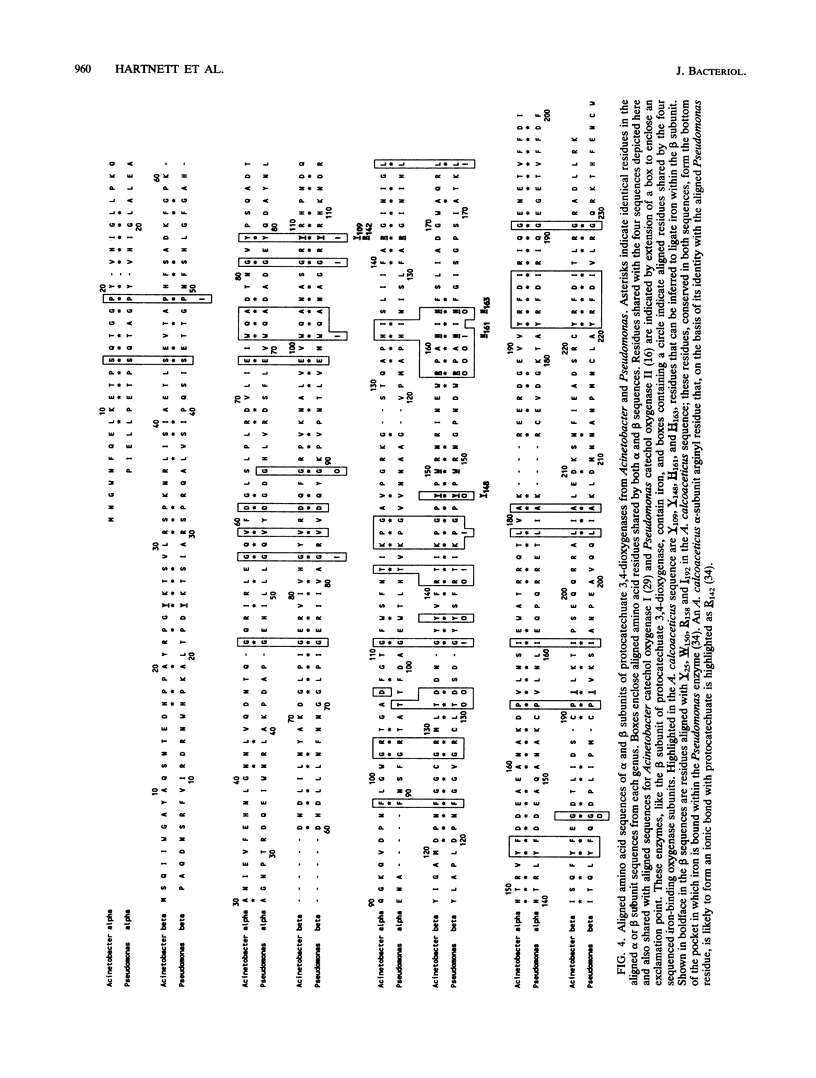
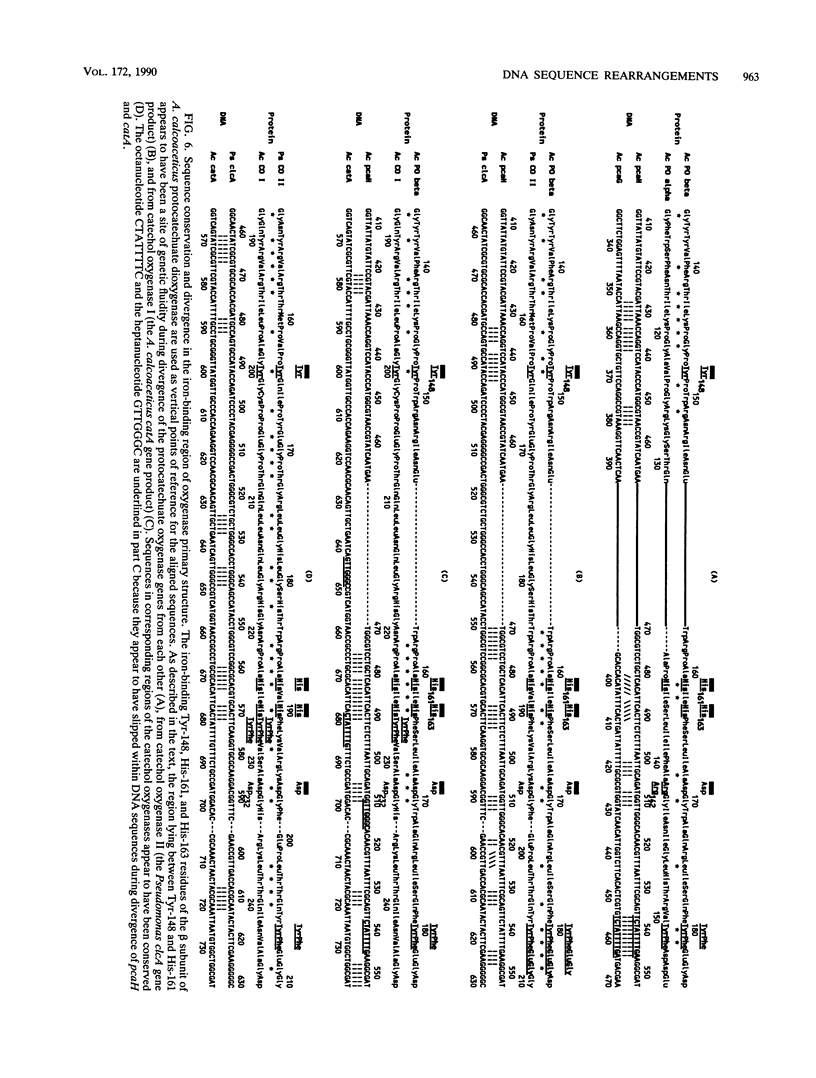
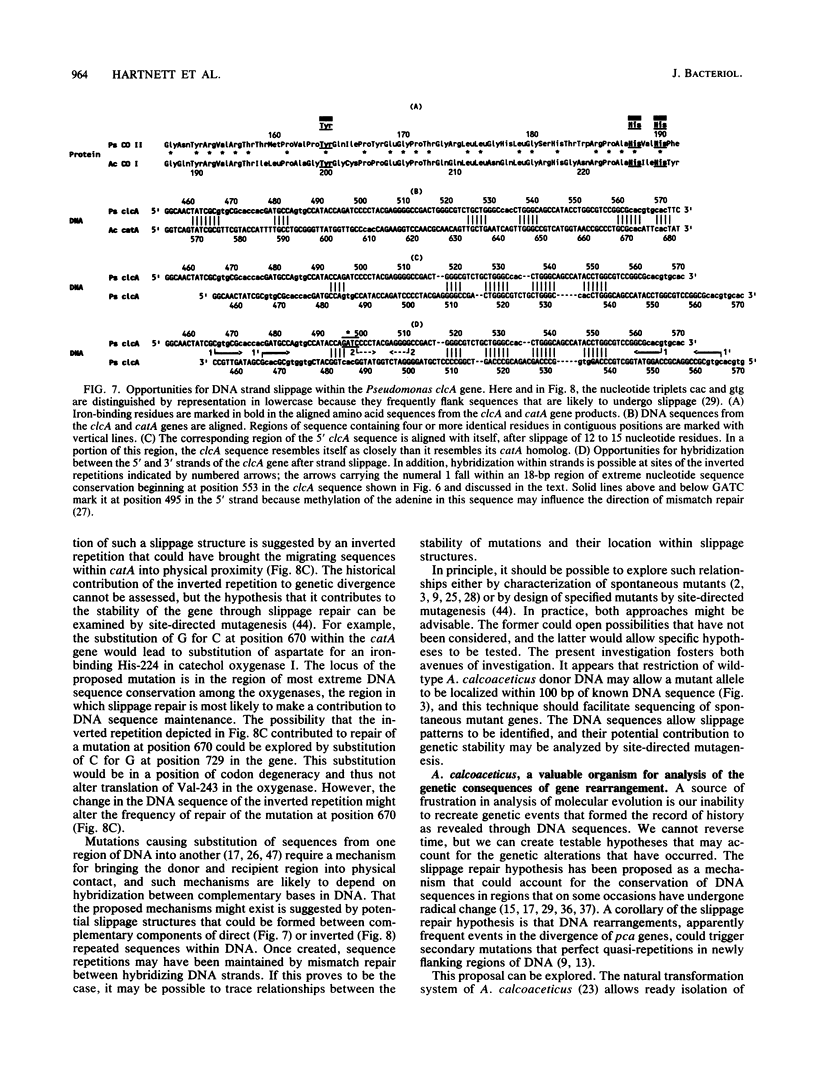
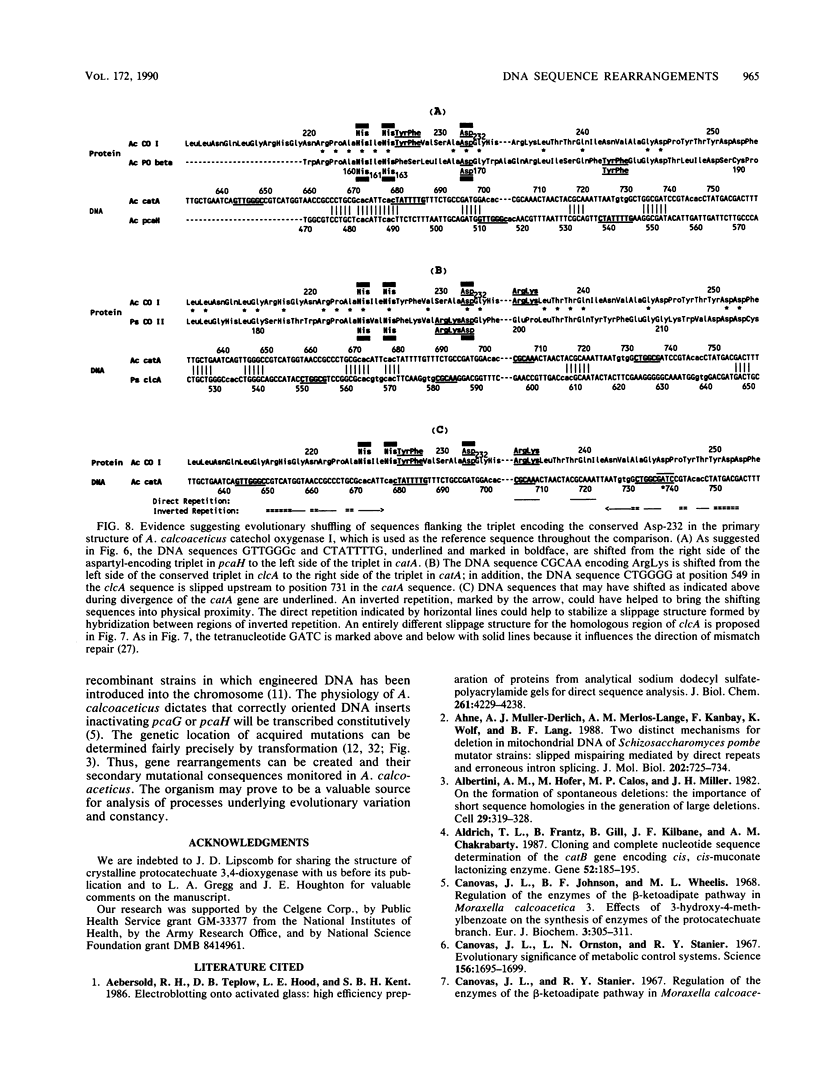
Abstract
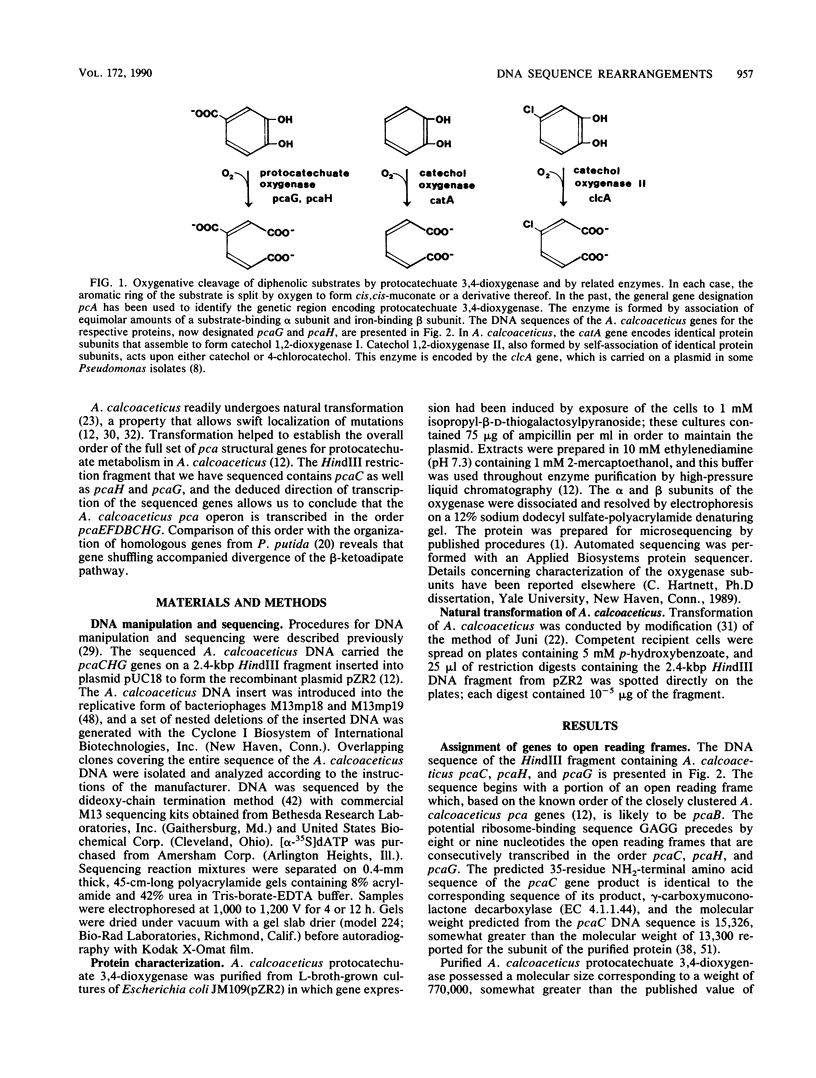
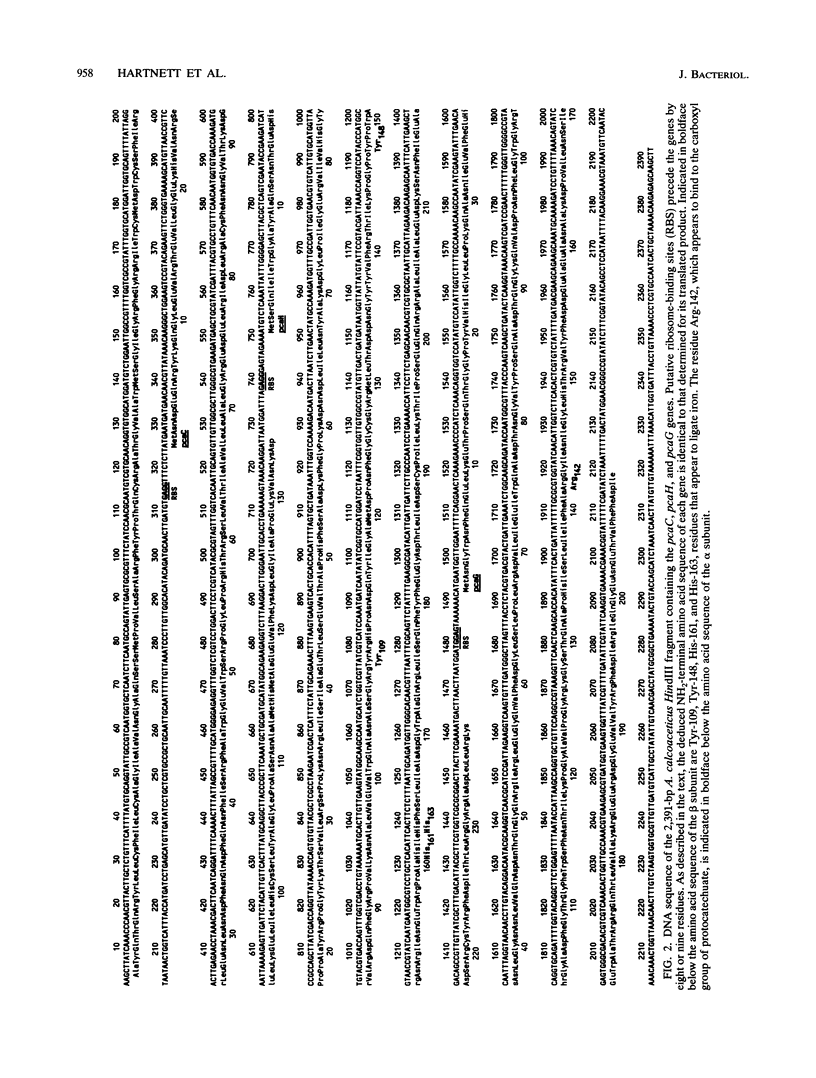
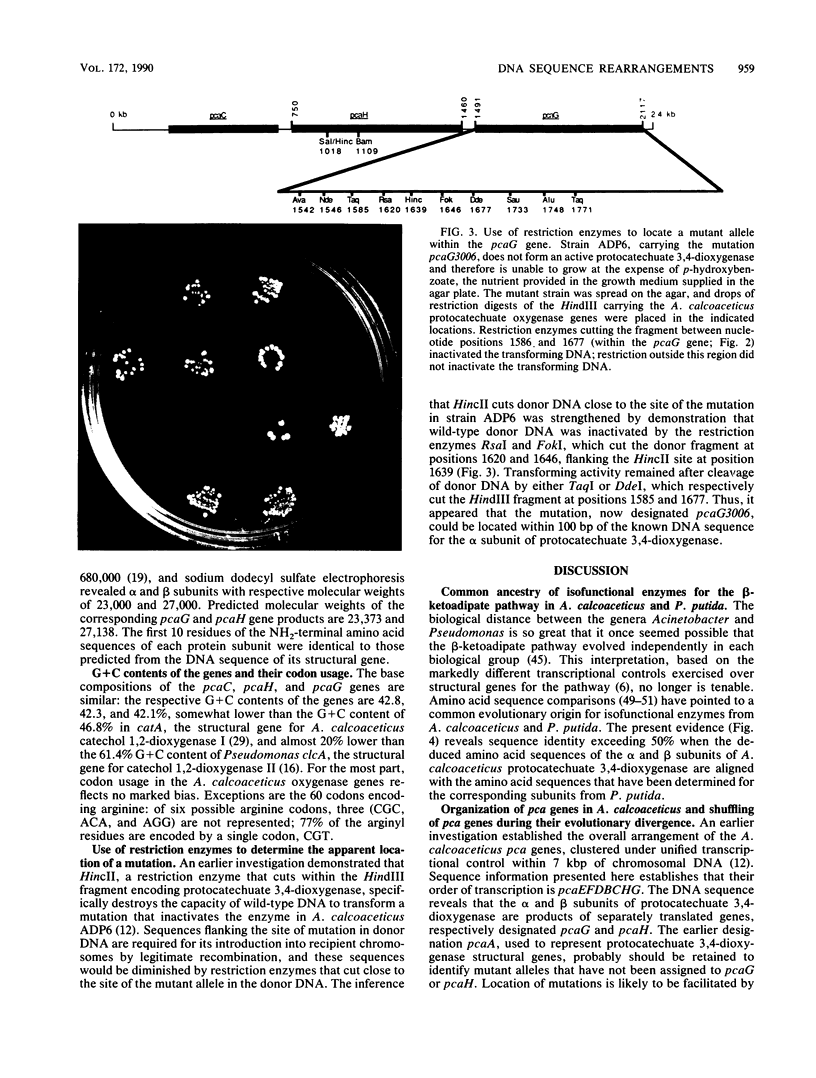
The DNA sequence of a 2,391-base-pair HindIII restriction fragment of Acinetobacter calcoaceticus DNA containing the pcaCHG genes is reported. The DNA sequence reveals that A. calcoaceticus pca genes, encoding enzymes required for protocatechuate metabolism, are arranged in a single transcriptional unit, pcaEFDBCHG, whereas homologous genes are arranged differently in Pseudomonas putida. The pcaG and pcaH genes represent separate reading frames respectively encoding the alpha and beta subunits of protocatechuate 3,4-dioxygenase (EC 1.13.1.3); previously a single designation, pcaA, had been used to represent DNA encoding this enzyme. The alpha and beta protein subunits appear to share common ancestry with each other and with catechol 1,2-dioxygenases from A. calcoaceticus and P. putida. Marked conservation of amino acid sequence is observed in a region containing two histidyl residues and two tyrosyl residues that appear to ligate iron within each oxygenase. In some regions within the aligned oxygenase sequences, DNA sequences appear to be conserved at a level beyond the extent that might have been demanded by selection at the level of protein. In other regions, divergence of DNA sequences appears to have been achieved by substitution of DNA sequence from one genetic segment into another. The results are interpreted to be the consequence of sequence exchange by gene conversion between slipped strands of DNA during evolutionary divergence; mismatch repair between slipped strands may contribute to the maintenance of DNA sequence in divergent genes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Ahne A., Müller-Derlich J., Merlos-Lange A. M., Kanbay F., Wolf K., Lang B. F. Two distinct mechanisms for deletion in mitochondrial DNA of Schizosaccharomyces pombe mutator strains. Slipped mispairing mediated by direct repeats and erroneous intron splicing. J Mol Biol. 1988 Aug 20;202(4):725–734. doi: 10.1016/0022-2836(88)90553-0. [DOI] [PubMed] [Google Scholar]
- Albertini A. M., Hofer M., Calos M. P., Miller J. H. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell. 1982 Jun;29(2):319–328. doi: 10.1016/0092-8674(82)90148-9. [DOI] [PubMed] [Google Scholar]
- Aldrich T. L., Frantz B., Gill J. F., Kilbane J. J., Chakrabarty A. M. Cloning and complete nucleotide sequence determination of the catB gene encoding cis,cis-muconate lactonizing enzyme. Gene. 1987;52(2-3):185–195. doi: 10.1016/0378-1119(87)90045-x. [DOI] [PubMed] [Google Scholar]
- Chatterjee D. K., Chakrabarty A. M. Restriction mapping of a chlorobenzoate degradative plasmid and molecular cloning of the degradative genes. Gene. 1984 Feb;27(2):173–181. doi: 10.1016/0378-1119(84)90138-0. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Johnson B. F., Wheelis M. L. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 3. Effects of 3-hydroxy-4-methylbenzoate on the synthesis of enzymes of the protocatechuate branch. Eur J Biochem. 1968 Jan;3(3):305–311. doi: 10.1111/j.1432-1033.1968.tb19530.x. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- Dorn E., Knackmuss H. J. Chemical structure and biodegradability of halogenated aromatic compounds. Two catechol 1,2-dioxygenases from a 3-chlorobenzoate-grown pseudomonad. Biochem J. 1978 Jul 15;174(1):73–84. doi: 10.1042/bj1740073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doten R. C., Gregg L. A., Ornston L. N. Influence of the catBCE sequence on the phenotypic reversion of a pcaE mutation in Acinetobacter calcoaceticus. J Bacteriol. 1987 Jul;169(7):3175–3180. doi: 10.1128/jb.169.7.3175-3180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doten R. C., Ngai K. L., Mitchell D. J., Ornston L. N. Cloning and genetic organization of the pca gene cluster from Acinetobacter calcoaceticus. J Bacteriol. 1987 Jul;169(7):3168–3174. doi: 10.1128/jb.169.7.3168-3174.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham D. R., Stirling L. A., Ornston L. N., Perry J. J. Intergeneric evolutionary homology revealed by the study of protocatechuate 3,4-dioxygenase from Azotobacter vinelandii. Biochemistry. 1980 Jan 8;19(1):149–155. doi: 10.1021/bi00542a023. [DOI] [PubMed] [Google Scholar]
- Edelman G. M., Gally J. A. Antibody structure, diversity, and specificity. Brookhaven Symp Biol. 1968 Jun;21(2):328–344. [PubMed] [Google Scholar]
- Frantz B., Chakrabarty A. M. Organization and nucleotide sequence determination of a gene cluster involved in 3-chlorocatechol degradation. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4460–4464. doi: 10.1073/pnas.84.13.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding G. B., Gearhart P. J., Glickman B. W. Patterns of somatic mutations in immunoglobulin variable genes. Genetics. 1987 Jan;115(1):169–176. doi: 10.1093/genetics/115.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding G. B., Glickman B. W. Sequence-directed mutagenesis: evidence from a phylogenetic history of human alpha-interferon genes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8577–8581. doi: 10.1073/pnas.82.24.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou C. T., Lillard M. O., Schwartz R. D. Protocatechuate 3, 4-dioxygenase from Acinetobacter calcoaceticus. Biochemistry. 1976 Feb 10;15(3):582–588. doi: 10.1021/bi00648a020. [DOI] [PubMed] [Google Scholar]
- Hughes E. J., Shapiro M. K., Houghton J. E., Ornston L. N. Cloning and expression of pca genes from Pseudomonas putida in Escherichia coli. J Gen Microbiol. 1988 Nov;134(11):2877–2887. doi: 10.1099/00221287-134-11-2877. [DOI] [PubMed] [Google Scholar]
- Iwaki M., Kagamiyama H., Nozaki M. The complete amino acid sequence of the beta-subunit of protocatechuate 3,4-dioxygenase from Pseudomonas aeruginosa. J Biochem. 1979 Oct;86(4):1159–1162. doi: 10.1093/oxfordjournals.jbchem.a132612. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juni E., Janik A. Transformation of Acinetobacter calco-aceticus (Bacterium anitratum). J Bacteriol. 1969 Apr;98(1):281–288. doi: 10.1128/jb.98.1.281-288.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohlmiller N. A., Howard J. B. The primary structure of the alpha subunit of protocatechuate 3,4-dioxygenase. I. Isolation and sequence of the tryptic peptides. J Biol Chem. 1979 Aug 10;254(15):7302–7308. [PubMed] [Google Scholar]
- Kunkel T. A., Soni A. Mutagenesis by transient misalignment. J Biol Chem. 1988 Oct 15;263(29):14784–14789. [PubMed] [Google Scholar]
- Levinson G., Gutman G. A. Slipped-strand mispairing: a major mechanism for DNA sequence evolution. Mol Biol Evol. 1987 May;4(3):203–221. doi: 10.1093/oxfordjournals.molbev.a040442. [DOI] [PubMed] [Google Scholar]
- Marinus M. G. DNA methylation in Escherichia coli. Annu Rev Genet. 1987;21:113–131. doi: 10.1146/annurev.ge.21.120187.000553. [DOI] [PubMed] [Google Scholar]
- McClain W. H. Specific duplications fostered by a DNA structure containing adjacent inverted repeat sequences. J Mol Biol. 1988 Nov 5;204(1):27–40. doi: 10.1016/0022-2836(88)90595-5. [DOI] [PubMed] [Google Scholar]
- Neidle E. L., Hartnett C., Bonitz S., Ornston L. N. DNA sequence of the Acinetobacter calcoaceticus catechol 1,2-dioxygenase I structural gene catA: evidence for evolutionary divergence of intradiol dioxygenases by acquisition of DNA sequence repetitions. J Bacteriol. 1988 Oct;170(10):4874–4880. doi: 10.1128/jb.170.10.4874-4880.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Ornston L. N. Benzoate and muconate, structurally dissimilar metabolites, induce expression of catA in Acinetobacter calcoaceticus. J Bacteriol. 1987 Jan;169(1):414–415. doi: 10.1128/jb.169.1.414-415.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Ornston L. N. Cloning and expression of Acinetobacter calcoaceticus catechol 1,2-dioxygenase structural gene catA in Escherichia coli. J Bacteriol. 1986 Nov;168(2):815–820. doi: 10.1128/jb.168.2.815-820.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Shapiro M. K., Ornston L. N. Cloning and expression in Escherichia coli of Acinetobacter calcoaceticus genes for benzoate degradation. J Bacteriol. 1987 Dec;169(12):5496–5503. doi: 10.1128/jb.169.12.5496-5503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai K. L., Ornston L. N. Abundant expression of Pseudomonas genes for chlorocatechol metabolism. J Bacteriol. 1988 May;170(5):2412–2413. doi: 10.1128/jb.170.5.2412-2413.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlendorf D. H., Lipscomb J. D., Weber P. C. Structure and assembly of protocatechuate 3,4-dioxygenase. Nature. 1988 Nov 24;336(6197):403–405. doi: 10.1038/336403a0. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Ornston L. N., Yeh W. K. Origins of metabolic diversity: evolutionary divergence by sequence repetition. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3996–4000. doi: 10.1073/pnas.76.8.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parke D. Structural comparison of gamma-carboxymuconolactone decarboxylase and muconolactone isomerase from Pseudomonas putida. Biochim Biophys Acta. 1979 May 23;578(1):145–154. doi: 10.1016/0005-2795(79)90122-3. [DOI] [PubMed] [Google Scholar]
- Patel R. N., Hou C. T., Felix A., Lillard M. O. Catechol 1,2-dioxygenase from Acinetobacter calcoaceticus: purification and properties. J Bacteriol. 1976 Jul;127(1):536–544. doi: 10.1128/jb.127.1.536-544.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley M. S., Neidle E. L., Parales R. E., Ornston L. N. Cloning and expression of Acinetobacter calcoaceticus catBCDE genes in Pseudomonas putida and Escherichia coli. J Bacteriol. 1986 Feb;165(2):557–563. doi: 10.1128/jb.165.2.557-563.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. In vitro mutagenesis. Annu Rev Genet. 1985;19:423–462. doi: 10.1146/annurev.ge.19.120185.002231. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Ornston L. N. The beta-ketoadipate pathway. Adv Microb Physiol. 1973;9(0):89–151. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Tautz D., Trick M., Dover G. A. Cryptic simplicity in DNA is a major source of genetic variation. Nature. 1986 Aug 14;322(6080):652–656. doi: 10.1038/322652a0. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yeh W. K., Durham D. R., Fletcher P., Ornston L. N. Evolutionary relationships among gamma-carboxymuconolactone decarboxylases. J Bacteriol. 1981 Apr;146(1):233–238. doi: 10.1128/jb.146.1.233-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston L. N. Evolutionary divergence of co-selected beta-ketoadipate enol-lactone hydrolases in Acinetobacter calcoaceticus. J Biol Chem. 1980 Jul 10;255(13):6342–6346. [PubMed] [Google Scholar]
- Yeh W. K., Fletcher P., Ornston N. Homologies in the NH2-terminal amino acid sequences of gamma-carboxymuconolactone decarboxylases and muconolactone isomerases. J Biol Chem. 1980 Jul 10;255(13):6347–6354. [PubMed] [Google Scholar]
- Yeh W. K., Ornston L. N. Origins of metabolic diversity: substitution of homologous sequences into genes for enzymes with different catalytic activities. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5365–5369. doi: 10.1073/pnas.77.9.5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer J. G., Ripley L. S. Demonstration of the production of frameshift and base-substitution mutations by quasipalindromic DNA sequences. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5528–5531. doi: 10.1073/pnas.81.17.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]