. P. Evolution of aminobenzoate synthases: nucleotide sequences of Salmonella typhimurium and Klebsiella aerogenes pabB. Mol Biol Evol. 1988 Sep;5(5):531–548. doi: 10.1093/oxfordjournals.molbev.a040512. [DOI] [PubMed] [Google Scholar]
- Gunsalus C., Gunsalus C. F., Chakrabarty A. M., Sikes S., Crawford I. P. Fine structure mapping of the tryptophan genes in Pseudomonas putida. Genetics. 1968 Nov;60(3):419–435. doi: 10.1093/genetics/60.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W., Morgan A. F. Genome organization in Pseudomonas. Annu Rev Microbiol. 1986;40:79–105. doi: 10.1146/annurev.mi.40.100186.000455. [DOI] [PubMed] [Google Scholar]
- Kaplan J. B., Nichols B. P. Nucleotide sequence of Escherichia coli pabA and its evolutionary relationship to trp(G)D. J Mol Biol. 1983 Aug 15;168(3):451–468. doi: 10.1016/s0022-2836(83)80295-2. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Keim P. S., Goto Y., Zalkin H., Heinrikson R. L. Anthranilate synthetase component II from Pseudomonas putida. Covalent structure and identification of the cysteine residue involved in catalysis. J Biol Chem. 1978 Jul 10;253(13):4659–4668. [PubMed] [Google Scholar]
- Manch J. N., Crawford I. P. Genetic evidence for a positive-acting regulatory factor mediating induction in the tryptophan pathway of Pseudomonas aeruginosa. J Mol Biol. 1982 Mar 25;156(1):67–77. doi: 10.1016/0022-2836(82)90459-4. [DOI] [PubMed] [Google Scholar]
- Matsui K., Sano K., Ohtsubo E. Complete nucleotide and deduced amino acid sequences of the Brevibacterium lactofermentum tryptophan operon. Nucleic Acids Res. 1986 Dec 22;14(24):10113–10114. doi: 10.1093/nar/14.24.10113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R., Crawford I. P. New regulatory mutation affecting some of the tryptophan genes in Pseudomonas putida. J Bacteriol. 1971 May;106(2):331–338. doi: 10.1128/jb.106.2.331-338.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer R., Crawford I. P. Properties and subunit structure of the B component of Pseudomonas putida tryptophan synthetase. Arch Biochem Biophys. 1971 May;144(1):193–203. doi: 10.1016/0003-9861(71)90468-1. [DOI] [PubMed] [Google Scholar]
- Nagahari K., Sano Y., Sakaguchi K. Derepression of E. coli trp operon on interfamilial transfer. Nature. 1977 Apr 21;266(5604):745–746. doi: 10.1038/266745a0. [DOI] [PubMed] [Google Scholar]
- Oppenheim D. S., Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980 Aug;95(4):785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paluh J. L., Zalkin H., Betsch D., Weith H. L. Study of anthranilate synthase function by replacement of cysteine 84 using site-directed mutagenesis. J Biol Chem. 1985 Feb 10;260(3):1889–1894. [PubMed] [Google Scholar]
- Queener S. W., Queener S. F., Meeks J. R., Gunsalus I. C. Anthranilate synthase from Pseudomonas putida. Purification and properties of a two-component enzyme. J Biol Chem. 1973 Jan 10;248(1):151–161. [PubMed] [Google Scholar]
- Reznikoff W. S., Siegele D. A., Cowing D. W., Gross C. A. The regulation of transcription initiation in bacteria. Annu Rev Genet. 1985;19:355–387. doi: 10.1146/annurev.ge.19.120185.002035. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Ina S. Genetic comparison of bacteriophage PS17 and Pseudomonas aeruginosa R-type pyocin. J Bacteriol. 1989 May;171(5):2287–2292. doi: 10.1128/jb.171.5.2287-2292.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomiya T., Shiga S., Kageyama M. Genetic determinant of pyocin R2 in Pseudomonas aeruginosa PAO. I. Localization of the pyocin R2 gene cluster between the trpCD and trpE genes. Mol Gen Genet. 1983;189(3):375–381. doi: 10.1007/BF00325898. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]