Abstract
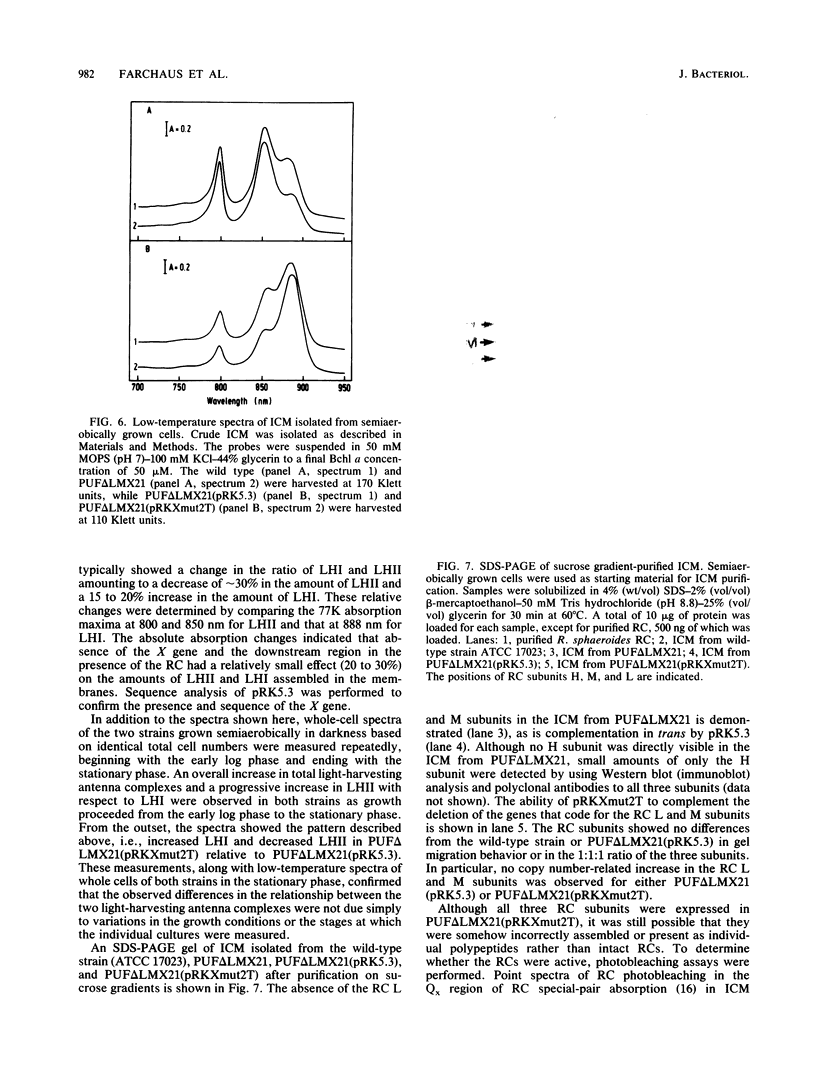
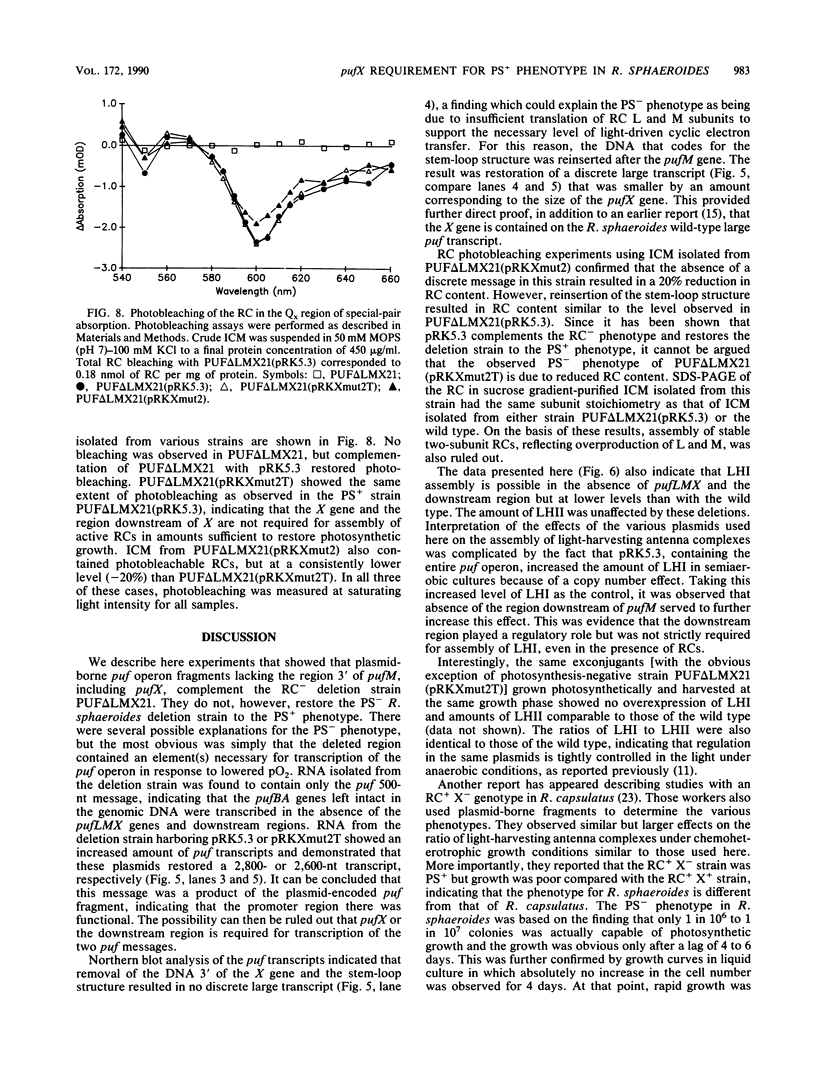
The puf operon in Rhodobacter sphaeroides is composed of the genes for the photosynthetic reaction center L and M subunits, light-harvesting antenna complex I, and one other open reading frame termed pufX. Complementation of a reaction center-deficient, photosynthetically incompetent pufLMX deletion strain in trans with a fragment containing the entire puf operon, including pufX and an additional 1,100 base pairs of DNA downstream of pufX, restored the reaction center and the photosynthesis-positive phenotype. Complementation of the same strain with pufBALM restores the reaction center to the level seen with the entire puf operon but not the photosynthesis-positive phenotype. Northern (RNA) blot analysis revealed that oxygen regulated transcription was not blocked in the absence of pufX and the downstream region. Spectroscopic and protein analyses indicated that the pigment-binding protein complexes, including the reaction center, were expressed and showed normal absorption characteristics. A 20% reduction in the amount of light-harvesting antenna complex II and a corresponding increase in the amount of light-harvesting antenna complex I were observed in the deletion strain harboring the plasmid with the puf insert lacking the pufX gene and the downstream region compared with those complemented with the entire puf operon and an additional downstream 1,100 base pairs.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. W., Forrest M. E., Cohen S. N., Beatty J. T. Structural and functional analysis of transcriptional control of the Rhodobacter capsulatus puf operon. J Bacteriol. 1989 Jan;171(1):473–482. doi: 10.1128/jb.171.1.473-482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Bauer C. E., Marrs B. L. Rhodobacter capsulatus puf operon encodes a regulatory protein (PufQ) for bacteriochlorophyll biosynthesis. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7074–7078. doi: 10.1073/pnas.85.19.7074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C. E., Young D. A., Marrs B. L. Analysis of the Rhodobacter capsulatus puf operon. Location of the oxygen-regulated promoter region and the identification of an additional puf-encoded gene. J Biol Chem. 1988 Apr 5;263(10):4820–4827. [PubMed] [Google Scholar]
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Belasco J. G., Chen C. Y. Mechanism of puf mRNA degradation: the role of an intercistronic stem-loop structure. Gene. 1988 Dec 10;72(1-2):109–117. doi: 10.1016/0378-1119(88)90132-1. [DOI] [PubMed] [Google Scholar]
- Bowyer J. R., Tierney G. V., Crofts A. R. Secondary electron transfer in chromatophores of Rhodopseudomonas capsulata A1a pho. Binary out-of-phase oscillations in ubisemiauinone formation and cytochrome b50 reduction with consective light flashes. FEBS Lett. 1979 May 1;101(1):201–206. doi: 10.1016/0014-5793(79)81326-5. [DOI] [PubMed] [Google Scholar]
- Broglie R. M., Hunter C. N., Delepelaire P., Niederman R. A., Chua N. H., Clayton R. K. Isolation and characterization of the pigment-protein complexes of Rhodopseudomonas sphaeroides by lithium dodecyl sulfate/polyacrylamide gel electrophoresis. Proc Natl Acad Sci U S A. 1980 Jan;77(1):87–91. doi: 10.1073/pnas.77.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech T. R., Tanner N. K., Tinoco I., Jr, Weir B. R., Zuker M., Perlman P. S. Secondary structure of the Tetrahymena ribosomal RNA intervening sequence: structural homology with fungal mitochondrial intervening sequences. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3903–3907. doi: 10.1073/pnas.80.13.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., Donohue T. J., Kaplan S. Construction, characterization, and complementation of a Puf- mutant of Rhodobacter sphaeroides. J Bacteriol. 1988 Jan;170(1):320–329. doi: 10.1128/jb.170.1.320-329.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHoff B. S., Lee J. K., Donohue T. J., Gumport R. I., Kaplan S. In vivo analysis of puf operon expression in Rhodobacter sphaeroides after deletion of a putative intercistronic transcription terminator. J Bacteriol. 1988 Oct;170(10):4681–4692. doi: 10.1128/jb.170.10.4681-4692.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G., Schmidhauser T., Yakobson E., Lu P., Liang X. W., Finlay D. R., Guiney D., Helinski D. R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmid. 1985 Mar;13(2):149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Donohue T. J., Hoger J. H., Kaplan S. Cloning and expression of the Rhodobacter sphaeroides reaction center H gene. J Bacteriol. 1986 Nov;168(2):953–961. doi: 10.1128/jb.168.2.953-961.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutton P. L., Petty K. M., Bonner H. S., Morse S. D. Cytochrome c2 and reaction center of Rhodospeudomonas spheroides Ga. membranes. Extinction coefficients, content, half-reduction potentials, kinetics and electric field alterations. Biochim Biophys Acta. 1975 Jun 17;387(3):536–556. doi: 10.1016/0005-2728(75)90092-4. [DOI] [PubMed] [Google Scholar]
- Farchaus J. W., Oesterhelt D. A Rhodobacter sphaeroides puf L, M and X deletion mutant and its complementation in trans with a 5.3 kb puf operon shuttle fragment. EMBO J. 1989 Jan;8(1):47–54. doi: 10.1002/j.1460-2075.1989.tb03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feick R. G., Fitzpatrick M., Fuller R. C. Isolation and characterization of cytoplasmic membranes and chlorosomes from the green bacterium Chloroflexus aurantiacus. J Bacteriol. 1982 May;150(2):905–915. doi: 10.1128/jb.150.2.905-915.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides light-harvesting B800-850-alpha and B800-850-beta genes. J Bacteriol. 1987 Jul;169(7):3268–3275. doi: 10.1128/jb.169.7.3268-3275.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Adams C. W., Belasco J., Doerge B., Cohen S. N. Biological consequences of segmental alterations in mRNA stability: effects of deletion of the intercistronic hairpin loop region of the Rhodobacter capsulatus puf operon. EMBO J. 1987 Nov;6(11):3515–3520. doi: 10.1002/j.1460-2075.1987.tb02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug G., Cohen S. N. Pleiotropic effects of localized Rhodobacter capsulatus puf operon deletions on production of light-absorbing pigment-protein complexes. J Bacteriol. 1988 Dec;170(12):5814–5821. doi: 10.1128/jb.170.12.5814-5821.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Fritz H. J. Oligonucleotide-directed construction of mutations via gapped duplex DNA. Methods Enzymol. 1987;154:350–367. doi: 10.1016/0076-6879(87)54084-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrs B., Kaplan S. 23 s precursor ribosomal RNA of Rhodopseudomonas spheroides. J Mol Biol. 1970 Apr 28;49(2):297–317. doi: 10.1016/0022-2836(70)90247-0. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks L. C., Niederman R. A. Membranes of Rhodopseudomonas sphaeroides. V. Identification of bacteriochlorophyll alpha-depleted cytoplasmic membrane in phototrophically grown cells. Biochim Biophys Acta. 1978 Jul 20;511(1):70–82. doi: 10.1016/0005-2736(78)90065-2. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Schumacher A., Drews G. The formation of bacteriochlorophyll.protein complexes of the photosynthetic apparatus of Rhodopseudomonas capsulata during early stages of development. Biochim Biophys Acta. 1978 Feb 9;501(2):183–194. doi: 10.1016/0005-2728(78)90025-7. [DOI] [PubMed] [Google Scholar]
- Stanssens P., Opsomer C., McKeown Y. M., Kramer W., Zabeau M., Fritz H. J. Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nucleic Acids Res. 1989 Jun 26;17(12):4441–4454. doi: 10.1093/nar/17.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwag E. J., Dahlberg A. E. Electrophoretic transfer of DNA, RNA and protein onto diazobenzyloxymethyl (DBM) - paper. Nucleic Acids Res. 1980 Jan 25;8(2):299–317. doi: 10.1093/nar/8.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Feher G. Primary structure of the reaction center from Rhodopseudomonas sphaeroides. Proteins. 1986 Dec;1(4):312–325. doi: 10.1002/prot.340010405. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Ismail S., Bylina E. J. Chromosomal deletion and plasmid complementation of the photosynthetic reaction center and light-harvesting genes from Rhodopseudomonas capsulata. Gene. 1985;38(1-3):19–30. doi: 10.1016/0378-1119(85)90199-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Cook D. N., Leach F., Armstrong G. A., Alberti M., Hearst J. E. Oxygen-regulated mRNAs for light-harvesting and reaction center complexes and for bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus during the shift from anaerobic to aerobic growth. J Bacteriol. 1986 Dec;168(3):1180–1188. doi: 10.1128/jb.168.3.1180-1188.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Hearst J. E. Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7613–7617. doi: 10.1073/pnas.83.20.7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kaplan S. Effects of light, oxygen, and substrates on steady-state levels of mRNA coding for ribulose-1,5-bisphosphate carboxylase and light-harvesting and reaction center polypeptides in Rhodopseudomonas sphaeroides. J Bacteriol. 1985 Jun;162(3):925–932. doi: 10.1128/jb.162.3.925-932.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. S., Kiley P. J., Donohue T. J., Kaplan S. Origin of the mRNA stoichiometry of the puf operon in Rhodobacter sphaeroides. J Biol Chem. 1986 Aug 5;261(22):10366–10374. [PubMed] [Google Scholar]