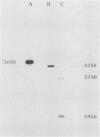
Abstract
The gene for a novel extracellular metalloprotease was cloned, and its nucleotide sequence was determined. The gene (mpr) encodes a primary product of 313 amino acids that has little similarity to other known Bacillus proteases. The amino acid sequence of the mature protease was preceded by a signal sequence of approximately 34 amino acids and a pro sequence of 58 amino acids. Four cysteine residues were found in the deduced amino acid sequence of the mature protein, indicating the possible presence of disulfide bonds. The mpr gene mapped in the cysA-aroI region of the chromosome and was not required for growth or sporulation.
Full text
PDF


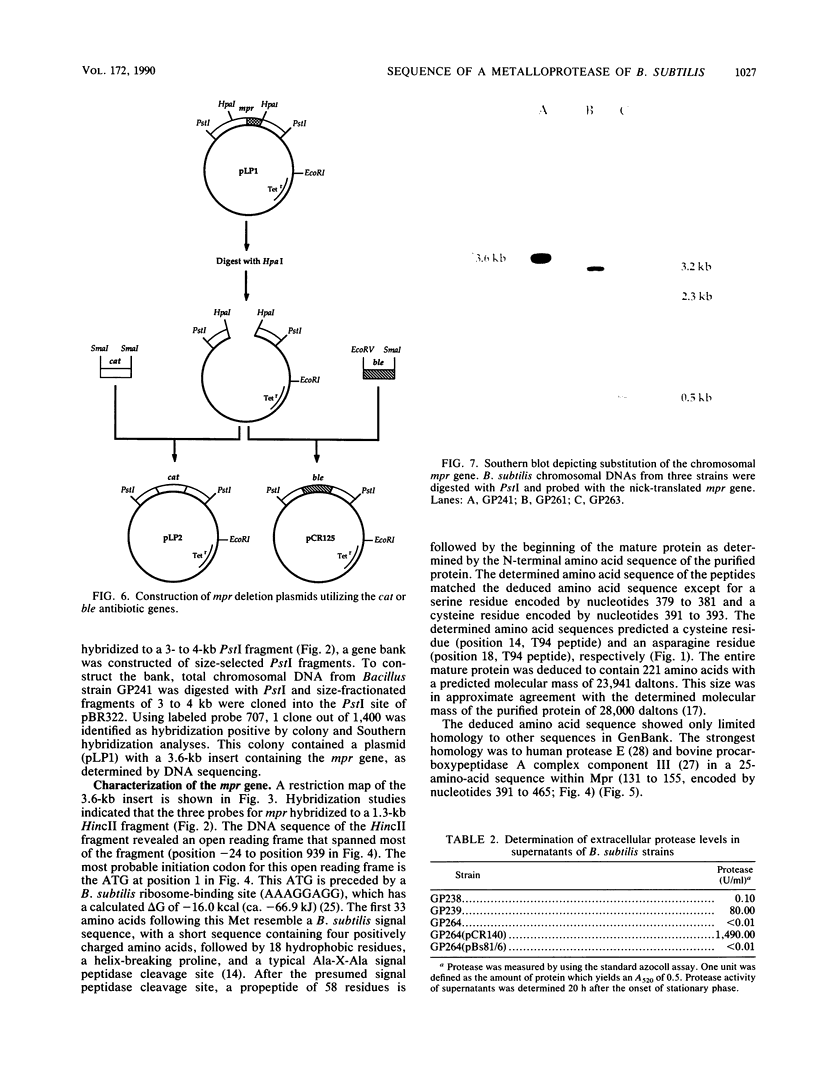
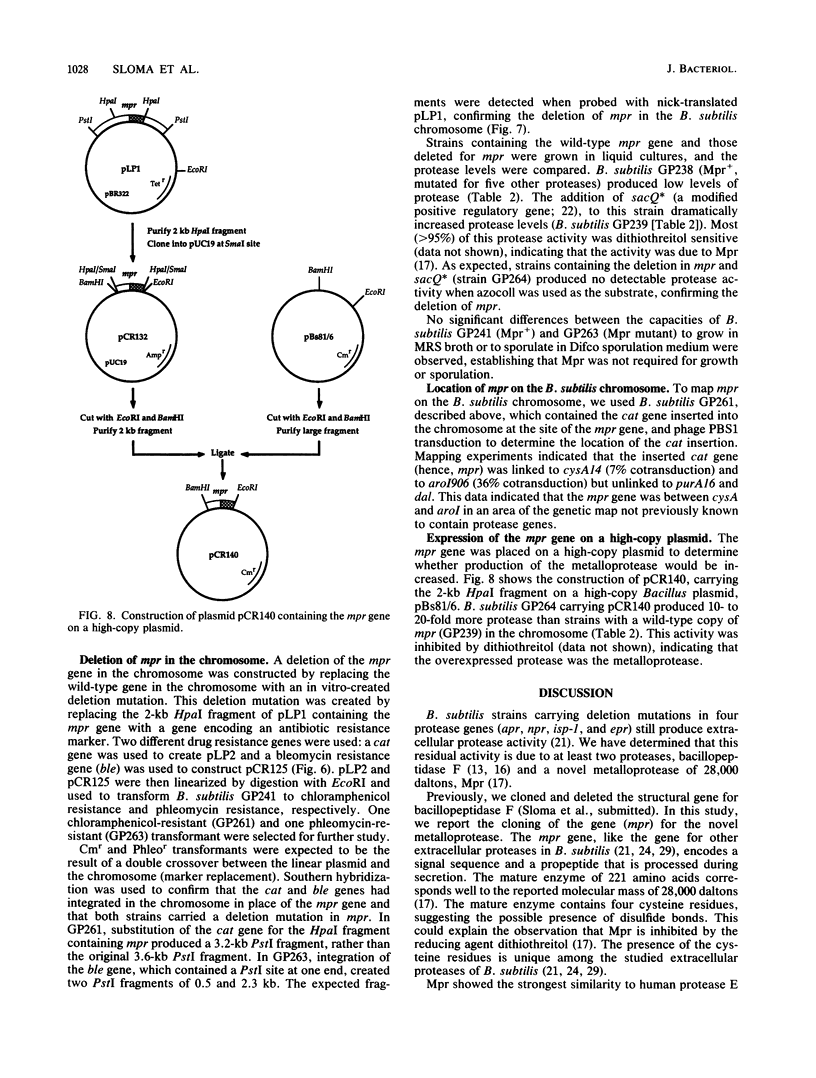

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedonder R. A., Lepesant J. A., Lepesant-Kejzlarová J., Billault A., Steinmetz M., Kunst F. Construction of a kit of reference strains for rapid genetic mapping in Bacillus subtilis 168. Appl Environ Microbiol. 1977 Apr;33(4):989–993. doi: 10.1128/aem.33.4.989-993.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J Mol Biol. 1971 Mar 14;56(2):209–221. doi: 10.1016/0022-2836(71)90460-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T., Shivakumar A. G., Dubnau D. Characterization of chimeric plasmid cloning vehicles in Bacillus subtilis. J Bacteriol. 1980 Jan;141(1):246–253. doi: 10.1128/jb.141.1.246-253.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higerd T. B., Hoch J. A., Spizizen J. Hyperprotease-producing mutants of Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):1026–1028. doi: 10.1128/jb.112.2.1026-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Doi R. H. Construction of a Bacillus subtilis double mutant deficient in extracellular alkaline and neutral proteases. J Bacteriol. 1984 Oct;160(1):442–444. doi: 10.1128/jb.160.1.442-444.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide Y., Nakamura A., Uozumi T., Beppu T. Cloning and sequencing of the major intracellular serine protease gene of Bacillus subtilis. J Bacteriol. 1986 Jul;167(1):110–116. doi: 10.1128/jb.167.1.110-116.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamas S., Millet J. Purification et propriétés d'une estérase excrétée pendant la sporulation de Bacillus subtilis. Biochimie. 1975;57(1):9–16. doi: 10.1016/s0300-9084(75)80104-0. [DOI] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. A putative signal peptidase recognition site and sequence in eukaryotic and prokaryotic signal peptides. J Mol Biol. 1983 Jun 25;167(2):391–409. doi: 10.1016/s0022-2836(83)80341-6. [DOI] [PubMed] [Google Scholar]
- Priest F. G. Extracellular enzyme synthesis in the genus Bacillus. Bacteriol Rev. 1977 Sep;41(3):711–753. doi: 10.1128/br.41.3.711-753.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitsch C. A., Hageman J. H. Bacillopeptidase F: two forms of a glycoprotein serine protease from Bacillus subtilis 168. J Bacteriol. 1983 Jul;155(1):145–152. doi: 10.1128/jb.155.1.145-152.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufo G. A., Jr, Sullivan B. J., Sloma A., Pero J. Isolation and characterization of a novel extracellular metalloprotease from Bacillus subtilis. J Bacteriol. 1990 Feb;172(2):1019–1023. doi: 10.1128/jb.172.2.1019-1023.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semon D., Movva N. R., Smith T. F., el Alama M., Davies J. Plasmid-determined bleomycin resistance in Staphylococcus aureus. Plasmid. 1987 Jan;17(1):46–53. doi: 10.1016/0147-619x(87)90007-2. [DOI] [PubMed] [Google Scholar]
- Shen W. F., Fletcher T. S., Largman C. Primary structure of human pancreatic protease E determined by sequence analysis of the cloned mRNA. Biochemistry. 1987 Jun 16;26(12):3447–3452. doi: 10.1021/bi00386a030. [DOI] [PubMed] [Google Scholar]
- Sloma A., Ally A., Ally D., Pero J. Gene encoding a minor extracellular protease in Bacillus subtilis. J Bacteriol. 1988 Dec;170(12):5557–5563. doi: 10.1128/jb.170.12.5557-5563.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stahl M. L., Ferrari E. Replacement of the Bacillus subtilis subtilisin structural gene with an In vitro-derived deletion mutation. J Bacteriol. 1984 May;158(2):411–418. doi: 10.1128/jb.158.2.411-418.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Uehara H., Yamane K., Maruo B. Thermosensitive, extracellular neutral proteases in Bacillus subtilis: isolation, characterization, and genetics. J Bacteriol. 1979 Aug;139(2):583–590. doi: 10.1128/jb.139.2.583-590.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venot N., Sciaky M., Puigserver A., Desnuelle P., Laurent G. Amino acid sequence and disulfide bridges of subunit III, a defective endopeptidase present in the bovine pancreatic 6 S procarboxypeptidase A complex. Eur J Biochem. 1986 May 15;157(1):91–99. doi: 10.1111/j.1432-1033.1986.tb09642.x. [DOI] [PubMed] [Google Scholar]
- Yang M. Y., Ferrari E., Henner D. J. Cloning of the neutral protease gene of Bacillus subtilis and the use of the cloned gene to create an in vitro-derived deletion mutation. J Bacteriol. 1984 Oct;160(1):15–21. doi: 10.1128/jb.160.1.15-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P., Perkins J. B., Losick R. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid. 1984 Jul;12(1):1–9. doi: 10.1016/0147-619x(84)90061-1. [DOI] [PubMed] [Google Scholar]