Abstract
Most of the γδ T cells in the intestinal epithelium of normal mice use the Vγ1 or the Vγ7 gene segments. However, the relative proportions of γδ intraepithelial lymphocytes expressing either the Vγ1 or the Vγ7 chain vary among different strains of mice whereas they are quite constant between different individuals of the same strain, suggesting that genetic factors, rather than environmental factors, are responsible for the observed differences. To analyze the genetic factors influencing the representation of different γδ T cell subsets in the intestinal epithelium, we used available anti-T cell antigen receptor (TCR) V region-specific mAbs against Vγ1, Vγ4, Vγ7, and Vδ4 to examine the TCR repertoire of intraepithelial γδ lymphocytes in a set of (C57BL/6 × DBA/2) recombinant inbred strains. Our results show that the representation of different Vγ and Vδ gene products among γδ intestinal intraepithelial lymphocytes is under a complex genetic control with a marked influence by genes closely linked to the TCRγ, TCRδ, and major histocompatibility complex loci.
Keywords: T cell selection, recombinant inbred strains, genetic control
Based on the chains they use to form their T cell antigen receptors (TCRs), T lymphocytes can be divided into two different populations: αβ T cells and γδ T cells. The TCR αβ allows for recognition of small peptides in association with products of the major histocompatibility complex (MHC) (1–4). This phenomenon, termed “MHC restriction,” requires a bias toward self-MHC in the TCR αβ repertoire, which is imposed in the thymus during the development of the αβ T cells by means of positive selection (5, 6). Developing αβ T cells also can be subject to negative selection pressures, which contribute to the establishment of tolerance (7, 8). Negative selection results in the deletion or inactivation of clones with high affinity for self-MHC in combination with a self-peptide. A different kind of interaction between certain Vβ products and endogenous retroviruses in combination with MHC class II molecules also results in the deletion of a large fraction of thymocytes expressing those Vβ products by a mechanism that appears to be similar to that observed in peptide-specific negative selection (9, 10).
Much less is known about the development and selection of γδ T cells. Studies with transgenic mice have shown that developing γδ T cells can be subject to both positive and negative selection pressures (11–14). In those studies, the transgenic TCRs were specific for the product of an MHC class Ib gene mapping to the T region of the MHC, and, consequently, positive and negative selection of the transgenic cells were shown to be MHC- dependent. In contrast, studies performed on β2m-deficient and MHC class II-deficient mice have shown that normal numbers of functionally competent γδ T cells are present in various lymphoid compartments in those mice, suggesting that γδ T cells can develop normally in the absence of MHC molecules (15). In particular, fetal γδ T cells expressing a unique TCR were shown to require an interaction involving their TCR for their development, but interactions with MHC molecules were not strictly required for their maturation (15, 16).
With the availability of Vγ- and Vδ-specific mAb, it has been possible to analyze the selection of γδ TCR repertoires in different organs. Thus, analysis of CD8+ γδ intestinal intraepithelial lymphocytes (i-IEL) in different strains of mice has shown a correlation between the presence of a high frequency of cells expressing a Vδ4-containing TCR and the expression of a gene closely linked to the MHC class II I-E molecule (17, 18). Furthermore, similar analysis performed with mAbs specific for the Vγ4 and the Vδ4 chains on a panel of recombinant inbred (RI) strains generated from C57BL/6 and DBA/2 founders (BXD) has shown that the relative representation of different splenic γδ T cell subsets defined by those mAbs is influenced by genes closely linked to the TCRγ and TCRδ loci (19). We recently produced an mAb specific for the Vγ1 chain and showed that Vγ1+ cells constitute a quantitatively important fraction of the mouse γδ i-IEL (20). With this and other mAbs specific for different Vγ and Vδ chains, we have analyzed the genes that influence the relative representation of different γδ i-IEL subsets using the same BXD RI lines. Our results show that, in those animals, the γδ i-IEL repertoire is influenced by genes linked to the MHC and TCR loci.
MATERIALS AND METHODS
Animals.
C57BL/6 (B6), DBA/2, and (C57BL/6 × DBA/2)F1 (B6D2F1) mice were obtained from The Jackson Laboratory or from Iffa Credo. All of the BXD RI strains were obtained from The Jackson Laboratory. Animals were used between 2 and 6 months of age.
Antibodies.
3A10, anti-pan-δ (21), and 2.11 anti-Vγ1 (20) were purified from culture supernatant by affinity chromatography on protein G-Sepharose (Pharmacia) and fluorescein isothiocyanate-labeled or biotinylated by standard procedures. Fluorescein isothiocyanate-labeled anti-Vγ7 (GL1) was a kind gift from L. Lefrancois, and biotin-labeled anti-Vδ4 (GL2) was purchased from PharMingen.
Cell Purifications, Immunofluorescence Staining, and Flow Cytometric Analysis.
The preparation of i-IELs has been described in detail (22). i-IEL (105–106) were incubated in staining buffer (PBS/3% fetal calf serum/0.1% NaN3) with the indicated labeled antibodies for 30 min. on ice. After two washes, the cells were incubated with streptavidin–phycoerythrin (Southern Biotechnology Associates) for 15 min on ice. After another two washes, 10,000 viable cells were analyzed using a FACScan flow cytometer (Becton Dickinson) and the lysys II program. Dead cells were gated out by staining with propidium iodide.
Statistical Analysis.
The mapmanager program containing the distribution in the BXD RI strains of all known polymorphic genes between the B6 and DBA/2 strains was obtained from Ken Manly (Roswell Park Cancer Institute, Buffalo, NY). For each identified gene, the BXD RI lines were distributed in two groups according to whether they had inherited the allele from the B6 or the DBA/2 founder strain, and the mean ± SD of all the lines in each group was calculated for each γδ i-IEL subset. These mean values were then compared by different statistical tests and sorted according to the Student’s t value. The t values distributed in a Gaussian-like curve, suggesting that most of the analyzed genes have no detectable effects on the studied phenotype. For all γδ i-IEL subsets analyzed, however, there were a number of genes for which the mean values of the B and D lines were statistically significant. All of the genes that seemed to influence a particular γδ i-IEL subset were linked on the same chromosome, suggesting that only one gene in this area was responsible for the observed differences.
RESULTS
Representation of Different γδ T Cell Subsets Among γδ i-IEL in B6, DBA/2, and B6D2F1 Mice.
Previous studies have shown that >80% of the γδ i-IEL of most common laboratory mouse strains use either the Vγ1 or the Vγ7 chain (17, 20). The relative proportion of γδ i-IEL expressing the Vγ1 or the Vγ7 chain, however, varies among different strains of mice whereas it remains quite constant among genetically identical individuals. Such strain dependence is maintained even when animals are housed under different conditions, suggesting that genetic factors, rather than environmental factors, are responsible for these differences. We chose to address the question of the putative genetic control of the TCR repertoire in γδ i-IEL using B6 and DBA/2 as prototype strains. These two strains differ in the relative representation of most γδ i-IEL subsets that can be defined with the available TCR V region specific mAbs, and a large number of RI strains generated from (B6 × DBA/2) crosses is available. Furthermore, analysis of the splenic γδ TCR repertoire in these RI lines already has been performed (19), allowing the comparison of the genetic elements influencing the i-IEL and the splenic γδ T cell populations.
The proportion of γδ i-IEL expressing the Vγ1, Vγ7, Vγ4, or the Vδ4 gene segments in B6, DBA/2, and B6D2F1 hybrid mice is shown in Fig. 1 (Upper). In all three groups of mice, Vγ4-bearing cells constituted ≈6% of the total γδ i-IEL (Fig. 1C). The relative proportions of γδ i-IEL expressing the Vγ1 or the Vγ7 chains are inversely correlated in B6 and DBA/2 strains. Thus, 28.2 ± 2.4% of the γδ i-IEL in B6 and 52.7 ± 3.8% of the γδ i-IEL in DBA/2 mice express the Vγ1 chain (A). Inversely, 57.9 ± 3% and 36.5 ± 3.9% of the γδ i-IEL express the Vγ7 in B6 and DBA/2, respectively (B). The fraction of γδ i-IEL expressing the Vδ4 chain also is different in the two strains of mice, with values of 25.5 ± 1.8% and 13.5 ± 1.3% in B6 and DBA/2, respectively (D). In all cases, the frequencies observed in B6D2F1 mice resemble those found in DBA/2 mice, demonstrating that the DBA/2 phenotype (high Vγ1, low Vγ7, and low Vδ4) is dominant. These data confirm and extend the clear strain dependence of γδ TCR subset representation in these strains (17, 19, 20).
Figure 1.
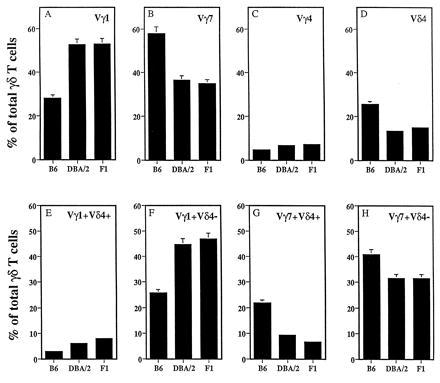
Representation of different γδ i-IEL subsets in B6, DBA/2, and B6D2F1 mice. (Upper) i-IEL from the three mouse strains were isolated and stained with fluorescein isothiocyanate-labeled anti-pan γδ mAb and biotin-labeled anti-Vγ1 (A), anti-Vγ7 (B), anti-Vγ4 (C), or anti-Vδ4 (D) mAbs followed by streptavidin–phycoerythrin and analyzed on a FACScan. Data are shown as the percentage of total γδ i-IEL expressing the indicated V region and correspond to the mean ± SD of six independent determinations using two to four animals per determination. (Lower) The same i-IEL preparations were stained with fluorescein isothiocyanate-labeled anti-Vγ1 (E and F) or anti-Vγ7 (G and H) mAbs together with biotin-labeled anti-Vδ4 mAb followed by streptavidin–phycoerythrin and analyzed as above.
Double staining of γδ i-IEL with Vγ1 or Vγ7 and Vδ4 specific mAbs allows the definition of four subsets, namely Vγ1+Vδ4+, Vγ1+Vδ4−, Vγ7+Vδ4+, and Vγ7+Vδ4−. The relative frequency of each of the four subsets also is different in B6 and DBA/2 mice (Fig. 1, Lower). Vγ1+Vδ4+ cells constitute a small fraction of γδ i-IEL in both strains (2.7 ± 0.9% in B6 and 5.6 ± 1.9% in DBA/2; Fig. 1E). Consequently, the levels of the Vγ1+Vδ4− cells are very similar to the total level of Vγ1 cells (25.3 ± 2.2% in B6 and 44.4 ± 4.3% in DBA/2; Fig. 1F). Thus, most of the differences in the level of Vγ1 cells among the two strains can be attributed to cells expressing Vδ chains other than Vδ4. The levels of Vγ7+Vδ4− cells were 40.5 ± 4.6% in B6 and 31.2 ± 1.9% in DBA/2 whereas the levels of Vγ7+Vδ4+ γδ cells were 21 ± 4.6% and 8.9 ± 1.3% in B6 and DBA/2, respectively. Thus, the differences in the frequency of γδ i-IEL expressing the Vδ4 chain are mainly due to the differences in the frequency of the Vγ7+Vδ4+ cells.
Analysis of Different γδ i-IEL Subsets in Various BXD RI Lines.
To genetically map the loci that control the levels of the different TCRγδ i-IEL subsets, their relative frequencies were analyzed in BXD RI lines (B6 and DBA/2 founders). The representation of all four γδ i-IEL subsets analyzed in the RI lines did not fall into distinct clusters (not shown). The values recorded in these lines formed a continuum from low to high levels, suggesting that multiple genes control the representation of the γδ i-IEL subsets. Close to 1000 polymorphic genes have been identified in the BXD RI lines. To study the possible influence of any of these genes on the levels of the different γδ i-IEL subsets, the BXD RI lines were distributed in two groups for each identified gene, according to whether they had inherited the allele from the B6 or the DBA/2 founder strain (B and D lines). For each γδ i-IEL subset, we calculated the mean ± SD of all the lines in each group. These mean values were then compared by different statistical tests and sorted according to the Student’s t test value. The t test values distributed in a Gaussian-like curve, suggesting that most of the analyzed genes have no detectable effects on the studied phenotype. For all γδ i-IEL subsets analyzed, however, there were a number of genes for which the mean values of the B and D lines were statistically significant. All of the genes that seemed to influence a particular γδ i-IEL subset were linked on the same chromosome, suggesting that only one locus in this area was responsible for the observed influences. Thus, the level of Vγ1+Vδ4+ cells correlates best with the TCRγ allotype (Fig. 2, Left). All lines that inherited the TCRγ locus from B6 expressed, like the founder strain, low levels of Vγ1+Vδ4+ cells (mean 3.14 ± 1.16%) whereas the lines that inherited the TCRγ locus from the DBA/2 founder expressed higher levels of Vγ1+Vδ4+ cells (mean 9.5 ± 4.6%). Only one line, BXD32, did not fall into the expected category. Similar distribution analysis showed that the level of Vγ7+Vδ4+ cells correlates best with the TCRδ allotype (Fig. 2, Right). All of the lines that inherited the TCRδ locus from DBA/2 expressed, like the founder strain, low levels of Vγ7+Vδ4+ cells (mean 8 ± 1.89%) whereas all lines carrying the B6 allele at the TCRδ locus expressed higher levels of Vγ1+Vδ4+ γδ i-IEL (mean 19 ± 6.3%).
Figure 2.
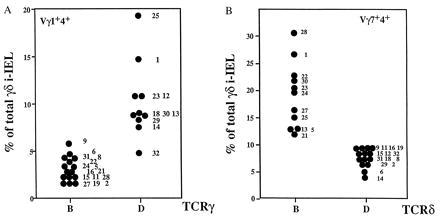
The representation of Vγ1+Vδ4+ and Vγ7+Vδ4+ i-IEL subsets in the BXD RI lines correlates with the TCRγ and TCRδ allotypes. (A) i-IEL from the indicated BXD strains were isolated and stained as in Fig. 1. Data shown are the percentage of total γδ i-IEL expressing the Vγ1+Vδ4+ TCR in the BXD lines distributed as to the TCRγ allotype. Each point corresponds to the mean value of two to three determinations of each BXD strain using two animals per determination. Numbers on the right of the dots denote the BXD strain number. B and D indicate the TCRγ locus inherited from the B6 and DBA/2 founder strains, respectively. The means of the values in TCRγ B and TCRγ D lines are 3.14 ± 1.16% and 9.5 ± 4.6%, respectively. According to Student’s t test, those values are different with a P < 0.001. (B) Data shown are the percentage of total γδ i-IEL expressing the Vγ7+Vδ4+ TCR in the BXD lines distributed as to the TCRδ allotype. B and D indicate the TCRδ locus inherited from the B6 and DBA/2 founder strains, respectively. Numbers on the right of the dots denote the BXD strain. The means of the values in TCRδ B and TCRδ D lines are 19 ± 6.3% and 8 ± 1.89%, respectively. According to Student’s t test, those values are not significantly different (P = 0.19).
The representation of the other two subsets, namely Vγ1+Vδ4− and Vγ7+Vδ4−, varied with an inverse relationship and correlated best with the MHC locus (Fig. 3). In concordance with the parental lines, H-2b lines expressed high levels of Vγ7+Vδ4− (mean 48 ± 7.8%) and low levels of Vγ1+Vδ4− (mean 32.7 ± 4.2%) whereas H-2d lines expressed lower levels of Vγ7+Vδ4− and higher levels of Vγ1+Vδ4− (means 27.06 ± 4.9% and 49 ± 9.9%, respectively). A closer scrutiny of the quantitative levels of these two subsets reflects well the complexity of the genetic control studied here. Thus, the comparison of the representation of Vγ7+Vδ4− and Vγ1+Vδ4− cells in the RI lines showed that, although related to each other, their levels are not strictly the reciprocal result of the same event (Fig. 4). This is due, in part, to the influence of the polymorphism at the TCRγ locus on the frequency of the Vγ7+Vδ4− subset in H-2b lines. As shown in Fig. 5, H-2b lines can be separated into two distinct groups whose levels of Vγ7+Vδ4− cells correlate well with the TCRγ allotype. The H-2b lines that inherited the TCRγ locus from the B6 founder strain show a higher frequency of Vγ7+Vδ4− γδ i-IEL than the H-2b lines that inherited the TCRγ locus from the DBA/2 founder strain (means 53.7 ± 2.4% and 41.2 ± 2.4%, respectively). Similarly, it seems clear that another locus (loci) regulates the level of this population in H-2d mice although no obvious linkage to any precise gene could be obtained with this kind of analysis.
Figure 3.
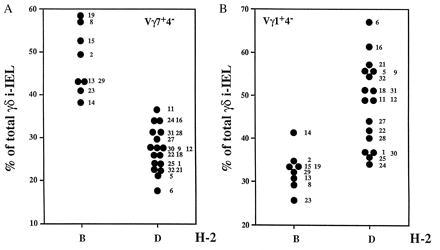
The representation of Vγ1+Vδ4− and Vγ7+Vδ4− i-IEL subsets in the BXD RI lines correlates with the MHC haplotype. i-IEL from the indicated BXD strains were isolated and stained as in Fig. 1. Data shown are the percentage of total γδ i-IEL expressing the Vγ7 chain but not the Vδ4 chain (A; Vγ7+Vδ4− cells) or the Vγ1 chain but not the Vδ4 chain (B; Vγ1+Vδ4− cells) in the BXD lines distributed as to the MHC haplotype. Each point corresponds to the mean value of two to three determinations of each BXD strain using two animals per determination. Numbers on the right of the dots denote the BXD strain number. B and D indicate the TCRγ locus inherited from the B6 and DBA/2 founder strains, respectively. The mean of the values of the Vγ7+Vδ4− in MHC B and MHC D lines are 48 ± 7.8% and 27 ± 4.9%, respectively. According to Student’s t test, those values are different with P < 0.001. The means of the values of the Vγ1+Vδ4− in MHC B and MHC D lines are 32.75 ± 4.2% and 49.1 ± 9.9%, respectively. According to Student’s t test, those values are different with a P < 0.02.
Figure 4.
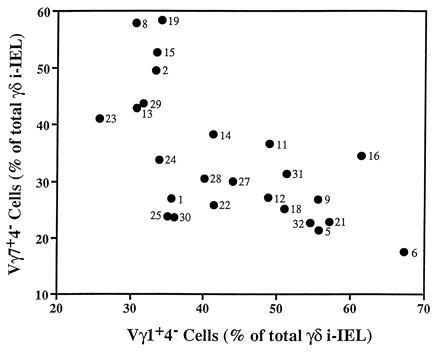
Lack of strict correlation between the representations of the Vγ1+Vδ4− and the Vγ7+Vδ4− i-IEL subsets. The percentages of Vγ1+Vδ4− and Vγ7+Vδ4− i-IELs from the BXD RI lines were plotted on the x and y axes, respectively. Numbers next to the symbols are the BXD strain number.
Figure 5.
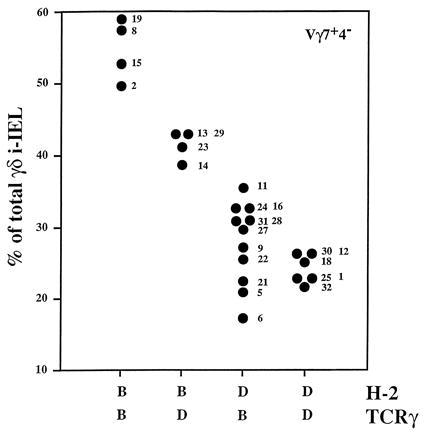
Differences in the representation of the Vγ7+Vδ4− i-IEL subset in BXD lines carrying the H-2b haplotype correlate with the TCRγ allotype. The same data as in Fig. 3 were plotted. The BXD lines were distributed as to their H-2 haplotype and TCRγ allotype. The mean values of the Vγ7+Vδ4− subset in H-2b animals expressing the TCRγ B or the TCRγ D were 53.7 ± 4% and 41.2 ± 2.4%, respectively. According to Student’s t test, those values are different with a P < 0.017.
DISCUSSION
In the present study, we have shown that the relative representation of different subsets of γδ i-IEL in B6 and DBA/2 mice is under a complex genetic control with marked influences by genes closely linked to the TCRγ, TCRδ, and MHC loci. Altogether these results suggest that the repertoire of γδ i-IEL is influenced by cellular selection and that MHC-linked genes play a major role in that selection. MHC-dependent selection of the γδ i-IEL repertoire has been shown in crosses between B6 and C3H mouse strains and in different intra-H-2 congenic strains in the C57BL/10 background (17). In those experiments, the relative representation of CD8+ γδ i-IEL expressing the Vδ4 chain was shown to correlate with the expression of the I-E molecule although further experiments indicated that expression of the I-E molecule was not sufficient to account for the increased proportion of Vδ4 cells (18, 23). In the experiments presented here, selection through MHC-linked genes is apparent when we analyze γδ T cells not bearing the Vδ4 chain. It is possible that, in the crosses between B6 and DBA/2 strains, MHC-linked selection affects the product of another Vδ gene although MHC-linked genes also appear responsible for the relative representation of cells expressing the Vγ1 or the Vγ7 chain.
The biological significance of the MHC-linked selection of the γδ i-IEL repertoire is not clear. One possibility is that, similar to αβ T cells, γδ T cells recognize their specific ligands in the context of polymorphic MHC molecules. Although γδ T cell clones recognizing MHC class Ib and II molecules have been described (24–28), they appear to be the exception rather than the rule, which makes this possibility unlikely. It is still possible, however, that certain TCRγ or TCRδ chains (or their combinations) interact with endogenous ligands in the context of MHC molecules in a “superantigen-like” fashion. An alternative possibility is that polymorphic MHC molecules select for different TCR αβ specificities that, in turn, select for different γδ TCR repertoires. Analysis of γδ T cell subsets in TCR αβ-deficient mice should help to clarify this issue once these mice are placed in the correct genetic backgrounds.
Studies of mice lacking MHC class I and II molecules have shown that their numbers of γδ T cells are similar to those found in normal animals (15, 29), suggesting that class I and II molecules are not necessary for the development of γδ T cells. Furthermore, it has been shown that the frequency of γδ i-IEL expressing the Vδ4 chain is not different between mutant and normal mice, leading to the suggestion that MHC class I and II genes did not play a major role in the selection of γδ TCR repertoires (23). Although it is possible that other MHC-linked gene(s) are responsible for the selection of γδ TCR repertoires, we believe that a role of MHC class I or II molecules in this selection has not been completely excluded. Thus, the relative representation of certain γδ T cell subsets can be influenced by different loci depending on the genetic background. For example, although the relative representation on Vγ1 i-IEL is controlled by H-2-linked genes in crosses between B6 and DBA/2 strains, we have shown that H-2b strains that are genetically close to the B6 strain, like C57BL/10, can be typed as Vγ1 high (20). This could be the result of two different kinds of phenomena. On the one hand, it appears clear that the γδ TCR repertoire is strongly influenced by the TCRγ and TCRδ haplotypes, suggesting that different TCRγ or TCRδ alleles can be submitted to different selective pressures (see below). On the other hand, the fact that few Vγ and Vδ genes are expressed by γδ i-IEL makes it difficult to define the selective pressures that influence the representation of a particular γδ subset. Selection of cells expressing a given Vγ or Vδ chain will clearly influence the representation of cells expressing other Vγ and Vδ chains. The total number of γδ i-IELs is limited, so increased or decreased representation of a given γδ subset will be compensated by decreased or increased representation of other subsets, respectively. Thus, the fact that two different strains of mice show the same TCR repertoire among γδ i-IELs does not suffice to make the conclusion that the γδ i-IELs in the two strains are subject to identical selective pressures.
The control of the γδ TCR repertoire by TCR genes may have two different, although not mutually exclusive, explanations. As stated before, it is possible that cellular selection is V gene allele-specific. The finding that the reactivity of Vγ1/Vδ6-expressing γδ hybridomas to heat-shock protein-derived peptides depends on the Vγ1–Cγ4 allele agrees well with this notion (30). Similar V gene allele specificity has been shown to operate in the selection of αβ T cells. Thus, studies performed with phylogenetically distant mice have shown that the reactivity of certain mouse mammary tumor virus products with different Vβ chains depends upon the TCRβ allele (31, 32). The greater polymorphism of TCRγ and TCRδ genes, compared with that of TCRβ genes in the mouse, would make this allele specificity more evident in γδ cells than in αβ cells.
A second, more simple explanation considers that the two mouse strains use a different number of TCR genes. This possibility is certainly not relevant for the TCRγ genes because 80–90% of the γδ i-IEL in both strains express the Vγ1 or the Vγ7 chain, but it could explain, at least in part, the linkage to the TCRδ locus. In our experiments, the higher frequency of Vδ4-positive γδ i-IEL in B6 mice compared with DBA/2 and B6D2F1 mice and the influence of the TCRδ locus found in the analysis of the RI strains could be simply explained, in the absence of any cellular selection, if γδ i-IEL from DBA/2 mice express more Vδ genes than γδ i-IEL from B6 mice. We are currently analyzing this possibility.
We have shown here that the higher representation of the Vγ1+Vδ4+ i-IEL subset correlates with the inheritance of the TCRγ allotype from the DBA/2 founder strain. Similar genetic control was found in the representation of Vγ4−Vδ4+ splenic cells (likely to represent Vγ1+Vδ4+ cells) in the same BXD RI lines (19). These results suggest that similar mechanisms control the relative size of the Vγ1+Vδ4+ subset in the spleen and in the intestinal epithelium.
From these and previous studies it appears likely that, besides the MHC locus and the TCRγ and δ loci, other gene products contribute to the differences observed in the γδ TCR repertoire. Because the frequencies observed for different subsets relate to each other, it is expected that the products of all three genes (MHC, TCRγ, and TCRδ) will, to some extent, control the level of each of the γδ T cell subsets. The fact that a linkage to those genes appears in the crosses analyzed suggests that few other gene products are involved in the selection of the γδ TCR repertoire. By fixing the TCRγ and TCRδ allotypes, it will be possible to further analyze the role of MHC-linked genes and to know whether the products of other loci have an important role in this selection. Thus, analysis, now in progress, of the representation of the different γδ T cell subsets in crosses between B6 and different BXD RI lines carrying the B6 TCRγ and TCRδ allotypes but segregating at other loci should help clarify these issues.
Acknowledgments
We thank Jorge Carneiro and John Stewart for their help with the statistical programs and for helpful discussions, A. Coutinho for critical reading of the manuscript, and K. Nagashima for excellent technical assistance. Supported by grants from Yakult Honsha and by National Institutes of Health Grants R37 and R35 to S.T. and by Grant 6969 from Association pour la Recherche sur le Cancer to P.P.
ABBREVIATIONS
- TCR
T cell antigen receptor
- MHC
major histocompatibility complex
- i-IEL
intestinal intraepithelial lymphocytes
- RI
recombinant inbred
References
- 1.Dembic Z, Haas W, Weiss S, McCubrey J, Keifer H, Von Boehmer H, Steinmetz M. Nature (London) 1986;320:232–238. doi: 10.1038/320232a0. [DOI] [PubMed] [Google Scholar]
- 2.Babbitt B, Allen P, Matsueda G, Haber E, Unanue E. Nature (London) 1985;317:359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- 3.Buus S, Colon S, Smith C, Freed J H, Miles C, Grey H. Proc Natl Acad Sci USA. 1986;83:3968–3971. doi: 10.1073/pnas.83.11.3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillet J G, Lai M Z, Briner T J, Smith J A, Gefter M L. Nature (London) 1986;324:260–262. doi: 10.1038/324260a0. [DOI] [PubMed] [Google Scholar]
- 5.Bevan M J. Nature (London) 1977;269:417–419. [Google Scholar]
- 6.Zinkernagel R M, G N C, Aalthage A, Cooper S, Klein P A, Klein J. J Exp Med. 1978;147:882–896. doi: 10.1084/jem.147.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kisielow P, Bluthmann H, Staerz U D, Steinmetz M, Von Boehmer H. Nature (London) 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 8.Sha W C, Nelson C A, Newberry R D, Kranz D M, Russel J H, Loh D Y. Nature (London) 1988;336:73–76. doi: 10.1038/336073a0. [DOI] [PubMed] [Google Scholar]
- 9.Kappler J W, Roehm N, Marrack P. Cell. 1987;49:273–280. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald H R, Schneider R, Lees R K, Howe R C, Acha-Orbea H, Festenstein H, Zinkernagel R M, Hengartner H. Nature (London) 1988;322:40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- 11.Bonneville M, Ishida I, Itohara S, Verbeek S, Berns A, Kanagawa O, Haas W, Tonegawa S. Nature (London) 1990;344:163–165. doi: 10.1038/344163a0. [DOI] [PubMed] [Google Scholar]
- 12.Dent A L, Matis L A, Hooshmand F, Widacki S M, Bluestone J A, Hedrick S M. Nature (London) 1990;343:714–719. doi: 10.1038/343714a0. [DOI] [PubMed] [Google Scholar]
- 13.Pereira P, Ziljstra M, McMaster J, Loring J M, Jaenisch R, Tonegawa S. EMBO J. 1992;11:25–31. doi: 10.1002/j.1460-2075.1992.tb05023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells F B, Gahm S J, Hedrick S M, Bluestone J A, Dent A, Matis L A. Science. 1991;253:903–905. doi: 10.1126/science.1831565. [DOI] [PubMed] [Google Scholar]
- 15.Correa I, Bik M, Liao N-S, Zijlstra M, Jaenisch R, Raulet D. Proc Natl Acad Sci USA. 1992;89:653–657. doi: 10.1073/pnas.89.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leclercq G, Plum J, Nandi D, De S M, Allison J P. J Exp Med. 1993;178:309–315. doi: 10.1084/jem.178.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lefrançois L, LeCorre R, Mayo J, Bluestone J A, Goodman T. Cell. 1990;63:333–340. doi: 10.1016/0092-8674(90)90166-c. [DOI] [PubMed] [Google Scholar]
- 18.Lefrançois L. Immunol Today. 1991;12:436–438. doi: 10.1016/0167-5699(91)90015-L. [DOI] [PubMed] [Google Scholar]
- 19.Sperling A I, Cron R Q, Decker D C, Stern D A, Bluestone J A. J Immunol. 1992;149:3200–3207. [PubMed] [Google Scholar]
- 20.Pereira P, Gerber D, Huang S Y, Tonegawa S. J Exp Med. 1995;182:1921–1930. doi: 10.1084/jem.182.6.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Itohara S, Nakanishi N, Kanagawa O, Kubo R, Tonegawa S. Proc Natl Acad Sci USA. 1989;86:5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishikawa H, Li Y, Yamamoto S, Kaufmann S H E, Tonegawa S. Proc Natl Acad Sci USA. 1993;90:8204–8208. doi: 10.1073/pnas.90.17.8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schleussner C, Ceredig R. Eur J Immunol. 1993;23:1615–1622. doi: 10.1002/eji.1830230733. [DOI] [PubMed] [Google Scholar]
- 24.Ito K, VanKear L, Bonneville M, Hsu M, Murphy D B, Tonegawa S. Cell. 1990;62:549–561. doi: 10.1016/0092-8674(90)90019-b. [DOI] [PubMed] [Google Scholar]
- 25.Houlden B A, Matis L A, Cron R Q, Widacki S M, Brown D, Pampeno C, Meruelo D, Bluestone J A. Cold Spring Harbor Symp Quant Biol. 1990;54:45–55. doi: 10.1101/sqb.1989.054.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Vidovic D, Roglic M, McKune K, Guerder S, MacKay C, Dembic Z. Nature (London) 1989;340:646–650. doi: 10.1038/340646a0. [DOI] [PubMed] [Google Scholar]
- 27.Matis L A, Cron R Q, Bluestone J A. Nature (London) 1987;330:262–264. doi: 10.1038/330262a0. [DOI] [PubMed] [Google Scholar]
- 28.Matis L A, Fry A M, Cron R Q, Cotterman M M, Dick R F, Bluestone J A. Science. 1989;245:746–749. doi: 10.1126/science.2528206. [DOI] [PubMed] [Google Scholar]
- 29.Bigby, M., Markowitz, J. S., Bleicher, P. A., Grubsby, M. J., Simha, S., Siebrecht, M., Wagner, M., Nagler-Anderson, C. & Glimcher, L. H. (1993) J. Immunol. (1993) 151, 4465–4475. [PubMed]
- 30.Kalataradi H, Eyster C L, Fry A, Vollmer M K, Fu Y-X, Born W B, O’Brien R L. J Immunol. 1994;153:1455–1465. [PubMed] [Google Scholar]
- 31.Cazenave P A, Marche P, Jouvin-Marche E, Voegtle D, Bonhomme F, Bandeira A, Coutinho A. Cell. 1990;63:717–728. doi: 10.1016/0092-8674(90)90138-5. [DOI] [PubMed] [Google Scholar]
- 32.Pullen A M, Potts W, Wakeland E K, Kappler J, Marrack P. J Exp Med. 1990;171:49–62. doi: 10.1084/jem.171.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]


