Abstract
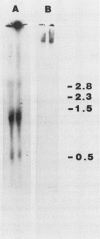
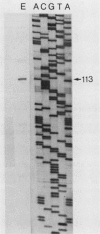
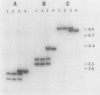
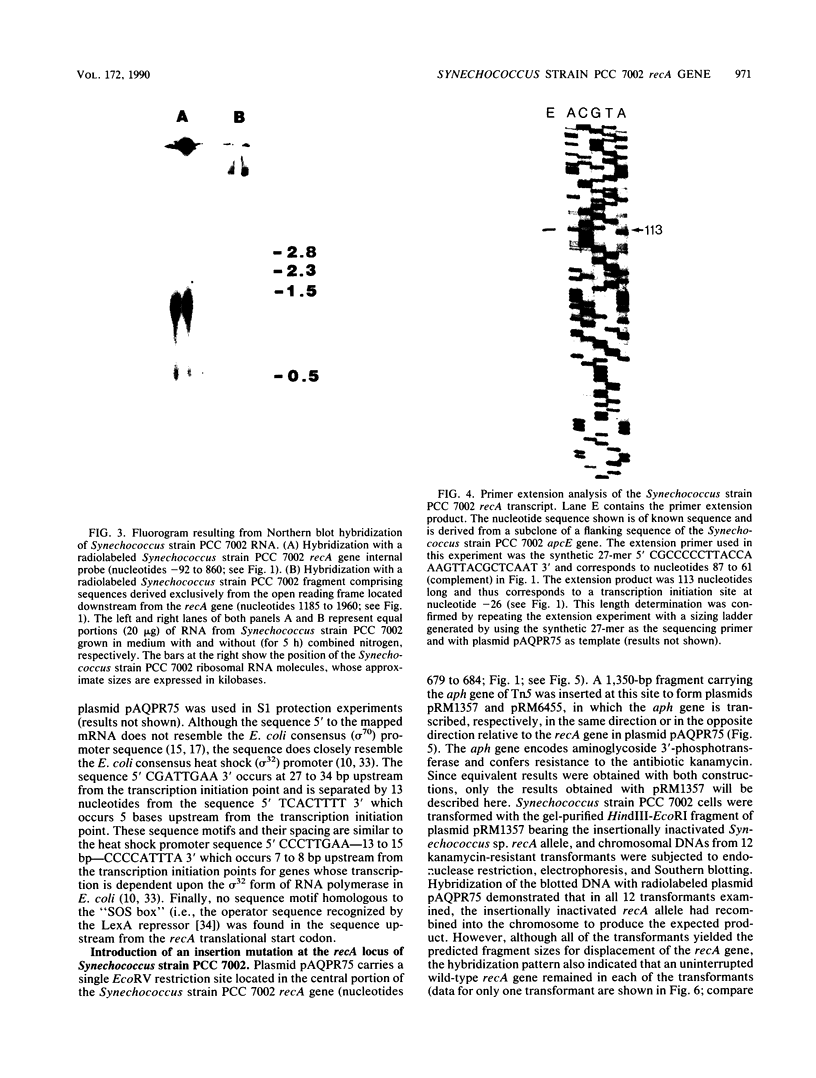
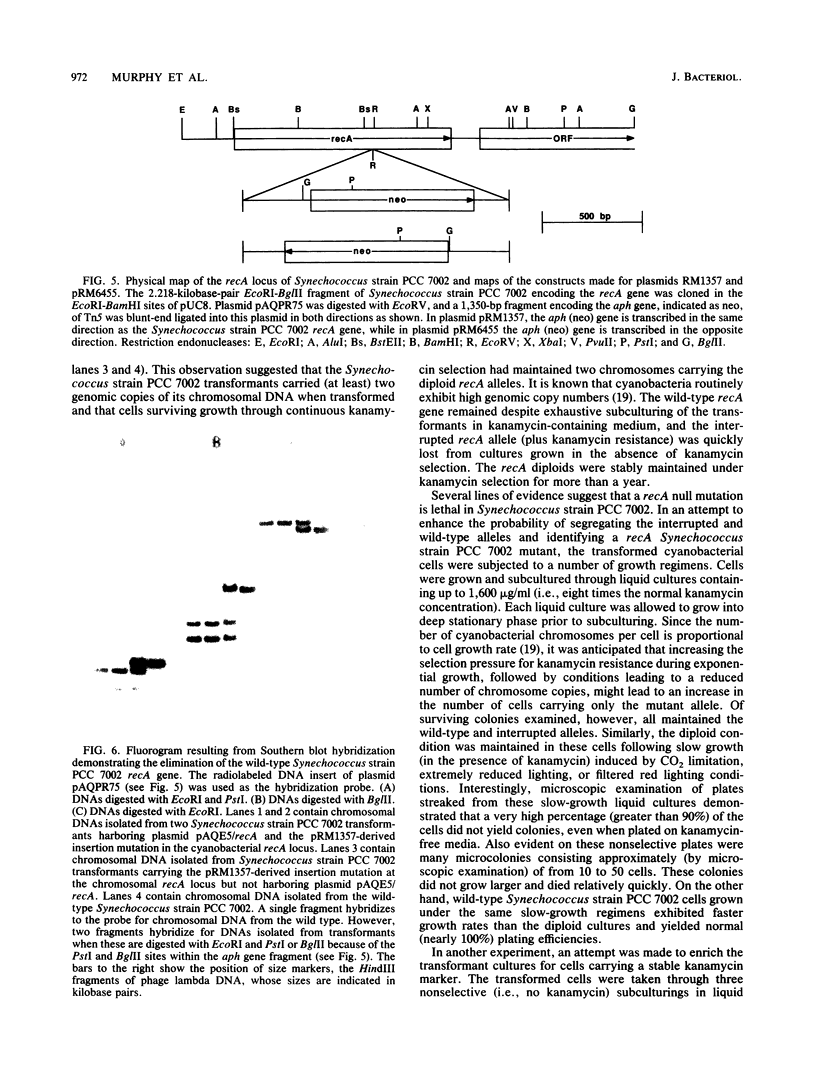
The nucleotide sequence and transcript initiation site of the Synechococcus sp. strain PCC 7002 recA gene have been determined. The deduced amino acid sequence of the RecA protein of this cyanobacterium is 56% identical and 73% similar to the Escherichia coli RecA protein. Northern (RNA) blot analysis indicates that the Synechococcus strain PCC 7002 recA gene is transcribed as a monocistronic transcript 1,200 bases in length. The 5' endpoint of the recA mRNA was mapped by primer extension by using synthetic oligonucleotides of 17 and 27 nucleotides as primers. The nucleotide sequence 5' to the mapped endpoint contained sequence motifs bearing a striking resemblance to the heat shock (sigma 32-specific) promoters of E. coli but did not contain sequences similar to the E. coli SOS operator recognized by the LexA repressor. An insertion mutation introduced into the recA locus of Synechococcus strain PCC 7002 via homologous recombination resulted in the formation of diploids carrying both mutant and wild-type recA alleles. A variety of growth regimens and transformation procedures failed to produce a recA Synechococcus strain PCC 7002 mutant. However, introduction into these diploid cells of the E. coli recA gene in trans on a biphasic shuttle vector resulted in segregation of the cyanobacterial recA alleles, indicating that the E. coli recA gene was able to provide a function required for growth of recA Synechococcus strain PCC 7002 cells. This interpretation is supported by the observation that the E. coli recA gene is maintained in these cells when antibiotic selection for the shuttle vector is removed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaboshi E., Yip M. L., Howard-Flanders P. Nucleotide sequence of the recA gene of Proteus mirabilis. Nucleic Acids Res. 1989 Jun 12;17(11):4390–4390. doi: 10.1093/nar/17.11.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks G. R., Sedgwick S. G. Direct ATP photolabeling of Escherichia coli recA proteins: identification of regions required for ATP binding. Biochemistry. 1986 Oct 7;25(20):5882–5889. doi: 10.1021/bi00368a007. [DOI] [PubMed] [Google Scholar]
- Benedict R. C., Kowalczykowski S. C. Increase of the DNA strand assimilation activity of recA protein by removal of the C terminus and structure-function studies of the resulting protein fragment. J Biol Chem. 1988 Oct 25;263(30):15513–15520. [PubMed] [Google Scholar]
- Buzby J. S., Porter R. D., Stevens S. E., Jr Plasmid transformation in Agmenellum quadruplicatum PR-6: construction of biphasic plasmids and characterization of their transformation properties. J Bacteriol. 1983 Jun;154(3):1446–1450. doi: 10.1128/jb.154.3.1446-1450.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyman J., Cunningham R. P. Escherichia coli K-12 mutants in which viability is dependent on recA function. J Bacteriol. 1987 Sep;169(9):4203–4210. doi: 10.1128/jb.169.9.4203-4210.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing D. W., Bardwell J. C., Craig E. A., Woolford C., Hendrix R. W., Gross C. A. Consensus sequence for Escherichia coli heat shock gene promoters. Proc Natl Acad Sci U S A. 1985 May;82(9):2679–2683. doi: 10.1073/pnas.82.9.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Froehlich B., Epstein W. Escherichia coli mutants in which transcription is dependent on recA function. J Bacteriol. 1981 Sep;147(3):1117–1120. doi: 10.1128/jb.147.3.1117-1120.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Genetic engineering of the cyanobacterial chromosome. Methods Enzymol. 1987;153:215–231. doi: 10.1016/0076-6879(87)53055-5. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Reynolds R. P. Analysis of E. coli promoter sequences. Nucleic Acids Res. 1987 Mar 11;15(5):2343–2361. doi: 10.1093/nar/15.5.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Márquez L. M., Chamberlin M. J. Cloning, sequencing, and disruption of the Bacillus subtilis sigma 28 gene. J Bacteriol. 1988 Apr;170(4):1568–1574. doi: 10.1128/jb.170.4.1568-1574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Whalley K., Jamieson D. J. Nucleotide binding by membrane components of bacterial periplasmic binding protein-dependent transport systems. EMBO J. 1985 Apr;4(4):1033–1039. doi: 10.1002/j.1460-2075.1985.tb03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kawashima H., Horii T., Ogawa T., Ogawa H. Functional domains of Escherichia coli recA protein deduced from the mutational sites in the gene. Mol Gen Genet. 1984;193(2):288–292. doi: 10.1007/BF00330682. [DOI] [PubMed] [Google Scholar]
- Knight K. L., Hess R. M., McEntee K. Conservation of an ATP-binding domain among RecA proteins from Proteus vulgaris, Erwinia carotovora, Shigella flexneri, and Escherichia coli K-12 and B/r. J Bacteriol. 1988 Jun;170(6):2427–2432. doi: 10.1128/jb.170.6.2427-2432.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight K. L., McEntee K. Nucleotide binding by a 24-residue peptide from the RecA protein of Escherichia coli. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9289–9293. doi: 10.1073/pnas.83.24.9289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasin F., Hutchinson F. Repair of DNA double-strand breaks in Escherichia coli, which requires recA function and the presence of a duplicate genome. J Mol Biol. 1977 Oct 15;116(1):81–98. doi: 10.1016/0022-2836(77)90120-6. [DOI] [PubMed] [Google Scholar]
- Miyata T., Miyazawa S., Yasunaga T. Two types of amino acid substitutions in protein evolution. J Mol Evol. 1979 Mar 15;12(3):219–236. doi: 10.1007/BF01732340. [DOI] [PubMed] [Google Scholar]
- Muller E. G., Buchanan B. B. Thioredoxin is essential for photosynthetic growth. The thioredoxin m gene of Anacystis nidulans. J Biol Chem. 1989 Mar 5;264(7):4008–4014. [PubMed] [Google Scholar]
- Murphy R. C., Bryant D. A., Porter R. D., de Marsac N. T. Molecular cloning and characterization of the recA gene from the cyanobacterium Synechococcus sp. strain PCC 7002. J Bacteriol. 1987 Jun;169(6):2739–2747. doi: 10.1128/jb.169.6.2739-2747.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Ogawa T. General recombination: functions and structure of RecA protein. Adv Biophys. 1986;21:135–148. doi: 10.1016/0065-227x(86)90019-5. [DOI] [PubMed] [Google Scholar]
- Porter R. D., Buzby J. S., Pilon A., Fields P. I., Dubbs J. M., Stevens S. E., Jr Genes from the cyanobacterium Agmenellum quadruplicatum isolated by complementation: characterization and production of merodiploids. Gene. 1986;41(2-3):249–260. doi: 10.1016/0378-1119(86)90105-8. [DOI] [PubMed] [Google Scholar]
- Porter R. D. Transformation in cyanobacteria. Crit Rev Microbiol. 1986;13(2):111–132. doi: 10.3109/10408418609108736. [DOI] [PubMed] [Google Scholar]
- Ramesar R. S., Abratt V., Woods D. R., Rawlings D. E. Nucleotide sequence and expression of a cloned Thiobacillus ferrooxidans recA gene in Escherichia coli. Gene. 1989 May 15;78(1):1–8. doi: 10.1016/0378-1119(89)90308-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Stachelek C., Konigsberg W., Rupp W. D. Sequences of the recA gene and protein. Proc Natl Acad Sci U S A. 1980 May;77(5):2611–2615. doi: 10.1073/pnas.77.5.2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano Y., Kageyama M. The sequence and function of the recA gene and its protein in Pseudomonas aeruginosa PAO. Mol Gen Genet. 1987 Jul;208(3):412–419. doi: 10.1007/BF00328132. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Connelly M. Isolation and characterization of a cyanophage infecting the unicellular blue-green algae A. nidulans and S. cedrorum. Virology. 1976 Jul 15;72(2):540–544. doi: 10.1016/0042-6822(76)90186-0. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Cohen-Bazire G. Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol. 1977;31:225–274. doi: 10.1146/annurev.mi.31.100177.001301. [DOI] [PubMed] [Google Scholar]
- Stevens S. E., Porter R. D. Transformation in Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6052–6056. doi: 10.1073/pnas.77.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Shiina T., Takahashi H. Multiple principal sigma factor homologs in eubacteria: identification of the "rpoD box". Science. 1988 Nov 18;242(4881):1040–1042. doi: 10.1126/science.3194753. [DOI] [PubMed] [Google Scholar]
- Uhlin B. E., Clark A. J. Overproduction of the Escherichia coli recA protein without stimulation of its proteolytic activity. J Bacteriol. 1981 Oct;148(1):386–390. doi: 10.1128/jb.148.1.386-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui A., Takao M., Oikawa A., Kiener A., Walsh C. T., Eker A. P. Cloning and characterization of a photolyase gene from the cyanobacterium Anacystis nidulans. Nucleic Acids Res. 1988 May 25;16(10):4447–4463. doi: 10.1093/nar/16.10.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]