Abstract
Leptin, a potent anorexigenic hormone is found in the anterior pituitary. The aim of this study was to determine if and how pituitary leptin-bearing cells were regulated by nutritional status. Male rats showed 64% reductions in pituitary leptin mRNA, but not serum leptin, 24 h after fasting, accompanied by significant 30-50% reductions in growth hormone (GH), prolactin, luteinizing hormone (LH), and 70-80% reductions in target cells for gonadotropin releasing hormone (GnRH) or growth hormone releasing hormone (GHRH). There was a 2—fold increase in corticotropes. Subsets (22%) of pituitary cells co-expressed leptin and GH and <5% co-expressed leptin and LH, prolactin, TSH, or ACTH. Fasting resulted in significant 55-75% losses in cells with leptin proteins or mRNA and GH or LH. To determine if restoration of serum glucose could rescue leptin, LH and GH, additional fasted rats were given 10% glucose water for 24 h. Restoring serum glucose in fasted rats resulted in pituitary cell populations with normal levels of leptin, GH, and LH cells. Similarly, LH and GH cells were restored, in vitro, after populations from fasted rats were treated for as little as 1 h in 10-100 pg/ml leptin. These correlative changes in pituitary leptin, LH and GH, coupled with leptin’s rapid restoration of GH and LH, in vitro, suggest that pituitary leptin may signal nutritional changes. Collectively, the findings suggest that pituitary leptin expression could be coupled to glucose sensors like glucokinase, to facilitate rapid responses by the neuroendocrine system to nutritional cues.
Keywords: Pituitary, Leptin, Growth Hormone, Luteinizing hormone, Adrenocorticotropin, Fasting, Glucose treatment, Cytochemistry, Cell culture, QRT-PCR, Rat
Introduction
Leptin is a product of the Ob gene that regulates satiety as well as energy expenditure (Rowland et al. 1996). Its presence was initially discovered in adipose tissue (Zhang et al. 1994); and it was first thought to be an appetite regulator by acting on specific neurons in the arcuate nucleus (Baranowska et al. 2001; Neary et al. 2004; Rowland et al. 1996; Vasselli 2001). Leptin directly stimulates the CART/POMC (cocaine and amphetamine RNA transcript / pro-opiomelanocortin) neurons in the arcuate nucleus and enhances the production of α-MSH (alpha-melanocyte-stimulating hormone), both appetite repressors. High leptin levels also inhibit functions of neurons producing neuropeptide Y/agouti related peptide (NPY/AgRP) neurons in the arcuate nucleus (Mizuno et al. 1999; Mizuno and Mobbs 1999), which are orexigenic in that they stimulate appetite (Chua et al. 1991; Ebihara et al. 1999; Korner et al. 2001; Mizuno et al. 1996; Mizuno et al. 1999; Mizuno and Mobbs 1999).
The ubiquitous distribution of leptin receptors has suggested that this cytokine performs additional functions throughout the body (Fruhbeck 2006). For example, leptin may regulate neuroendocrine systems, particularly in the reproductive system (Ahima et al. 1996; Casanueva and Dieguez 1999; Moschos et al. 2002). Rats without functional leptin receptors (Zucker fa/fa) and mice without functional leptin proteins (ob/ob) both exhibit characteristics of morbid obesity, insulin resistance, delayed or impaired pubertal development, and pituitaries with low numbers of somatotropes or gonadotropes (Isozaki et al. 1999; Popovic et al. 2001). Leptin levels are permissive for puberty, as levels of fat stores must reach a threshold for the animal to reproduce (Ahima et al. 1996; Casanueva and Dieguez 1999; Gonzalez et al. 1999; Mann and Plant 2002; Nagatani et al. 1998; Nagatani et al. 2000; Urbanski 2001). Without leptin proteins or receptors, rats will not reach full reproductive maturity, but exogenous leptin injections given to leptin deficient animals will restore fertility (Barash et al. 1996; Cheung et al. 1997).
Leptin regulates reproduction by direct effects on the hypothalamic-pituitary axis. It stimulates the secretion of both Luteinizing hormone (LH) and Gonadotropin releasing hormone (GnRH) (Finn et al. 1998; Gonzalez et al. 1999; Nagatani et al. 1998; Nagatani et al. 2000; Tezuka et al. 2002), and restores LH secretion in fasted mice, rats, hamsters and monkeys (Ahima et al. 1996; Finn et al. 1998; Gonzalez et al. 1999; Nagatani et al. 1998; Nagatani et al. 2000; Schneider et al. 2000; Schneider et al. 2002; Schneider et al. 1998; Schneider and Zhou 1999). Leptin also directly stimulates LH secretion from pituitary cells in vitro (De Biasi et al. 2001; Ogura et al. 2001; Walczewska et al. 1999; Yu et al. 1997a; Yu et al. 1997b). In addition, leptin also regulates Growth hormone (GH) (Casanueva and Dieguez 1999), although there is lack of agreement about the exact direction of its regulation (Aubert et al. 1998; Casanueva and Dieguez 1999; Pombo et al. 1999; Roh et al. 2001; Vuagnat et al. 1998). Leptin stimulates GH secretion mediated by GH releasing peptide (GHRP (Chen et al. 2001; Roh et al. 2001) and restores GH pulses in fasted rats (Pombo et al. 1999; Vuagnat et al. 1998).
A growing body of evidence suggests, however, that the adipocyte source of leptin may be too sluggish to regulate rapid neuroendocrine responses. For example, the rise in serum leptin is too slow for it to be a regulator of the LH surge (Akhter et al. 2007). Also, Schneider et al report that LH pulses lost by fasting, could be restored within a few hours, by nutrition alone (Schneider et al. 2000; Schneider et al. 2002). The timing of this response was too rapid for it to be due to the restoration of adipocyte leptin (Bronson 1986; Bronson and Heideman 1990; Cameron 1996).
This evidence led to the hypothesis that local sources of leptin, such as those in the pituitary, might be part of a neuroendocrine regulatory circuit. Supportive evidence from our laboratory demonstrated a sharp rise in pituitary leptin just before the LH surge and that GnRH could stimulate pituitary leptin secretion (Akhter et al. 2007). To further investigate its potential role in the pituitary, we tested additional in vivo and in vitro conditions to determine states that might regulate leptin.
We hypothesized that pituitary leptin might be regulated by the nutrient status. It was already known that significant changes in the adipocyte leptin mRNA expression can be seen as early as 8 hours (Gui et al. 2003; Kowalska et al. 1999; Zhang et al. 1994), with losses in leptin mRNA of 50% (Igel et al. 1996) or 85-90% (MacDougald et al. 1995; Mizuno et al. 1996) in 24 h. Therefore, we first developed an in vivo model of food deprivation in which the expression of pituitary leptin was selectively reduced, without changes in serum leptin. After the initial studies showed parallel reductions in pituitary leptin, LH and GH, the experiments were expanded to determine if changing nutrients, such as serum glucose, in vivo, or exogenous leptin, in vitro could restore the losses in gonadotropes and somatotropes. This report presents these findings, which support the hypothesis that pituitary leptin is regulated by nutrient status and that as little as 10 pg/ml exogenous leptin for 1 h, in vitro can restore expression of GH and LH in cells from fasted animals.
Materials and Methods
Collection of Pituitaries
Male and female Sprague-Dawley rats obtained from Harlan Sprague Dawley (Indianapolis, IN) were used throughout this study. Animals were housed three per cage with a 12-hr light-dark cycled maintained (lights on at 0600) at a constant room temperature of 68C. A standard pellet chow diet (Harlan Teklad-Rodent diet #8640; Madison WI) and water were available ad libitum (in all animals in all experiments except for the fasting experiments). Animals were allowed to become acclimated for approximately 2 weeks before any experiments commenced. The rats were anesthetized with IP injections of sodium pentobarbital (24 mg/kg or 0.06ml/250g rat) and then sacrificed via guillotine. The animal care protocol was approved, annually, by the Animal Use and Care Protocol Committee.
Fasting Experiments
At least 12 major groups of weight and age-matched male rats were used for these studies. Some of the groups of rats were taken to provide whole anterior pituitaries for either protein or mRNA extracts; others provided pituitary cells for the analysis of hormone stores by cytochemistry. The experimental design for the fasting was similar, irrespective of the end point assays.
At 0900 male rats were divided into two or three separate groups with 2-3 rats in each group. The animals were weighed and given a known amount of water (controls and fasted). In the last 4 groups, the third group of animals were given glucose water (glucose fasted). All rats were kept in separate cages for a period of 24hr. For the controls a standard pellet chow diet (Harlan Teklad-Rodent) and water were available ad libitum. The animals that were strictly fasted were only allowed water ad libitum, and the fasted glucose rats were only allowed a 5% or 10% glucose (Sigma G7021-1KG, St. Louis, MO) solution ad libitum. After a glucometer detected changes in serum glucose in these rats, we used only those treated with 10% glucose, because rats given 5% glucose did not have normal serum glucose. These data will be shown in Table 1.
TABLE 1.
| Changes ina | ||
|---|---|---|
| Serum Glucose (mg/dl)b | Weight (g)c | |
| Fed/Control | 166 ±4 | +11 ±4 |
| Fasted | 71 ± 5 | −18 ± 2.6 |
| Fasted+Glucose | 144 ± 16 | −9 ± 1 |
Average of three experiments ± SEM; nine rats/group.
Fasted<fed or fasted+glucose: p<0.007.
Fasted or fasted+glucose<fed: p<0.001.
The fasted and fasted glucose conditions were held for a period of 24 hours. Pituitaries were taken during the morning 24 hours prior to the start of the fast. At 0900 the next day the rats were anesthetized, re-weighed, and sacrificed. Trunk blood was taken and the amount of water consumed was recorded. Serum was collected for leptin EIA (ALPCO Diagnostics, Salem, NH). Serum glucose was also measured by a glucometer, as stated above.
Dispersion of Pituitary Cells
Pituitaries from the rats were removed and dispersed into single suspensions by the following protocol. After gently teasing away and discarding the neurointermediate lobe, the remaining APs were chopped into small pieces and transferred to a 15-ml tube that contained DME and then centrifuged for 1 min at 400 rpm. The anterior pituitaries were then dispersed, as previously described (Akhter et al. 2007; McDuffie et al. 2004), and plated for 1 h on glass coverslips coated with poly-D-Lysine to enhance cell adhesion in Dulbeccos Modified Eagle’s media (DME), which is serum free. Viability tests showed 99-100% viability during this brief culture period, and the cells remained rounded after settling on the coverslips. These very short term (1 h) cultures are used for reasons outlined in our recent report (Akhter et al. 2007). To avoid stimulation of leptin by additives in the media, the cultures were allowed to settle in as little time as possible in basic DME. The cells were fixed with 2.5% glutaraldehyde diluted in 0.1 M of phosphate buffer for 30 min at room temperature (RT). Fixation was followed by washes with 0.1M phosphate buffer containing 4.5% sucrose and glycine 4 times for 15 min.
Cytochemical Labeling Protocols
In situ hybridization involved the application of pre-hybridization reagents followed by 10-100 ng/ml biotinylated probe complementary to leptin mRNA, as described recently (Akhter et al. 2007; McDuffie et al. 2004). GH mRNA was detected with 5-10 ng/ml of biotinylated probe, according to our recent studies (Childs et al. 2005; Childs et al. 2000; Iruthayanathan et al. 2005) and the LHβ mRNA was detected with 20-50 ng/ml biotinylated probe, as previously described (Childs et al. 1992; Childs et al. 1994b). The probes were produced by GeneDetect.com (www.GeneDetect.com), by Greenstar® protocols that provided high levels of biotin per probe. After 12-15 h hybridization at 39.6° C (leptin probe) or 35.6° C (GH or LHβ probes), the probes were detected by monoclonal anti-biotin and streptavidin peroxidase in a sandwich technique, as described previously (Akhter et al. 2007; Childs et al. 2005; Childs et al. 1992; Childs et al. 1994b; Iruthayanathan et al. 2005; McDuffie et al. 2004). Controls included the substitution of sense probes for the antisense probes, or the omission of the antisense probe in the sequence.
Immunocytochemical labeling for leptin proteins involved the protocol described in our previous publications (Akhter et al. 2007; McDuffie et al. 2004) with 1:37,000 anti-rat leptin (Sigma-Aldridge, St. Louis, Mo). The controls for leptin immunolabeling are reported and illustrated in our recent publications (Akhter et al. 2007; McDuffie et al. 2004). Dual labeling detected leptin with ACTH, TSH, LH, GH or prolactin according to recent studies (Childs et al. 2005). The antisera dilutions used are 1: 7,000 anti-rat prolactin (Childs et al. 1999); 1:70,000 anti-rat TSH-β (Childs et al. 1989); 1:200,000 anti-rat GH (NIH, Hormone Distribution Program)(Childs et al. 2005; Childs et al. 1999; Childs et al. 1994b; Iruthayanathan et al. 2005; McDuffie et al. 2004) ; 1:80,000-100,000 anti 17-39ACTH (Childs et al. 1989) ; and 1:100,000 anti-bLHβ (Akhter et al. 2007; Childs et al. 1992; Childs et al. 1999; Childs et al. 1994b) (a gift from Dr. JG Pierce). The controls for each of these antisera are described in each of these previous studies (Akhter et al. 2007; Childs et al. 2005; Childs et al. 1992; Childs et al. 1999; Childs et al. 1994b; Childs et al. 1989; Iruthayanathan et al. 2005; McDuffie et al. 2004).
Affinity Cytochemical labeling for biotinylated analogs of GnRH or GHRH was done, as described in recent studies (Childs et al. 1994a; Childs et al. 1999). Freshly dispersed, living cultures were incubated in biotinylated GnRH or biotinylated GHRH for 10 min and then fixed in 2% glutaraldehyde for 30 min. These analogs have a potency equal to that of native GnRH or GHRH and only bind receptors in healthy, living pituitary cells (Childs et al. 1994a; Childs et al. 1999) The target cells for each of the analogs were detected by avidin-biotin-peroxidase complex (ABC; Vector Laboratories; Burlingame, CA) and nickel intensified diaminobenzidine, as described previously (Childs et al. 1994a; Childs et al. 1999). Over 90% of target cells for biotinylated GnRH store gonadotropins (Childs et al. 1994a), although a subset of these are somatogonadotropes and express GH. Similarly, greater than 90% of target cells for GHRH store GH (Childs et al. 2005; Childs et al. 1999), although a subset of GHRH target cells express gonadotropins and prolactin (Childs et al. 1999). Controls for these protocols have been described in previous studies (Childs et al. 2005; Childs et al. 1994a; Childs et al. 1999). They demonstrate that 10-100 fold excess of non-biotinylated GnRH or GHRH compete successfully for the receptor site and neutralize labeling for their respective biotinylated neuropeptide. However, the same concentrations of other neuropeptides do not compete for the receptors and labeling is unaffected.
Image Analysis
The Bioquant Nova image analysis system (Bioquant, Nashville, Tenn) was used to digitize the images (taken at 40 X) and analyze the density of the label, as described previously (Akhter et al. 2007; Iruthayanathan et al. 2005). In addition, counts of labeled cells were done following single or dual labeling, as described previously (Akhter et al. 2007; Childs et al. 2005; Childs et al. 1992; Childs et al. 1994a; Childs et al. 1999; Childs et al. 1994b; Childs et al. 1989; Iruthayanathan et al. 2005; McDuffie et al. 2004) to learn if the treatment affected percentages of cells bearing leptin with and without pituitary hormones.
Immunoassays
The leptin mouse/rat Enzyme Immunoassay (EIA) kit by American Laboratory Products Company (ALPCO Diagnostics, Salem, NH), was used to detect serum leptin proteins, which were frozen in small aliquots after they were collected. In addition, some serum samples were sent to LINCO Diagnostics for radioimmunoassay (RIA). These assays are described in previous studies (Akhter et al. 2007). Interassay and intra-assay variation coefficients were < 4.7% and < 4.4%, respectively.
Protein Extraction
Pituitary cells from 3 rats/group (described above) were dispersed and resuspended in 2000 μl of DME as previously described (McDuffie et al. 2004). The cells were spun at 4400 rpm for 10 min and then resuspended in 100 μl of NP40 lysis buffer, containing 1μl each of the following freshly added protease inhibitors: aprotinin, leupeptin, and phenylmethylsulphonylfluoride (PMSF). The cell lysate was mixed with a pipette and aliquoted in small tubes. It was then spun at 4° C for 10 min, the supernatant was collected, and frozen for storage at -80° C.
RNA extraction, cDNA synthesis and QRT-PCR
RNA extraction and cDNA synthesis
Whole anterior pituitaries from 3 rats/ group X 3 groups were placed in RLT buffer (Qiagen) containing β mercaptoethanol (as per manufacturer’s protocol) and homogenized. The extraction and cDNA synthesis are as described recently (Akhter et al. 2007; Iruthayanathan et al. 2005). Leptin mRNA was assayed by QRT-PCR as described in previous studies (Akhter et al. 2007; Iruthayanathan et al. 2005), in a Roche Light Cycler 1.5 (Roche, Indianapolis, IN) with the FAST-START DNA Master SYBR Green I enzyme mix (Roche, Indianapolis, IN). Housekeeping genes used to normalize the readings were rat ribosomal protein subunit 9 (Rps9) or hypoxanthine guanine phosphoribosyltransferase (Hprt). Tests of each housekeeping gene showed no changes in expression with fasting and the primers for these genes are described in a previous publication (Iruthayanathan et al. 2005).
The forward primer for rat leptin was 5’-CCAGGATCAATGACATTTCACA-3’ (179 to 200) and the reverse primer was 5’-AATGAAGTCCAAACCGGTGA-3’ (230 to 249) (Accession number NM_013076) (Akhter et al. 2007). The primers and conditions for GH are described previously (Iruthayanathan et al. 2005). Values were expressed as a ratio of the number of transcripts of the gene to that of one of the housekeeping genes.
Statistics
One-Way ANOVA was used to detect differences in a given set of experimental groups. Significant differences were then detected by the Fisher’s Least Significant differences post-hoc test, or Student’s T test. p<0.05 was considered significant. A posthoc Power Analysis was done to establish the number of replicates needed, as described previously (Akhter et al. 2007).
Results
Pituitary Cell Types Affected by Food Deprivation
The pilot studies done on the first 3 groups of rats (5 rats/group) showed a weight loss in the fasted rats of 30 gm over the 24 h period. Serum leptin in the fed group averaged 1.866 ± 0.467 ng/ml and that in the fasted group averaged 2.3 ± 0.7 ng/ml. There were no significant differences in serum leptin between the two groups.
The first objective of this study was to determine the effect of short term fasting on the pituitary cells themselves. Figure 1 illustrates the cell counts, showing that fasting caused declines in percentages of pituitary cells with GH (by 31%, p<0.01), prolactin (by 53% p<.001) and LH (by 50%, p<0.001). In contrast however, fasting increased percentages of corticotropes over 2X (p<0.001) from 10 to 21% of AP cells. The percentages of thyrotropes appeared unaffected by fasting. Figures 1B-G illustrate the changes in immunolabeled corticotropes, somatotropes and gonadotropes.
Figure 1. Effect of Food Deprivation on Each Pituitary Cell type.
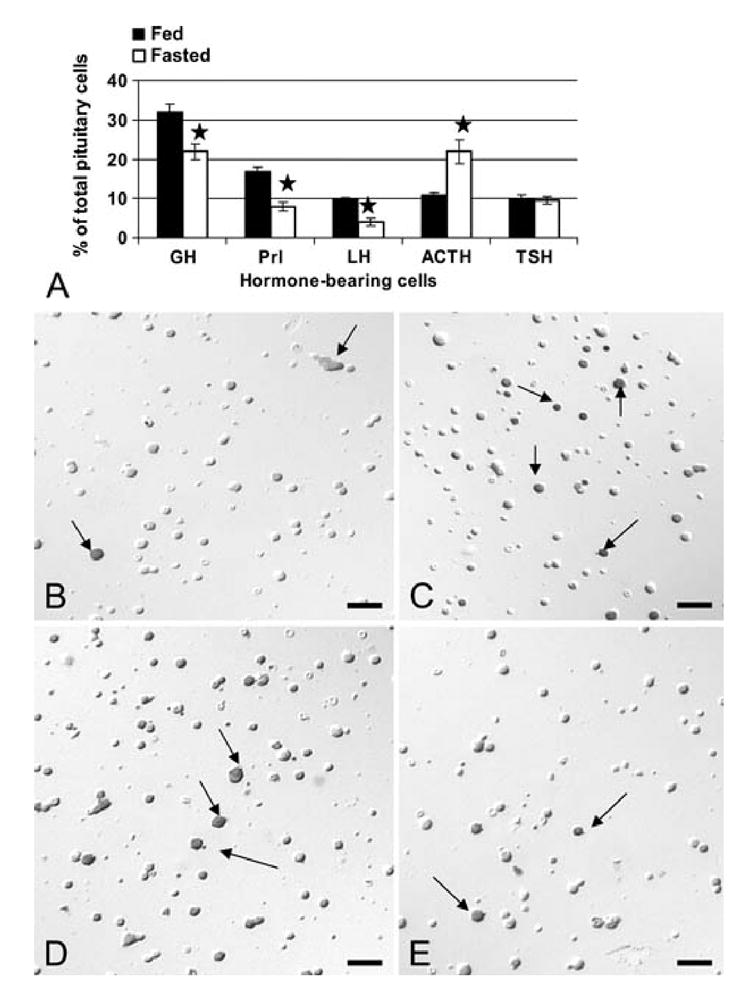
Counts and photographs show the effects of a 24 h food deprivation on pituitary cells detected by immunolabeling. Figure 1A shows the analysis of changes in the percentages of each cell type in the anterior pituitary (AP). The stars indicate a significant difference when compared with the populations from fed rats. The photographs were taken of freshly dispersed cells fixed after 1 h in culture and immunolabeled for one of the 6 pituitary hormones. The fields were imaged and photographed under Nomarski optics. Therefore, whereas a gray shadow may provide image depth showing the cells in 3-D, the immunolabeling itself is black (arrows). Figure 1B and C illustrates the increased percentages of corticotropes immunolabeled with anti- 17-39ACTH comparing fed (Fig 1B) and fasted (Fig 1C) rats. Figures 1D and E illustrate immunolabeled GH cells in fed (Fig 1D) and fasted (Fig 1E) rats. Figures 1F and G depict immunolabeled LH cells in fed (Fig 1F) and fasted (Fig 1G) rats. Arrow=immunolabeled cell. Bar=15 μm
Focused Studies of Changes in Gonadotropes and Somatotropes
The studies then focused on other changes in gonadotropes and somatotropes, because they were affected most severely by the food deprivation. Detection of GH mRNA showed that somatotropes had not disappeared from the population. The counts showed a slight increase from 33 ± 2% to 40 ± 2% (SD, p<.001) of anterior pituitary cells. In contrast, cells with LH mRNA detected by in situ hybridization showed a 50% decline in expression from 14 ± 2% to 7 ± 3% (±SD, p<.001).
Tests of gonadotrope and somatotrope function also included the detection of changes in binding sites for GnRH or GHRH with protocols that detect biotinylated analogs of GnRH or GHRH (Childs et al. 1983a, 1983b; Childs et al. 1994a; Childs et al. 1999). Figure 2 shows that fasting caused an 80% reduction in expression of GHRH receptive cells (p<0.001) and a 73% reduction in GnRH receptive cells (p<0.001). Figures 2B and C illustrate these cell populations in fed and fasted rat pituitary populations.
Figure 2. Effect of Food Deprivation on the Expression of Pituitary GHRH or GnRH receptors.
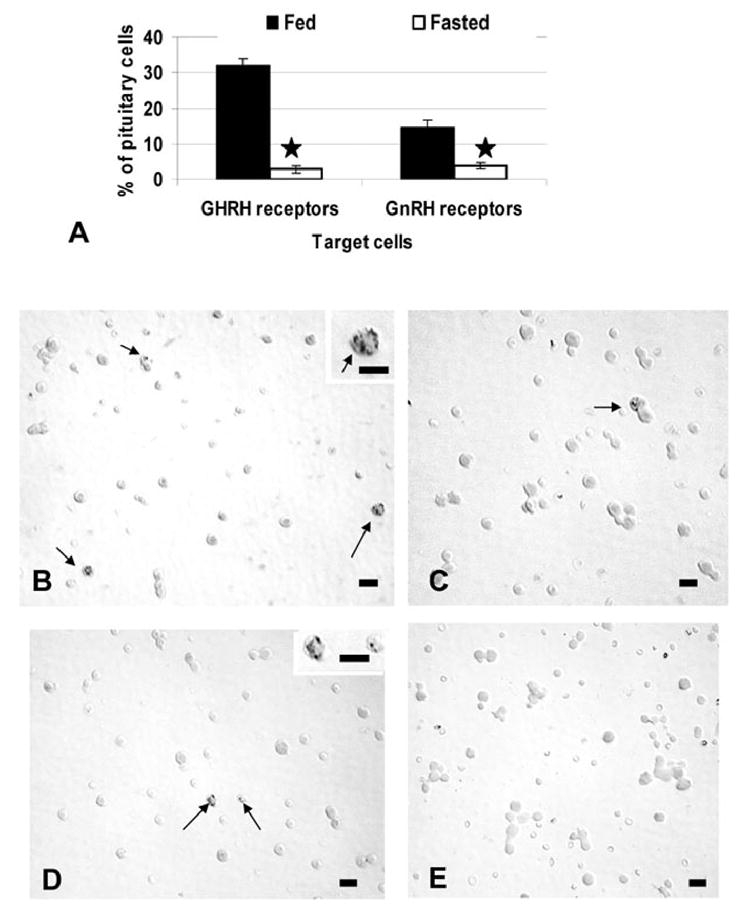
Living, freshly collected AP cells were plated for 45 min and exposed to 1 nM biotinylated GnRH or GHRH for 10 min. After fixation, the biotinylated analogs were detected at their binding sites by avidin biotin complex and counts of labeled target cells were then performed. Figure 2a illustrates the loss in the percentages of GHRH or GnRH target cells with fasting. (stars=significant reduction). Figures 2b and 2c compare fields labeled for biotinylated GHRH in fed (Fig 2b) and fasted (Fig 2c) animals. At least three labeled GHRH target cells are seen in the population from fed animals (arrows) and those from fasted animals may have 1 or less/field. Starting levels of GnRH receptive cells are lower (15% of the population) and Figures 2d and 2e compare fields from fed (Fig 2d) and fasted (Fig 2e) male rats to show how infrequent the GnRH target cells are in the fasted group. Bar=15 μm.
Fasting Effects on Leptin Proteins and mRNA
Food deprivation brought about a significant 64% reduction in the overall percentages of pituitary cells with leptin mRNA from 33 ± 2 to 12 ± 1% of anterior pituitary cells (p<0.001). Image analyses also showed a 40% reduction in the integrated optical density of label (p=0.03) (data not shown). In these first groups of fasted animals, there was also a 22% reduction in the percentages of cells with leptin proteins, from 36 ± 2% to 29 ± 2 % of AP cells (±SE; p<0.029) (Figure 3A). Figures 3B-G illustrate the in situ hybridization and the reduction in cells with leptin mRNA.
Figure 3. Effect of Food Deprivation on Pituitary Cells with Leptin mRNA or Proteins.
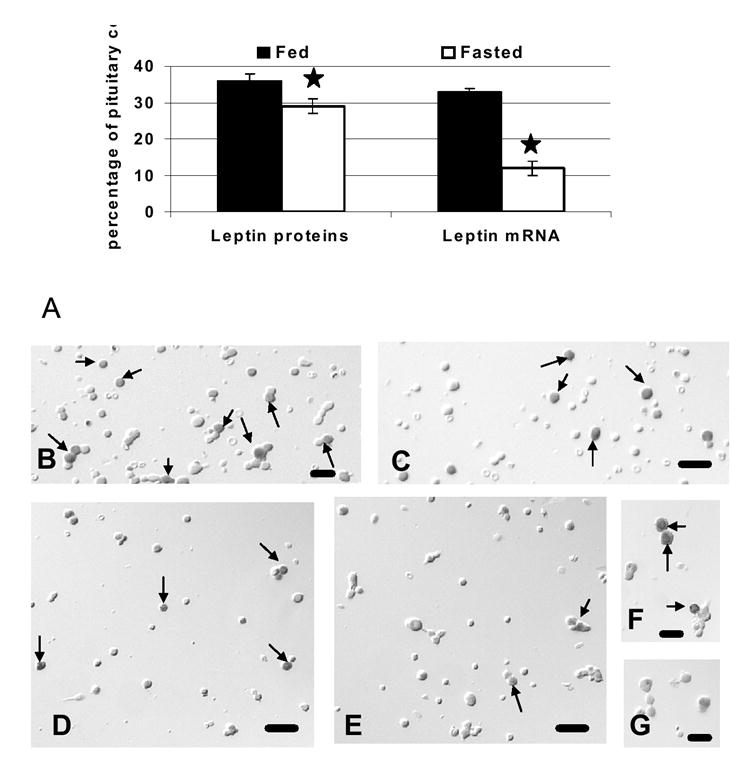
Analysis of the expression of pituitary leptin after 24 h of food deprivation. Figure 3A shows changes in the percentages of cells with leptin proteins or mRNA. Figure 3B shows changes in pituitary leptin mRNA, detected by QRT-PCR as a ratio of leptin mRNA/Hprt housekeeping gene mRNA. The stars indicate values different from those of fed rat cell populations. Figures 3C-H include photographs taken of freshly dispersed pituitary cells with Nomarski optics. Figures 3C and 3D show leptin protein bearing cells in populations from fed (Fig 3C) and fasted (Fig 3D) rats. Figures 3E and 3F illustrate leptin mRNA-bearing cells in fed (Fig 3E) and fasted (Fig 3F) rats. Figures 3G and 3H compare higher magnifications of cells with leptin mRNA showing the loss in individual cells in the population from fasted rats (Fig 3H) compared with that from fed rats (Fig 3G). Bar=15 μm.
Effect of Fasting on Differential Expression of Leptin by AP Cells
The next objective of these studies was to learn which pituitary cell types were most affected by the food deprivation, specifically in their production of leptin. Figure 4 illustrates the changes in the percentages of cells with leptin and each pituitary hormone in the anterior pituitary. In normal, fed populations, cells that co-express leptin and GH are 22% of the anterior pituitary population and this subset represents 84% of GH cells; cells that co-express leptin with LH, or TSH, or ACTH are 2-3% of the pituitary population (20-30% of each of these cell types); those with leptin and prolactin constitute only 1% of pituitary cells or 0.6% of prolactin cells..
Figure 4.
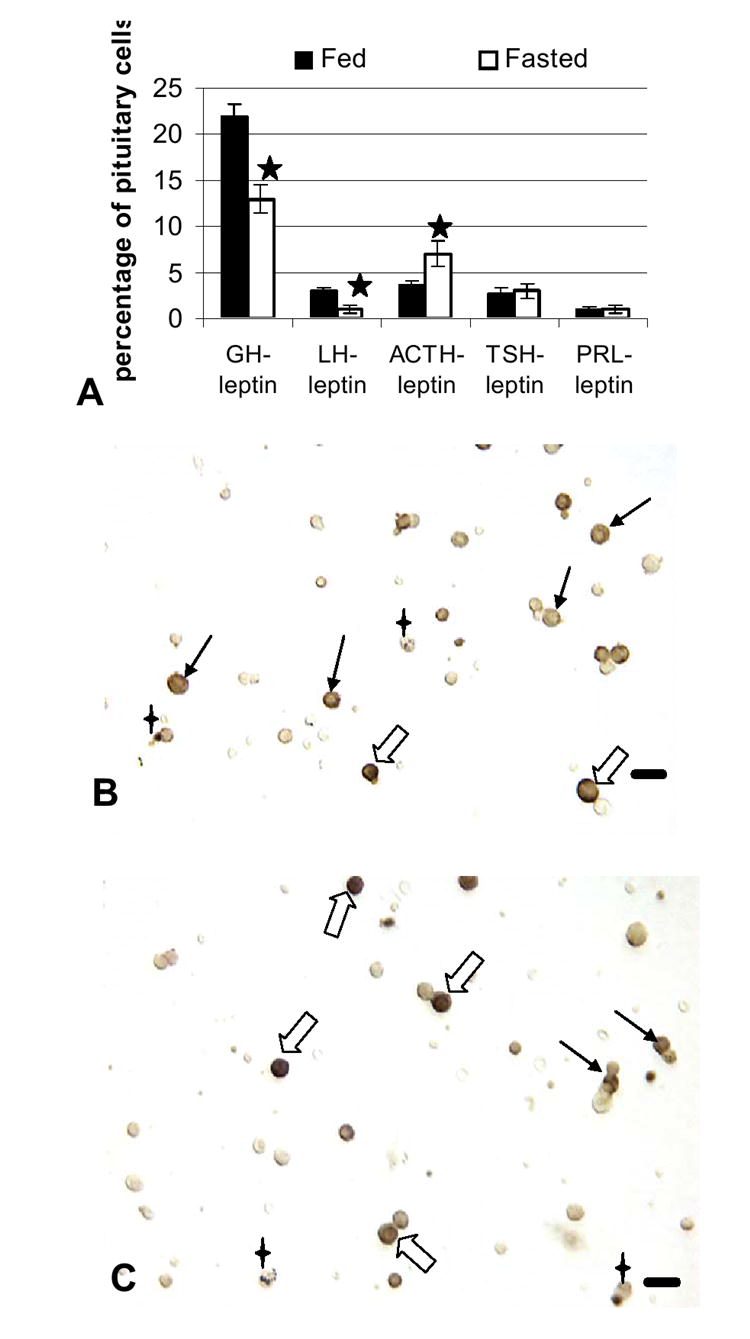
Pituitary cells that express leptin: Effects of food deprivation Dual labeling for leptin proteins and each of the pituitary hormones was done on freshly dispersed pituitary cells. Figure 4A shows a significant decrease in percentages of AP cells with leptin proteins and GH or LH and an increase in percentages of cells with leptin proteins and ACTH. Stars=values different from those of fed rat cell populations. Figure 4B illustrates a field dual labeled for leptin (black) and ACTH (orange) proteins. Black, filled arrows show corticotropes labeled for only ACTH and the 4 pointed stars indicate cells labeled for only leptin. Dual-labeled corticotropes are indicated by the hollow arrows. Figure 4C shows a field from fasted animals, similarly labeled for leptin and ACTH. The dual labeled cells (hollow arrows) are more darkly labeled for leptin, making the ACTH label difficult to see in a lower magnification image. Cells that express only leptin (4 point star) or ACTH (black arrows) remain in the populations from both groups. Bar=15 μm.
When the analysis focused on the population of leptin-bearing cells, the dual labeling accounted for over 90% of cells with leptin proteins, with 61% of leptin-bearing cells co-expressing GH, 8-10% co-expressing LH, TSH, or ACTH and only 3% co-expressing prolactin. The remaining 10% could be folliculostellate cells, or monohormonal FSHβ cells, which were not detected in these dual labeling studies.
Figure 4A also shows that food deprivation brought about major losses in leptin protein expression by somatotropes or gonadotropes, reducing the percentages of AP cells with leptin and GH from 22% to 13% and those with leptin and LH from 3% to 1% (p<0.001). In contrast, corticotropes maintained their expression of leptin at 30% of ACTH cells. The overall increase in corticotropes resulted in a 1.94 increase in AP cells with leptin and ACTH, from 3.6% to 7% of AP cells (p=0.03). Figure 4 illustrates this increased expression of leptin proteins in corticotropes. The analysis of leptin in fasted rat pituitary populations accounted for 86% of the leptin cell population, with 45% of leptin cells storing GH (reduced from 61%) and 24% storing ACTH (increased from 8%).
Because gonadotropes and somatotropes were most severely affected by the food deprivation, the studies then determined if the reduced leptin mRNA was seen in these populations. Figure 5 shows an 80% decline in cells that co-expressed leptin mRNA and GH proteins from 25 ± 3% to 5 ± 2% of AP cells (p=<0.014). Similarly, fasting caused a significant decline in the co-expression of leptin mRNA in gonadotropes from 3 ± 0.5% of AP cells to 0.7 ± 0.02% of AP cells (p<0.029). The sum of the losses in gonadotropes and somatotropes accounted for the overall loss in leptin mRNA. Figures 5b and c illustrate the dual labeling for leptin mRNA and GH in fed and fasted rat populations.
Figure 5. Effect of fasting on % of AP cells with leptin mRNA and GH or LH.
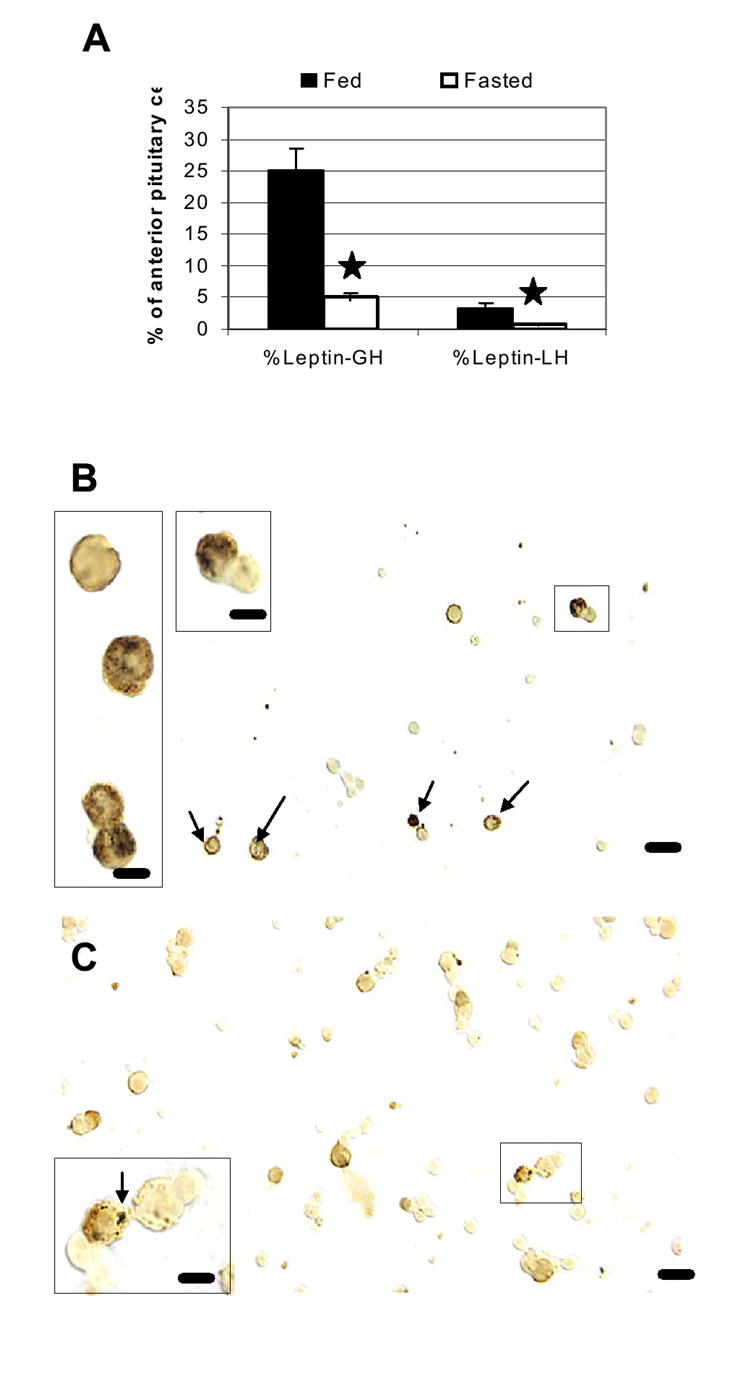
Dual labeling for leptin mRNA and GH or LH antigens was done to learn if the losses were focused in the somatotrope or gonadotrope population. Figure 5A shows the graph of the dramatic reduction in GH cells with leptin mRNA or the few LH with leptin mRNA. Stars indicate the significant differences when fed and fasted groups were compared. Figure 5B depicts a field from a fed male rat and labeling for leptin mRNA (black) and GH proteins (orange). In the fed rat, a number of GH cells express leptin mRNA. However, in the fasted rat (Fig 5C), their numbers are greatly reduced, and the area of label for leptin mRNA in individual GH cells is also reduced. The cells indicated by a box are depicted at higher magnification in the insets in Figures 5B and 5C. Bar=15 μm
Effects of Glucose on Pituitary Leptin-bearing Cells, in vivo
After the parallel fasting-induced reductions in leptin, LH and GH were detected, the experiments were expanded to test the hypothesis that nutrients, such as glucose might regulate this system. This involved the addition of a third set of fasted animals provided with 10% glucose water during the period of food deprivation. Table 1 shows data from 3 groups of rats (3 rats/group). Both groups of fasted rats lost weight, but serum glucose was restored in the animals given 10% glucose water.
Whole pituitaries from 3 separate sets of animals were committed to assays for mRNA by QRT-PCR, in order to obtain the entire pituitary for the mRNA extracts. This precluded any sampling errors that might be caused by regional differences in the pituitary. Figure 6A shows that leptin mRNA detected by QRT-PCR was reduced in the fasted animals (p<0.026) and restored in the fasted animals given 10% glucose water (p<0.03). The data are the ratio of number of leptin transcripts to the number of transcripts of the Hprt housekeeping gene. [A similar result was seen if Rps9 was used as the housekeeping gene; data not shown]. Glucose also restored leptin mRNA, detected by in situ hybridization (p<.001 fasted vs glucose-fasted) (Fig 6B).. Figure 6B also shows a parallel restoration of LH mRNA, as detected by in situ hybridization and counts of mRNA-bearing cells (p=0.02 fed vs fasted and p=0.004 fasted vs glucose-fasted). QRT-PCR assays of GH mRNA showed that there were no significant changes with fasting or with glucose water (data not shown).
Figure 6. Effect of Food Deprivation With or Without Glucose Water on Pituitary Leptin mRNA and Cells with Leptin mRNA.
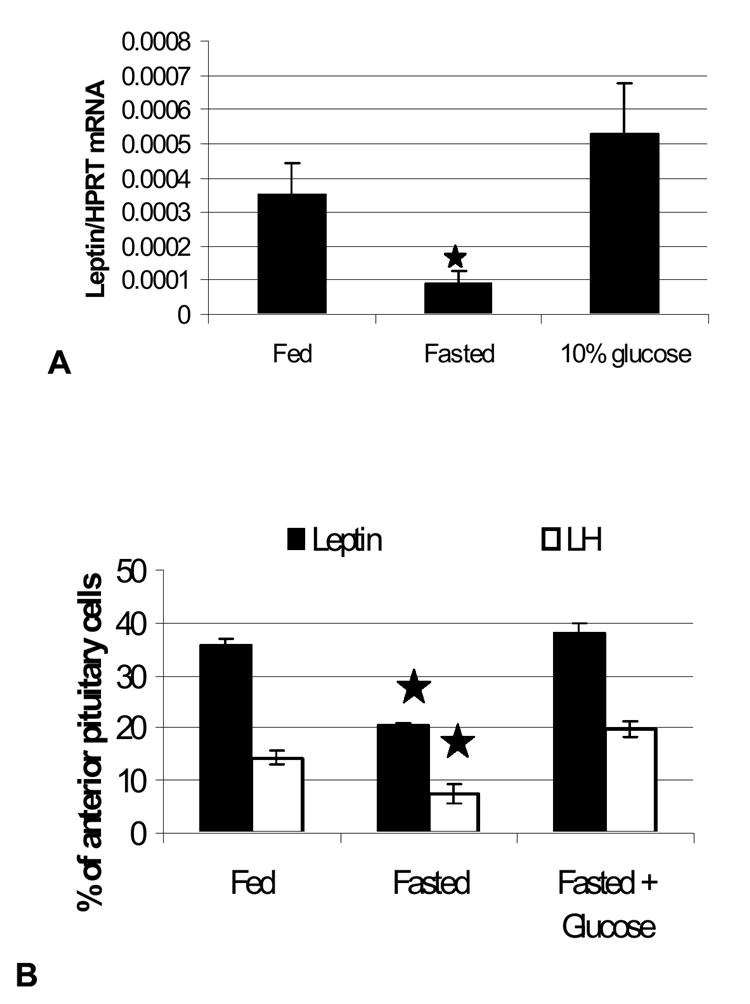
Tests of a second group of fasted rats that received 10% glucose water during the 24 h period of food deprivation. Figure 6A illustrates the recovery in leptin mRNA assayed by QRT-PCR in whole pituitary mRNA extracts from these groups. Figure 6B illustrates the losses in leptin and LH mRNA, as detected by in situ hybridization and the recovery in fasted rats receiving glucose.
Finally, when additional sets of fresh cultures of pituitary cells were committed to immunolabeling for leptin, LH and GH, glucose also restored expression of all three protein hormones, as detected by cell counts. Figure 7A illustrates this restoration graphically. Note that the fasting-mediated reduction in leptin bearing cells in these groups of rats is more dramatic than in the first group (Figure 3A). Figures 7B-E depict immunolabeled cultures from fasted rats with and without glucose water, showing the restoration of somatotropes (Figs 7B and 7D) and gonadotropes (Figs 7C and 7E) in the population from fasted rats given 10% glucose.
Figure 7. Restorative effects of glucose, in vivo, or leptin, in vitro, on GH, LH and leptin expression.
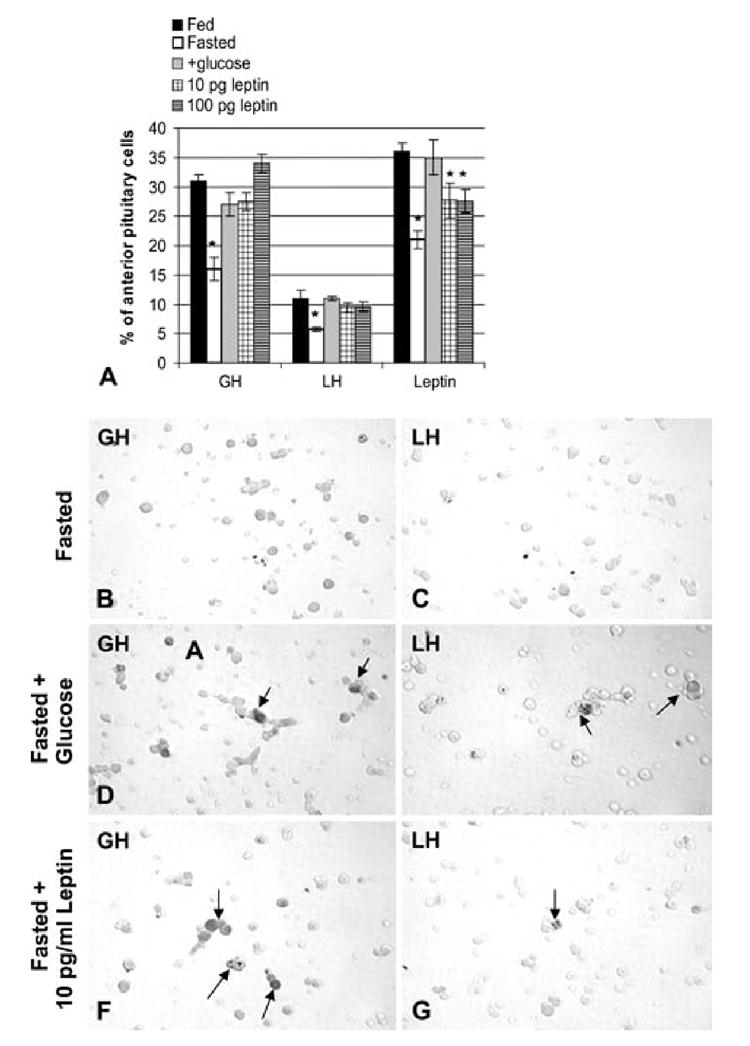
Counts of cells with leptin, GH, and LH proteins labeled by immunocytochemistry. In Figure 7A, these counts represent the latest groups of fasted animals, including those receiving glucose. Note that the decline in cells with leptin proteins is greater in this group of fasted rats, than in the first group shown in Figure 3A. All three gene products are recovered in the fasted rats receiving glucose (+glucose). Figure 7A also illustrates the restoration of GH and LH cells in cells from fasted rats, that received 10 pg/ml or 100 pg/ml leptin for 1 h, in vitro. Cells bearing leptin are partially restored in the presence of leptin. Figures 7B and C illustrate pituitary cells from fasted rats immunolabeled for GH (Fig 7B) or LH (Fig 7C). Figures 7D and 7E illustrate fields from fasted rats receiving glucose water immunolabeled for GH (Fig 7D) or LH (Fig 7E) showing the recovery in GH or LH cells. A similar recovery, in vitro, is illustrated in Figures 7F (GH cells) or 7G(LH cells). These cultures were from fasted rats receiving 10 pg/ml leptin for 1 h, in vitro. The vehicle control for this group is identical to the cells depicted in Figures 7B and 7C, having few GH or LH cells after 1 h in culture. This 1 h treatment, in vitro, with exogenous leptin has the same restorative effect on GH and LH cells as glucose, in vivo. Bar=21 micrometers.
Effects of Leptin on Pituitary Cells, in vitro
The final set of experiments tested the efficacy of leptin, in vitro on the possible restoration of GH and LH hormone expression in 3 groups of freshly dispersed cells from additional fasted animals (6 rats total). Leptin was added for 1 h, with a concentration range (10-100 pg/ml) that matched those assayed in pituitary cell culture media (Akhter et al. 2007). Figure 7A shows that, as little as 10 pg/ml leptin for 1 h restored the percentages of LH and GH hormone-bearing cells to values not different from those in cultures from fed or fasted rats treated with glucose, in vivo. Exogenous leptin for 1 h partially restored the percentages of leptin-bearing cells, with values midway between those from fed rats and fasted rats. Figures 7F and G illustrate the restoration of GH or LH cells in the fasted groups treated with leptin, in vitro.
Discussion
Development of the Short-Term Fasting Model
The overall objective of our ongoing studies has been to determine the physiological significance of pituitary leptin. This is difficult to do, in vivo because of the abundance of circulating leptin from adipocytes, and the presence of both leptin and leptin receptors in all pituitary cell types. Animal models with leptin or leptin receptor deficiencies have other confounding variables, like hyperglycemia and diabetes, which affect pituitary cells independently. In addition, these models are deficient in gonadotropes and somatotropes and do not respond normally to tests like fasting (Hardie et al. 1996).
Selective knockouts in the pituitary would be needed to fully test leptin’s significance. While these are being developed, this in vivo study was designed to test conditions that might change pituitary leptin in normal rats. The present report is the first to show that pituitary leptin mRNA and proteins are reduced 24 h after fasting in directions similar to those seen in adipocytes (Dallman et al. 1999). As the model was developed, we recognized that, if serum leptin was also reduced, we could not discriminate between effects of pituitary leptin and serum leptin. However, the male Sprague Dawley rats used in our study did not show lower serum leptin after the short 24 h fasting period. Thus, the timing of the decrease in pituitary leptin mRNA and proteins could be correlated with changes in the different pituitary cell types to begin to develop a hypotheses about a role for pituitary leptin.
The first set of results with fasted animals showed that somatotropes and gonadotropes were most severely reduced. The losses in GH or LH proteins, LH mRNA, and GnRH or GHRH receptor expressing cells correlate well with the absence of GH or LH pulses during food deprivation described by others (Ahima et al. 1996; Finn et al. 1998; Gonzalez et al. 1999; Maciel et al. 2004; Nagatani et al. 1998; Nagatani et al. 2000; Pombo et al. 1999; Schneider et al. 2000; Schneider et al. 2002; Schneider et al. 1998; Schneider and Zhou 1999; Vuagnat et al. 1998). In addition, this study is the first to show selective losses of leptin mRNA and proteins in gonadotropes, somatotropes and lactotropes with fasting. As stated previously, all of these changes were in the absence of changes in serum leptin.
We recognize that most previous studies of fasted or food deprived animals have found reductions in serum leptin, however, they tested periods longer than 24 h (Ahima et al. 1996; Casanueva and Dieguez 1999; Dallman et al. 1999; Luque et al. 2007; Maciel et al. 2004; Schneider et al. 2000; Schneider et al. 2002; Schneider and Zhou 1999). Fasting mediated reductions in serum leptin after 24 h were reported, however, in lean Zucker rats (Hardie et al. 1996), or sham, brain-lesioned female Sprague Dawley rats (Suga et al. 1999). In both of these studies, the control rats had higher resting levels of leptin (4 ng/ml) than are found in our Sprague Dawley rats (2 ng/ml). The differences in resting leptin could relate to the differences in the strain, gender or, in the latter study, (Suga et al. 1999) to the stress of sham surgery. Normal females are known to have higher circulating leptin than males and our present studies suggest that surgically stressed females might have more leptin from an expanded population of corticotropes.
Responses by Pituitary Cells to Fasting
As stated above, the 24 h fast produced reductions in somatotrope and gonadotrope functions, and at the same time, the short term fast resulted in a nearly 2—fold increase in corticotropes. This increase correlates well with previous reports that show higher spikes of ACTH secretion during the first 24 hours of food deprivation. (Akana et al. 1994). Mechanisms behind this increase could also stem from the increased CRH mRNA assayed 24 h after fasting (Luque et al. 2007). At this point, it is not clear if this increase is from mitotic activity (Childs et al. 1995) or from the differentiation of corticotrope precursors, however the increase is similar to that seen following a period of acute stress (Childs 1992).
The Fasting Model Tests the Significance of Pituitary Leptin
The fact that the fasting-mediated reduction in pituitary leptin mRNA and proteins occurred, rapidly, before serum leptin had declined implicates it as a potential regulator in the early responses to fasting. If pituitary leptin functions as a metabolic signal for nutritional changes, the findings in the present study favor this source because of its rapid responses. In addition, the dual labeling evidence shows that the reduction in leptin following food deprivation is mostly in somatotropes. Perhaps somatotrope leptin is uniquely responsive to changes in the nutritional state. Future studies of somatotrope regulators (including Ghrelin and GHRH) will be needed to provide more clues about regulators for this response..
In contrast, leptin was not reduced in the expanding population of corticotropes. This suggests that the regulation of the leptin gene is not uniform across all pituitary cell types. It should be noted that the corticotrope expansion added only 4% leptin-bearing cells to the population, while over 10-15% leptin-bearing were lost from somatotropes. Thus, assuming the new corticotropes could secrete their leptin in this environment, it was clearly not sufficient to correct for the losses in somatotropes and gonadotropes.
However, the losses in somatotropes or gonadotropes could be corrected rapidly by a 1 h incubation in as little as 10 pg/ml exogenous leptin. These levels match the levels secreted by normal rat pituitary cultures. Higher levels (100 pg/ml) did not cause further increases in LH or GH cells. (Note: A full set of dose response tests in normal rat pituitary cells (1 pg/ml to 1 ng/ml) showed that 10 pg/ml was a plateau point for increases in GH cells and 100 pg/ml was the plateau point for increases in LH cells.). Many previous studies have demonstrated exogenous leptin restoration of LH or GH pulses, in vivo, (Ahima et al. 1996; Finn et al. 1998; Gonzalez et al. 1999; Nagatani et al. 2000; Pombo et al. 1999; Vuagnat et al. 1998) with the assumption that its actions are mainly on the hypothalamus. Our studies suggest that leptin may also act directly on pituitary target cells and that restoration is not limited to actions on the hypothalamus.
Exogenous leptin was not able to fully restore the expression of pituitary leptin, although there was a partial recovery with 100 pg/ml leptin. This points to independent regulators for pituitary leptin and our previous studies show that they include estrogen and GHRH (McDuffie et al. 2004) and GnRH (Akhter et al. 2007). These regulators would be reduced in a fasted state (Ahima et al. 1996; Finn et al. 1998; Gonzalez et al. 1999; Luque et al. 2007; Maciel et al. 2004; Nagatani et al. 2000; Pombo et al. 1999; Vuagnat et al. 1998).
The parallel restoration of leptin and LH mRNA, or leptin, LH and GH proteins in the glucose treated rats provided circumstantial evidence that further supports an association between these gene products in the pituitary. Food deprivation (signaled by a drop in nutrients, like glucose) is known to result in the attenuation of LH or GH pulses and it is thought that some of this is signaled by a drop in serum leptin. In vivo studies have shown that the LH pulses can be restored within hours of giving nutrition. It can also be restored, in vivo, in fasted animals by exogenous leptin as long as glucose can be utilized (Schneider et al. 2000; Schneider et al. 2002; Schneider and Zhou 1999). Thus, workers have theorized that leptin reports nutritional information to the hypothalamic and pituitary cells, permitting reproduction and normal GH or LH cell pulses if nutrition is adequate.
However, this present study reports new and unexpected findings in which gonadotropes and somatotropes show deficiencies in response to food deprivation in the face of a decline in pituitary, but not serum leptin. Perhaps pituitary leptin is needed to maintain LH and GH cell functions. If so, our dual labeling studies suggest that important sources would be the somatotropes or gonadotropes themselves. These findings lead us to hypothesize that a decline in somatotrope or gonadotrope leptin below certain threshold levels might signal nutritional distress and cause reductions in LH or GH cell functions. Endogneous pituitary leptin could thus serve as either a paracrine, or an autocrine regulator.
The studies of glucose restoration confirmed this hypothesis as it showed parallel increases in pituitary leptin, GH and LH, once serum glucose was restored to normal levels. Serum leptin remained unchanged (data not shown). This suggests that pituitary leptin might serve as a glucostat, which would provide one mechanism by which it senses the nutritional state. Recent landmark studies (Sorenson et al. 2007; Zelent et al. 2006) reported glucokinase expression in a subset of pituitary cells including thyrotropes and gonadotropes. This important glucose sensor may allow gonadotropes to monitor the changes in serum glucose. Our studies suggest that leptin may also be affected by this sensor and may play a role in facilitating gonadotrope responses.
Finally, as stated in the beginning of this discussion, we recognize that the evidence for pituitary leptin’s involvement in the regulation of LH and GH in this in vivo model is circumstantial at this point. Selective knockouts that remove leptin or its receptor from the pituitary would be needed to test the hypothesis about pituitary leptin directly. However, the present studies provide an important basis for such tests. They also add to the growing body of evidence that suggests local roles for leptin as an important cytokine in multiple organs and systems. Finally, the differential responses to fasting by corticotropes and somatotropes indicate that any future studies of pituitary leptin must recognize that overall changes in pituitary leptin reflect the net result of changes in diverse anterior pituitary cell types. They may not represent the unique responses seen by each of the cell types.
Acknowledgments
The authors thank the NIDDK Hormone Distribution office and Dr. A Parlow for the anti-rat GH, TSHβ, FSHβ, prolactin, and anti-rat/mouse leptin serum. They also thank JG Pierce, Ph.D. for the anti-bovine LHβ. The authors appreciate that help and advice of Paul Hughes of www.GeneDetect.com, in the design, preparation and application of the oligonucleotide probes.
This study was submitted in partial fulfillment for the degree of M.S. by C. Crane. It was presented as a poster at the 89th Annual Meetings of the Endocrine society, Toronto, Ca, June, 2007.
This publication was made possible by studies funded by NSF IBN 0240907, NIH R03 HD 44875, and 1 P20 RR020146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
References
- 1.Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 2.Akana SF, Strack AM, Hanson ES, Dallman MF. Regulation of activity in the hypothalamo-pituitary-adrenal axis is integral to a larger hypothalamic system that determines caloric flow. Endocrinology. 1994;135:1125–1134. doi: 10.1210/endo.135.3.8070356. [DOI] [PubMed] [Google Scholar]
- 3.Akhter N, Johnson BW, Crane C, Iruthayanathan M, Zhou YH, Kudo A, Childs GV. Anterior Pituitary Leptin Expression Changes in Different Reproductive States: Stimulation, In Vitro, by Gonadotropin Releasing Hormone (GnRH) J Histochem Cytochem. 2007;55:151–166. doi: 10.1369/jhc.6A7072.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aubert ML, Pierroz DD, Gruaz NM, d’Alleves V, Vuagnat BA, Pralong FP, Blum WF, Sizonenko PC. Metabolic control of sexual function and growth: role of neuropeptide Y and leptin. Mol Cell Endocrinol. 1998;140:107–113. doi: 10.1016/s0303-7207(98)00058-6. [DOI] [PubMed] [Google Scholar]
- 5.Baranowska B, Chmielowska M, Wolinska-Witort E, Roguski K, Wasilewska- Dziubinska E. The relationship between neuropeptides and hormones in starvation. Neuro Endocrinol Lett. 2001;22:349–355. [PubMed] [Google Scholar]
- 6.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–3147. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 7.Bronson FH. Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology. 1986;118:2483–2487. doi: 10.1210/endo-118-6-2483. [DOI] [PubMed] [Google Scholar]
- 8.Bronson FH, Heideman PD. Short-term hormonal responses to food intake in peripubertal female rats. Am J Physiol. 1990;259:R25–31. doi: 10.1152/ajpregu.1990.259.1.R25. [DOI] [PubMed] [Google Scholar]
- 9.Cameron JL. Regulation of reproductive hormone secretion in primates by short-term changes in nutrition. Rev Reprod. 1996;1:117–126. doi: 10.1530/ror.0.0010117. [DOI] [PubMed] [Google Scholar]
- 10.Casanueva FF, Dieguez C. Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol. 1999;20:317–363. doi: 10.1006/frne.1999.0187. [DOI] [PubMed] [Google Scholar]
- 11.Chen C, Roh SG, Nie GY, Loneragan K, Xu RW, Ruan M, Clarke LJ, Goding JW, Gertler A. The in vitro effect of leptin on growth hormone secretion from primary cultured ovine somatotrophs. Endocrine. 2001;14:73–78. doi: 10.1385/endo:14:1:073. [DOI] [PubMed] [Google Scholar]
- 12.Cheung CC, Thornton JE, Kuijper JL, Weigle DS, Clifton DK, Steiner RA. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–858. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 13.Childs GV. Structure-function correlates in the corticotropes of the anterior pituitary. Front Neuroendocrinol. 1992;13:271–317. [PubMed] [Google Scholar]
- 14.Childs GV, Iruthayanathan M, Akhter N, Unabia G, Whitehead-Johnson B. Bipotential effects of estrogen on growth hormone synthesis and storage in vitro. Endocrinology. 2005;146:1780–1788. doi: 10.1210/en.2004-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Childs GV, Naor Z, Hazum E, Tibolt R, Westlund KN, Hancock MB. Cytochemical characterization of pituitary target cells for biotinylated gonadotropin releasing hormone. Peptides. 1983a;4:549–555. doi: 10.1016/0196-9781(83)90061-x. [DOI] [PubMed] [Google Scholar]
- 16.Childs GV, Naor Z, Hazum E, Tibolt R, Westlund KN, Hancock MB. Localization of biotinylated gonadotropin releasing hormone on pituitary monolayer cells with avidin-biotin-peroxidase complexes. J Histochem Cytochem. 1983b;31:1422–1425. doi: 10.1177/31.12.6195217. [DOI] [PubMed] [Google Scholar]
- 17.Childs GV, Rougeau D, Unabia G. Corticotropin-releasing hormone and epidermal growth factor: mitogens for anterior pituitary corticotropes. Endocrinology. 1995;136:1595–1602. doi: 10.1210/endo.136.4.7895669. [DOI] [PubMed] [Google Scholar]
- 18.Childs GV, Unabia G, Lee BL, Rougeau D. Heightened secretion by small and medium-sized luteinizing hormone (LH) gonadotropes late in the cycle suggests contributions to the LH surge or possible paracrine interactions. Endocrinology. 1992;130:345–352. doi: 10.1210/endo.130.1.1727708. [DOI] [PubMed] [Google Scholar]
- 19.Childs GV, Unabia G, Miller BT. Cytochemical detection of gonadotropin-releasing hormone-binding sites on rat pituitary cells with luteinizing hormone, follicle-stimulating hormone, and growth hormone antigens during diestrous up-regulation. Endocrinology. 1994a;134:1943–1951. doi: 10.1210/endo.134.4.8137763. [DOI] [PubMed] [Google Scholar]
- 20.Childs GV, Unabia G, Miller BT, Collins TJ. Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J Endocrinol. 1999;162:177–187. doi: 10.1677/joe.0.1620177. [DOI] [PubMed] [Google Scholar]
- 21.Childs GV, Unabia G, Rougeau D. Cells that express luteinizing hormone (LH) and follicle-stimulating hormone (FSH) beta-subunit messenger ribonucleic acids during the estrous cycle: the major contributors contain LH beta, FSH beta, and/or growth hormone. Endocrinology. 1994b;134:990–997. doi: 10.1210/endo.134.2.8299592. [DOI] [PubMed] [Google Scholar]
- 22.Childs GV, Unabia G, Wu P. Differential expression of growth hormone messenger ribonucleic acid by somatotropes and gonadotropes in male and cycling female rats. Endocrinology. 2000;141:1560–1570. doi: 10.1210/endo.141.4.7429. [DOI] [PubMed] [Google Scholar]
- 23.Childs GV, Westlund KN, Unabia G. Characterization of anterior pituitary target cells for arginine vasopressin: including cells that store adrenocorticotropin, thyrotropin-beta, and both hormones. Endocrinology. 1989;125:554–559. doi: 10.1210/endo-125-1-554. [DOI] [PubMed] [Google Scholar]
- 24.Chua SC, Jr, Leibel RL, Hirsch J. Food deprivation and age modulate neuropeptide gene expression in the murine hypothalamus and adrenal gland. Brain Res Mol Brain Res. 1991;9:95–101. doi: 10.1016/0169-328x(91)90134-j. [DOI] [PubMed] [Google Scholar]
- 25.Dallman MF, Akana SF, Bhatnagar S, Bell ME, Choi S, Chu A, Horsley C, Levin N, Meijer O, Soriano LR, Strack AM, Viau V. Starvation: early signals, sensors, and sequelae. Endocrinology. 1999;140:4015–4023. doi: 10.1210/endo.140.9.7001. [DOI] [PubMed] [Google Scholar]
- 26.De Biasi SN, Apfelbaum LI, Apfelbaum ME. In vitro effect of leptin on LH release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur J Endocrinol. 2001;145:659–665. doi: 10.1530/eje.0.1450659. [DOI] [PubMed] [Google Scholar]
- 27.Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayase M, Matsuoka N, Aizawa-Abe M, Yoshimasa Y, Nakao K. Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes. 1999;48:2028–2033. doi: 10.2337/diabetes.48.10.2028. [DOI] [PubMed] [Google Scholar]
- 28.Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA. The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology. 1998;139:4652–4662. doi: 10.1210/endo.139.11.6297. [DOI] [PubMed] [Google Scholar]
- 29.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochem J. 2006;393:7–20. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez LC, Pinilla L, Tena-Sempere M, Aguilar E. Leptin(116-130) stimulates prolactin and luteinizing hormone secretion in fasted adult male rats. Neuroendocrinology. 1999;70:213–220. doi: 10.1159/000054479. [DOI] [PubMed] [Google Scholar]
- 31.Gui Y, Silha JV, Mishra S, Murphy LJ. Changes in adipokine expression during food deprivation in the mouse and the relationship to fasting-induced insulin resistance. Can J Physiol Pharmacol. 2003;81:979–985. doi: 10.1139/y03-103. [DOI] [PubMed] [Google Scholar]
- 32.Hardie LJ, Rayner DV, Holmes S, Trayhurn P. Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem Biophys Res Commun. 1996;223:660–665. doi: 10.1006/bbrc.1996.0951. [DOI] [PubMed] [Google Scholar]
- 33.Igel M, Kainulainen H, Brauers A, Becker W, Herberg L, Joost HG. Long-term and rapid regulation of ob mRNA levels in adipose tissue from normal (Sprague Dawley rats) and obese (db/db mice, fa/fa rats) rodents. Diabetologia. 1996;39:758–765. doi: 10.1007/s001250050508. [DOI] [PubMed] [Google Scholar]
- 34.Iruthayanathan M, Zhou YH, Childs GV. Dehydroepiandrosterone restoration of growth hormone gene expression in aging female rats, in vivo and in vitro: evidence for actions via estrogen receptors. Endocrinology. 2005;146:5176–5187. doi: 10.1210/en.2005-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isozaki O, Tsushima T, Miyakawa M, Nozoe Y, Demura H, Seki H. Growth hormone directly inhibits leptin gene expression in visceral fat tissue in fatty Zucker rats. J Endocrinol. 1999;161:511–516. doi: 10.1677/joe.0.1610511. [DOI] [PubMed] [Google Scholar]
- 36.Korner J, Savontaus E, Chua SC, Jr, Leibel RL, Wardlaw SL. Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol. 2001;13:959–966. doi: 10.1046/j.1365-2826.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- 37.Kowalska I, Straczkowski M, Gorski J, Kinalska I. The effect of fasting and physical exercise on plasma leptin concentrations in high-fat fed rats. J Physiol Pharmacol. 1999;50:309–320. [PubMed] [Google Scholar]
- 38.Luque RM, Park S, Kineman RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone in the fasted mouse: potential role of neuropeptide Y and corticotropin-releasing hormone. Endocrinology. 2007;148:300–309. doi: 10.1210/en.2006-0592. [DOI] [PubMed] [Google Scholar]
- 39.MacDougald OA, Hwang CS, Fan H, Lane MD. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1995;92:9034–9037. doi: 10.1073/pnas.92.20.9034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maciel MN, Zieba DA, Amstalden M, Keisler DH, Neves JP, Williams GL. Leptin prevents fasting-mediated reductions in pulsatile secretion of luteinizing hormone and enhances its gonadotropin-releasing hormone-mediated release in heifers. Biol Reprod. 2004;70:229–235. doi: 10.1095/biolreprod.103.021345. [DOI] [PubMed] [Google Scholar]
- 41.Mann DR, Plant TM. Leptin and pubertal development. Semin Reprod Med. 2002;20:93–102. doi: 10.1055/s-2002-32500. [DOI] [PubMed] [Google Scholar]
- 42.McDuffie IA, Akhter N, Childs GV. Regulation of leptin mRNA and protein expression in pituitary somatotropes. J Histochem Cytochem. 2004;52:263–273. doi: 10.1177/002215540405200214. [DOI] [PubMed] [Google Scholar]
- 43.Mizuno TM, Bergen H, Funabashi T, Kleopoulos SP, Zhong YG, Bauman WA, Mobbs CV. Obese gene expression: reduction by fasting and stimulation by insulin and glucose in lean mice, and persistent elevation in acquired (diet-induced) and genetic (yellow agouti) obesity. Proc Natl Acad Sci U S A. 1996;93:3434–3438. doi: 10.1073/pnas.93.8.3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV. Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology. 1999;140:4551–4557. doi: 10.1210/endo.140.10.6966. [DOI] [PubMed] [Google Scholar]
- 45.Mizuno TM, Mobbs CV. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 46.Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: a review. Fertil Steril. 2002;77:433–444. doi: 10.1016/s0015-0282(01)03010-2. [DOI] [PubMed] [Google Scholar]
- 47.Nagatani S, Guthikonda P, Thompson RC, Tsukamura H, Maeda KI, Foster DL. Evidence for GnRH regulation by leptin: leptin administration prevents reduced pulsatile LH secretion during fasting. Neuroendocrinology. 1998;67:370–376. doi: 10.1159/000054335. [DOI] [PubMed] [Google Scholar]
- 48.Nagatani S, Zeng Y, Keisler DH, Foster DL, Jaffe CA. Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in the sheep. Endocrinology. 2000;141:3965–3975. doi: 10.1210/endo.141.11.7762. [DOI] [PubMed] [Google Scholar]
- 49.Neary NM, Goldstone AP, Bloom SR. Appetite regulation: from the gut to the hypothalamus. Clin Endocrinol (Oxf) 2004;60:153–160. doi: 10.1046/j.1365-2265.2003.01839.x. [DOI] [PubMed] [Google Scholar]
- 50.Ogura K, Irahara M, Kiyokawa M, Tezuka M, Matsuzaki T, Yasui T, Kamada M, Aono T. Effects of leptin on secretion of LH and FSH from primary cultured female rat pituitary cells. Eur J Endocrinol. 2001;144:653–658. doi: 10.1530/eje.0.1440653. [DOI] [PubMed] [Google Scholar]
- 51.Pombo M, Pombo CM, Astorga R, Cordido F, Popovic V, Garcia-Mayor RV, Dieguez C, Casanueva FF. Regulation of growth hormone secretion by signals produced by the adipose tissue. J Endocrinol Invest. 1999;22:22–26. [PubMed] [Google Scholar]
- 52.Popovic V, Damjanovic S, Dieguez C, Casanueva FF. Leptin and the pituitary. Pituitary. 2001;4:7–14. doi: 10.1023/a:1012938308654. [DOI] [PubMed] [Google Scholar]
- 53.Roh SG, Nie GY, Loneragan K, Gertler A, Chen C. Direct modification of somatotrope function by long-term leptin treatment of primary cultured ovine pituitary cells. Endocrinology. 2001;142:5167–5171. doi: 10.1210/endo.142.12.8559. [DOI] [PubMed] [Google Scholar]
- 54.Rowland NE, Morien A, Li BH. The physiology and brain mechanisms of feeding. Nutrition. 1996;12:626–639. doi: 10.1016/s0899-9007(96)00227-4. [DOI] [PubMed] [Google Scholar]
- 55.Schneider JE, Blum RM, Wade GN. Metabolic control of food intake and estrous cycles in syrian hamsters. I. Plasma insulin and leptin. Am J Physiol Regul Integr Comp Physiol. 2000;278:R476–485. doi: 10.1152/ajpregu.2000.278.2.R476. [DOI] [PubMed] [Google Scholar]
- 56.Schneider JE, Buckley CA, Blum RM, Zhou D, Szymanski L, Day DE, Bartness TJ. Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. Eur J Neurosci. 2002;16:377–379. doi: 10.1046/j.1460-9568.2002.02118.x. [DOI] [PubMed] [Google Scholar]
- 57.Schneider JE, Goldman MD, Tang S, Bean B, Ji H, Friedman MI. Leptin indirectly affects estrous cycles by increasing metabolic fuel oxidation. Horm Behav. 1998;33:217–228. doi: 10.1006/hbeh.1998.1453. [DOI] [PubMed] [Google Scholar]
- 58.Schneider JE, Zhou D. Interactive effects of central leptin and peripheral fuel oxidation on estrous cyclicity. Am J Physiol. 1999;277:R1020–1024. doi: 10.1152/ajpregu.1999.277.4.R1020. [DOI] [PubMed] [Google Scholar]
- 59.Sorenson RL, Stout LE, Brelje TC, Jetton TL, Matschinsky FM. Immunohistochemical evidence for the presence of glucokinase in the gonadotropes and thyrotropes of the anterior pituitary gland of rat and monkey. J Histochem Cytochem. 2007;55:555–566. doi: 10.1369/jhc.6A7117.2007. [DOI] [PubMed] [Google Scholar]
- 60.Suga A, Hirano T, Kageyama H, Kashiba M, Oka J, Osaka T, Namba Y, Tsuji M, Miura M, Adachi M, Inoue S. Rapid increase in circulating leptin in ventromedial hypothalamus-lesioned rats: role of hyperinsulinemia and implication for upregulation mechanism. Diabetes. 1999;48:2034–2038. doi: 10.2337/diabetes.48.10.2034. [DOI] [PubMed] [Google Scholar]
- 61.Tezuka M, Irahara M, Ogura K, Kiyokawa M, Tamura T, Matsuzaki T, Yasui T, Aono T. Effects of leptin on gonadotropin secretion in juvenile female rat pituitary cells. Eur J Endocrinol. 2002;146:261–266. doi: 10.1530/eje.0.1460261. [DOI] [PubMed] [Google Scholar]
- 62.Urbanski HF. Leptin and puberty. Trends Endocrinol Metab. 2001;12:428–429. doi: 10.1016/s1043-2760(01)00505-7. [DOI] [PubMed] [Google Scholar]
- 63.Vasselli JR. Behavioral and biological determinants of leptin resistance. Appetite. 2001;37:115–117. doi: 10.1006/appe.2001.0418. [DOI] [PubMed] [Google Scholar]
- 64.Vuagnat BA, Pierroz DD, Lalaoui M, Englaro P, Pralong FP, Blum WF, Aubert ML. Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology. 1998;67:291–300. doi: 10.1159/000054326. [DOI] [PubMed] [Google Scholar]
- 65.Walczewska A, Yu WH, Karanth S, McCann SM. Estrogen and leptin have differential effects on FSH and LH release in female rats. Proc Soc Exp Biol Med. 1999;222:170–177. doi: 10.1046/j.1525-1373.1999.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 66.Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM. Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci U S A. 1997a;94:1023–1028. doi: 10.1073/pnas.94.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu WH, Walczewska A, Karanth S, McCann SM. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology. 1997b;138:5055–5058. doi: 10.1210/endo.138.11.5649. [DOI] [PubMed] [Google Scholar]
- 68.Zelent D, Golson ML, Koeberlein B, Quintens R, van Lommel L, Buettger C, Weik-Collins H, Taub R, Grimsby J, Schuit F, Kaestner KH, Matschinsky FM. A glucose sensor role for glucokinase in anterior pituitary cells. Diabetes. 2006;55:1923–1929. doi: 10.2337/db06-0151. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]


