Abstract
Ras proteins are distributed in distinct plasma-membrane microdomains and endomembranes. The biochemical signals generated by Ras therein differ qualitatively and quantitatively, but the extent to which this spatial variability impacts on the genetic program switched-on by Ras is unknown. We have used microarray technology to identify the transcriptional targets of localization-specific Ras subsignals in NIH3T3 cells expressing H-RasV12 selectively tethered to distinct cellular microenvironments. We report that the transcriptomes resulting from site-specific Ras activation show a significant overlap. However, distinct genetic signatures can also be found for each of the Ras subsignals. Our analyses unveil 121 genes uniquely regulated by Ras signals emanating from plasma-membrane microdomains. Interestingly, not a single gene is specifically controlled by lipid raft-anchored Ras. Furthermore, only 9 genes are exclusive for Ras signals from endomembranes. Also, we have identified 31 genes common to the site-specific Ras subsignals capable of inducing cellular transformation. Among these are the genes coding for Vitamin D receptor and for p120-GAP and we have assessed their impact in Ras-induced transformation. Overall, this report reveals the complexity and variability of the different genetic programs orchestrated by Ras from its main sublocalizations.
Keywords: Ras, Compartmentalization, Gene microarrays, Transformation
1. Introduction
Ras GTPases operate as molecular switches that convey signals from surface receptors to the interior of the cell, thereby regulating essential processes including proliferation, differentiation and survival [1]. Ras implication in the origin and progression of pathological conditions like cancer is also extensively documented [2]. The mechanisms whereby Ras promotes malignant transformation have been subject of exhaustive cellular and biochemical studies. Recently, the development of DNA microarray technologies has allowed genome-wide analyses of the alterations in gene expression profiles resulting from changes in Ras status. Extensive data has been accumulated on the transcriptional networks associated to the transformation of different cell lines by oncogenic Ras proteins [3-9]. Likewise, the expression profiles resulting from the ablation of H-Ras and N-Ras in murine fibroblasts have also been reported [10].
Ras proteins are segregated in plasma-membrane (PM) microdomains like lipid rafts and disordered membrane (DM) [11]. Furthermore, Ras is also present in endomembranes like endosomes, endoplasmic reticulum (ER) and the Golgi complex, where it can productively engage downstream effectors [12,13]. The presence of Ras in various compartments could be intended to generate variability in its biochemical and biological outputs. In support of this notion, recent findings indicate that the microenvironment in which Ras signals originate determines effector usage and subsequent biological outcomes [12,14].
Herein, we have extended these observations by analyzing the gene expression profiles resulting from Ras activity in its main signaling platforms, namely: DM, lipid rafts, Golgi complex and ER. Our data unveils the existence of distinct transcriptional networks that depend on the compartment at which Ras signals originate, further endorsing the concept of the microenvironment as a key regulator of the biochemical, genetic and biological outcomes of Ras signals. Furthermore, by focusing on the common elements among the transcriptomes of the site-specific Ras signals capable of inducing transformation, we have identified novel participants in this phenomenon.
2. Materials and methods
2.1. Microarray experiments and data analysis
Total RNAs from triplicates of exponentially growing NIH3T3 cell lines stably expressing H-RasV12 and the tethered H-RasV12 proteins [14], were collected using the RNEasy method (Quiagen). The quantity and quality of the RNAs was determined using 6000 Nano Chips (Agilent Technologies). RNA samples (4 μg) were processed for hybridization on MGU75Av2 microarrays (Affymetrix) following manufacturer’s recommendations. Normalization, filtering and analysis of the raw data, was performed using the Bioconductor software (www.bioconductor.com) using de ReadAffy package and the robust-multiarray analysis (RMA) application. The RMA algorithm was selected for its precision in signal detection to achieve adequate normalization of multiple microarrays, especially in cases of low levels of expression [15]. A gene was considered to be differentially expressed relative to the parental cell line when exhibiting a signal ≥100 and fulfilling the following criteria: a gene was regarded as “common” to all Ras mutants when: (i) It showed a fold change ≥±1.5 in all the cell lines analyzed when compared to the parental cell line; (ii) the fold change values obtained had statistical P-values ≤0.01. A gene was regarded as differentially expressed in some specific localization(s) when it did not undergo significant fold changes or when those changes had P values ≥0.01. A gene was uniquely regulated by a Ras protein when: (i) the fold change in the expression levels of is transcript in a given localization was ≥±1.5 with P-value P≤0.01; (ii) the fold change, if any, obtained in the other cell lines had P-values ≤0.01. Statistical analyses were performed using F-statistics. For the graphical presentation of microarray data, we performed hierarchical clustering analysis using the WPGA average linkage and the standard correlation similarity metric method with the J-Express application (2.1). Functional annotation of gene functions was performed manually using internet-available databases. The identification of proteins interactive networks was done using the Ingenuity Pathways Analysis program (www.ingenuity.com). We considered a network significant when it fulfilled the following criteria: (i) to have a minimal score of 15. (ii) to have at least 20 proteins participating in direct functional interactions inside the network.
2.2. Cellular transformation assays
Performed exactly as described [14].
2.3. Antibodies and other reagents
Rabbit polyclonal antibody anti-Vitamin D receptor and the mammalian expression vector encoding for this receptor were gifts from Dr. Ana Aranda (Madrid, Spain). Rabbit polyclonal antibodies against p120-GAP, Myc and ERK2 were from Santa Cruz Technologies.
2.4. Immunoblotting
Exactly as described previously [16].
2.5. Real-time PCR
Verification experiments using real-time PCR were performed using a commercial kit (Quiagen), following manufacturer’s instructions. The PCR primer sets sequences utilized correspond to those used in the Affymetrix arrays. The housekeeping gene GAPDH was used as internal control.
3. Results
3.1. Global analysis of Ras site-specific transcriptomes
To conduct this study, we used NIH3T3 cell lines expressing H-RasV12 selectively tethered to defined subcellular compartments by specific localization signals: the avian infectious bronchitis virus M1 protein (for ER localization), the LCK myristoylation signal (for lipid raft anchoring), the CD8α transmembrane domain (for DM localization) and the KDEL receptor N193D mutant (for Golgi complex localization). The validity of this approach has been described previously at the cell biology and signaling level [14]. A cell line expressing untargeted H-RasV12 was also included (see Supplementary Text, Section I). The gene expression profiles of these cell lines were compared to that of parental NIH3T3 cells, using Affymetrix microarrays. We observed that, in total, H-RasV12 signals emanating from its distinct sublocalizations induced changes in 8.8% (1074 genes) of the genes interrogated in the arrays (Fig. 1A, Supplementary Table S1). Verification by RT-PCR and/or immunoblotting of 20 randomly chosen samples confirmed the microarray data (data not shown). Hierarchical clustering of the microarray data sets yielded a dendrogram with two main vertical branches (Fig 1A): one corresponding to the untethered H-RasV12-dependent transcriptome and the other one clustering those generated by the site-specific H-RasV12 constructs. Interestingly, the transcriptome of H-RasV12 at the DM diverged from the expression profiles generated by H-RasV12 at lipid rafts, ER and the Golgi complex, which were grouped into a separate branch (Fig 1A). 329 genes were common to all the cases analyzed. Among these, essential genes involved in the regulation of proliferation and survival such as: PDGFα, TGFβ, FGFr, Integrin β5, Rho-C, PAK-3, MKK6, PTEN, MKP-1, c-Jun, Jun-B, Fra-1, Cyclin D1 and NFκB (Supplementary Table S2).
Fig. 1. Global analysis of Ras site-specific transcriptomes.
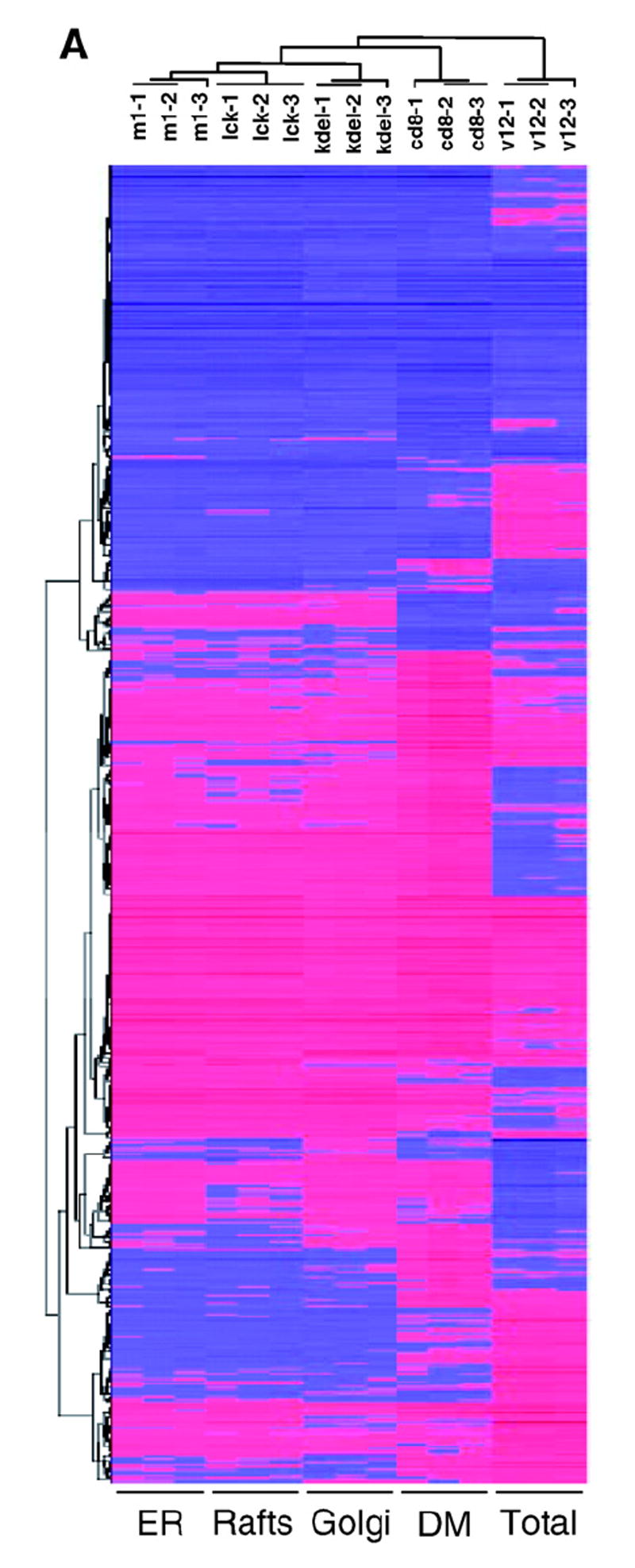
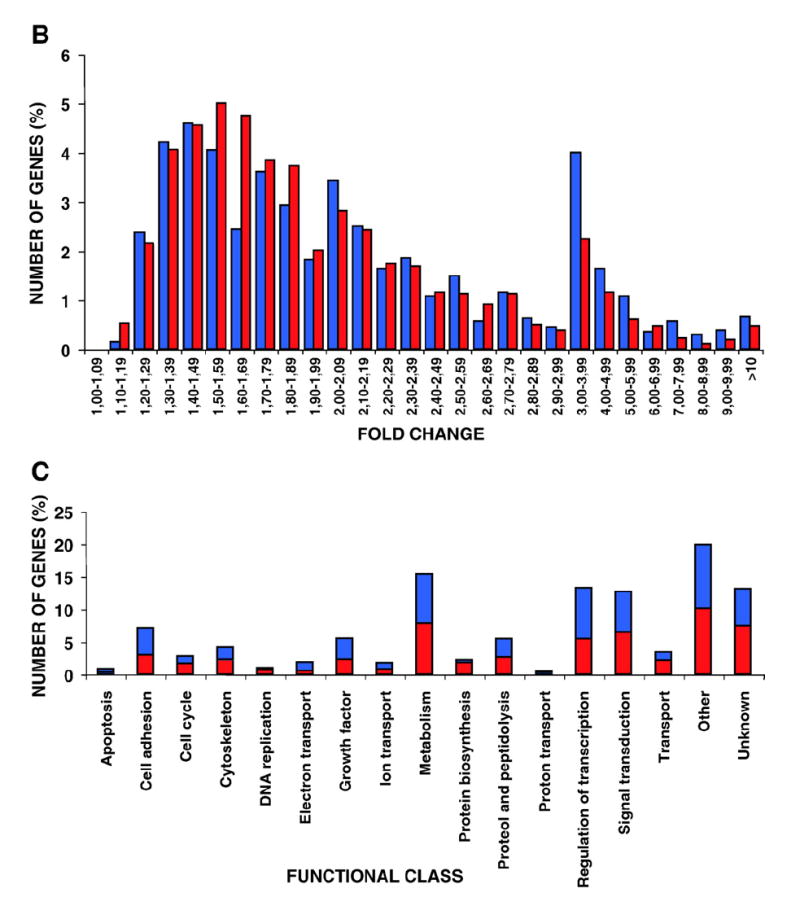
A) Hierarchical clustering of the genes regulated by site-specific Ras proteins: untagged H-RasV12 (V12) and ER- (M1), lipid raft- (LCK), DM- (CD8) and Golgi-tethered (KDEL) H- RasV12 proteins. Horizontal rows represent single gene probe sets. Vertical columns represent single microarray hybridizations. B) Histogram showing the number of up- (red) and down-regulated (blue) genes with a given expression fold change value compared to that of the parental cell line. Classifications of the genes according to general biological functions: C) Total number of genes, up- (red) and down-regulated (blue). D) Number of genes regulated from PM sublocalizations (LCK+CD8) and from endomembranes microdo-mains (M1+KDEL). E) Number of genes regulated from each of the Ras sublocalizations. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Further analysis of the transcriptomes revealed that the fold changes of the regulated genes followed a Poisson curve (Fig. 1B). Most of the genes experienced changes between 1.2 and 2.5 fold. Up-regulated and down-regulated genes followed similar patterns. Segregation of the 1074 identified genes into 17 different functional classes revealed that the higher numbers of genes were involved in transcriptional regulation, metabolism and signal transduction (Fig. 1C) (see Supplementary Text, Section I). In general, the regulation of the genes included in these functional classes did not reveal any spatial specificity. H-RasV12 signals generated at the plasma-membrane or at endomembranes were equally effective (Fig. 1D). Furthermore, in general, most gene functional groups were represented in similar proportions in the different compartment-specific transcriptomes (Fig. 1E).
3.2. Singularities of Ras site-specific transcriptomes
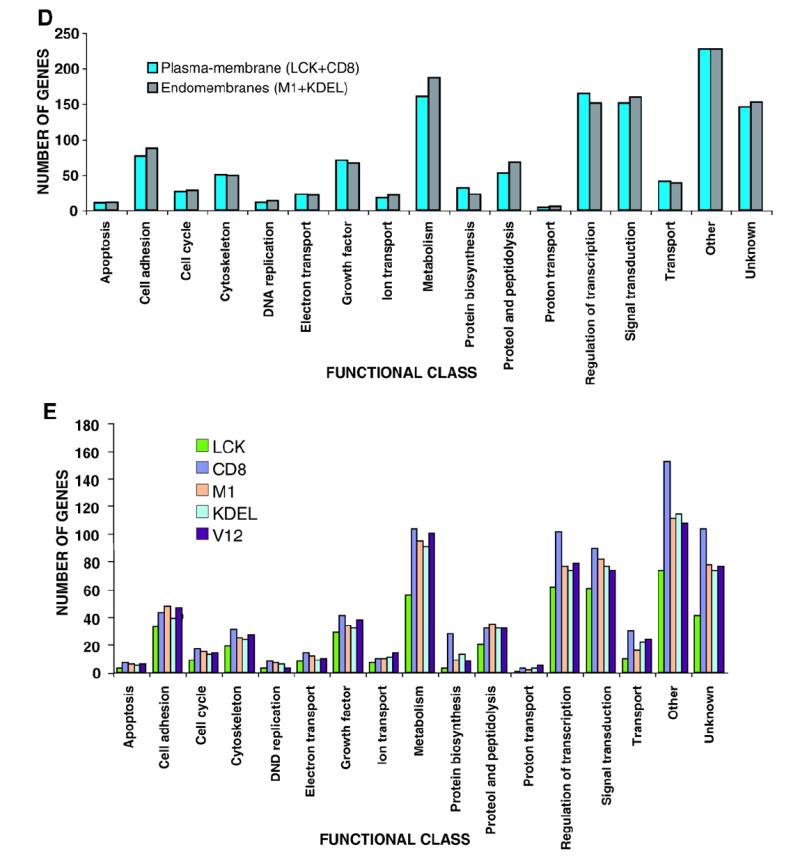
We next evaluated the possible transcriptomal specificities of the site-specific Ras mutants. Within the PM, it was observed that the DM-localized H-RasV12 protein (CD8) modulated a total of 858 genes (504 up-regulated, 354 down-regulated) (Fig. 2A, see also Supplementary Table S1). Furthermore, this subsignal contained the greatest number of specific genes of all the H-RasV12 signals studied (108 genes, 90 up-regulated, 18 down-regulated) (Fig. 2B and Table 1). Genes in this category include loci important for proliferation and survival such as TRAF1, RASSF1 and c-Myc. A significant number of angiogenesis-related genes were also detected in this DM-specific transcriptome (see Supplementary Text, Section II). The DM-specific transcriptome was further characterized using Ingenuity (www.ingenuity.com), a web-based software that identifies molecular networks by relating each microarray gene entry with an extensive database of reported physical, transcriptional or enzymatic interactions for ≈ 8000 mouse proteins. This analysis revealed the existence of four main interactive networks radiating from the nuclear proteins c-Myc, NFκB, c-Jun and HIF-1 (Fig. 2C). Keeping our focus on the events unfolding from the PM, we analyzed the genetic profile emerging from Ras activation at lipid rafts (LCK). This sublocalization regulated the lowest number of genes, barely 442 (189 up-regulated, 253 down-regulated) (Fig. 2A and Supplementary Table S1). Most interestingly, not a single gene was specifically regulated from this compartment (Fig. 2B). In addition to the genes that exhibited specific regulation from each of these PM sublocalizations, another 13 genes could be regulated from either compartment. Of these, 3 were up-regulated, 3 down-regulated and 6 genes showed opposite regulation, among them the transcription factor Ets2 (Fig. 2B, Supplementary Table S2). Overall, our analyses unveiled that 121 genes were specifically regulated by Ras signals emanating from PM microdomains.
Fig. 2. Singularities of Ras site-specific H-RasV12 transcriptomes.

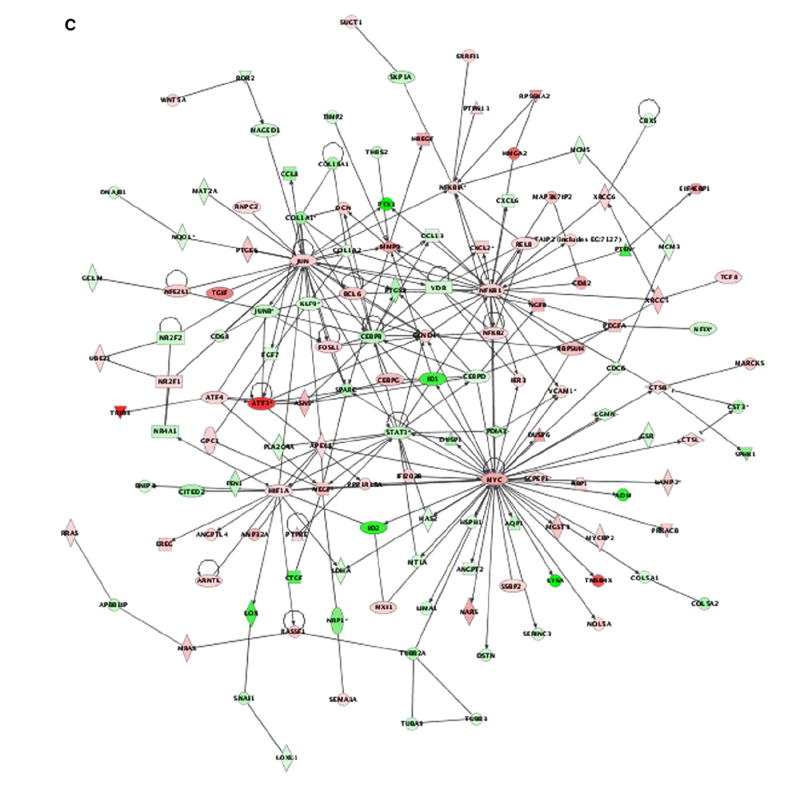
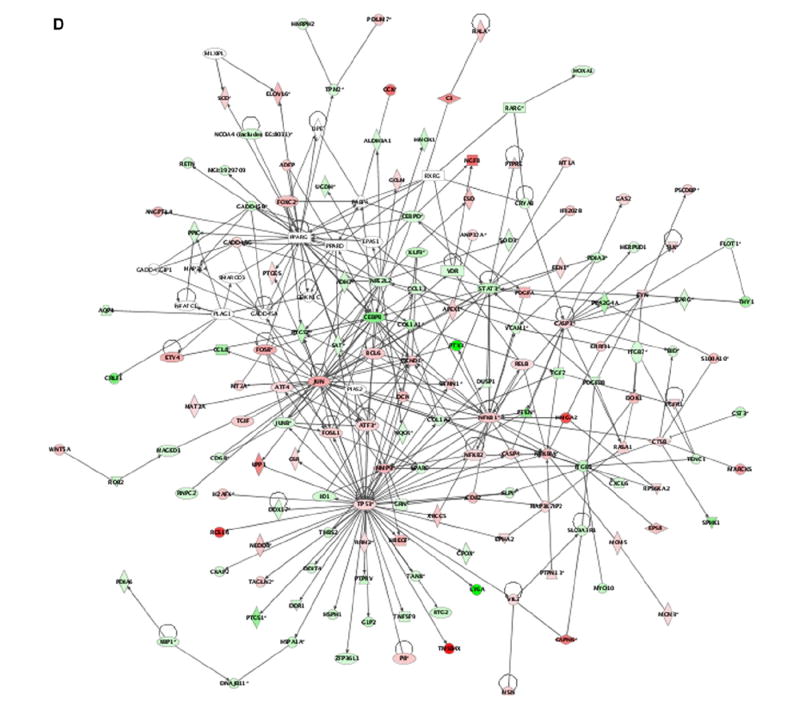
A) Total number of genes in the site-specific Ras transcriptomes. up- (red), down-regulated (blue). B) Quantification of the specific genes present in each of the Ras site-specific transcriptomes and of those specific for double combinations of transcriptomes. C) Functional networks in the disordered membrane Ras transcriptome identified using the Ingenuity database. Nodes are color-coded in red (up-regulated) or green (down-regulated) according to their fold change values. D) Functional network related to survival and apoptosis present in the Ras ER-signal transcriptome, identified using the Ingenuity database. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Table 1.
Genes specifically regulated from Disordered Membrane (CD8)
| ID probe set | R-fold | Gene symbol | Functional class Gene name | |
|---|---|---|---|---|
| Growth factor | ||||
| 103520_at | 2.64 | Vegfa | Vascular endothelial growth factor A | |
| 102624_at | 2.07 | Stc2 | Stanniocalcin 2 | |
| 92578_at | 1.76 | Scye1 | Small inducible cytokine figure2dsubfamily E, member 1 | |
| Signal transduction | ||||
| 101441_i_at | 4.28 | Itpr5 | Inositol 1,4,5-triphosphate receptor 5 | |
| 102379_at | 2.47 | Rassf1 | Ras association (RalGDS/AF-6) domain family 1 | |
| 93116_at | 2.37 | Prkacb | Protein kinase, cAMP dependent, catalytic, beta | |
| 100014_at | 2.34 | Tlk2 | Tousled-like kinase 2 | |
| 97740_at | 2.18 | Dusp16 | Dual specificity phosphatase 16 | |
| 94186_at | 2.18 | Traf1 | Tnf receptor-associated factor 1 | |
| 101937_s_a | 1.72 | Clk4 | CDC like kinase 4 | |
| 94394_at | 1.63 | Rras | Harvey rat sarcoma oncogene, subgroup R | |
| 160876_at | 1.47 | Bcap29 | B-cell receptor-associated protein 29 | |
| Regulation of transcription | ||||
| 104712_at | 3.64 | Myc | Myelocytomatosis oncogene | |
| 97893_at | 2.81 | Tbpl1 | TATA box binding protein-like 1 | |
| 101902_at | 2.8 | Rbpsuh | Recombining binding protein suppressor of hairless | |
| 101995_at | 2.55 | Sqstm1 | Sequestosome 1 | |
| 160535_at | 2.42 | Nfe2l1 | Nuclear factor, erythroid derived 2,-like 1 | |
| 93697_at | 2.07 | Cbx4 | Chromobox homolog 4 | |
| (Drosophila Pc class) | ||||
| 104215_at | 1.89 | Atf6 | Activating transcription factor 6 | |
| 95521_s_at | 1.64 | Zfp68 | Zinc finger protein 68 | |
| 103006_at | 1.51 | Atf5 | Activating transcription factor 5 | |
| 101958_f_at | 0.53 | Tfdp1 | Transcription factor Dp 1 | |
| 101959_r_at | 0.53 | Tfdp1 | Transcription factor Dp 2 | |
| 100307_at | 0.51 | Nfix | Nuclear factor I/X | |
| 95791_s_at | 0.43 | Sfrs2 | Splicing factor, arginine/serine-rich 2 | |
| 99607_at | 0.39 | Skp1a | S-phase kinase-associated protein 1A | |
| DNA replication | ||||
| 104096_at | 2.37 | Orc4l | origin recognition complex subunit 4 | |
| Cell cycle | ||||
| 101429_at | 9 | Ddit3 | DNA-damage inducible transcript 3 | |
| Cytoskeleton | ||||
| 103330_at | 2.22 | Spnr | Spermatid perinuclear RNA-binding protein | |
| 101578_f_at | 0.58 | Actb | Actin, beta, cytoplasmic | |
| 160462_f_at | 0.49 | Tubb3 | Tubulin, beta 3 | |
| 100342_i_at | 0.48 | Tuba1 | Tubulin, alpha 1 | |
| Protein biosynthesis | ||||
| 104144_at | 3.34 | Gtpbp2 | GTP binding protein 2 | |
| 98936_at | 2.74 | Sars1 | Seryl-aminoacyl-tRNA synthetase 1 | |
| 93564_at | 2.69 | Yars | Tyrosyl-tRNA synthetase | |
| 93270_at | 2.55 | Gars | Glycyl-tRNA synthetase | |
| 103630_at | 2.54 | Lars | Leucyl-tRNA synthetase | |
| 96693_at | 2.5 | Rars | Arginyl-tRNA synthetase | |
| 94494_at | 2.41 | Farsl | Phenylalanine-tRNA synthetase-like, beta subunit | |
| 93752_at | 2.38 | E430001P04Rik | Isoleucine-tRNA synthetase | |
| 95054_at | 2.35 | Tars | Threonyl-tRNA synthetase | |
| 98605_at | 2.16 | Wars | Tryptophanyl-tRNA synthetase | |
| 94484_at | 1.97 | Hbs1l | Hbs1-like | |
| Protein biosynthesis | ||||
| 95159_at | 1.91 | Mrps18b | Mitochondrial ribosomal protein S18B | |
| 95067_at | 1.52 | Mrpl2 | Mitochondrial ribosomal protein L2 | |
| Proteol and peptidolysis | ||||
| 160328_at | 4.18 | Prss15 | Protease, serine, 15 | |
| 96920_at | 2.1 | Prss11 | Protease, serine, 11 (Igf binding) | |
| 98906_at | 0.41 | Fbxo9 | f-box only protein 9 | |
| Metabolism | ||||
| 161221_f_at | 3.11 | Asns | Asparagine synthetase | |
| 95738_at | 2.82 | Pycs | Pyrroline-5-carboxylate synthetase | |
| 94369_at | 2.19 | Gnpnat1 | Glucosamine-phosphate N-acetyltransferase 1 | |
| 160647_at | 2.15 | Car6 | Carbonic anhydrase 6 | |
| 104343_f_at | 2.13 | Pla2g12 | Phospholipase A2, group XIIA | |
| 97560_at | 2.01 | Psap | Prosaposin | |
| 160770_at | 0.53 | Mvd | Mevalonate (diphospho) decarboxylase | |
| 94325_at | 0.26 | Hmgcs1 | 3-hydroxy-3-methylglutaryl-Coenzyme A synthase 1 | |
| Transport | ||||
| 104221_at | 3.4 | Slc7a5 | Solute carrier family 7 (cationic amino acid transporter, y+ system), member 5 | |
| 96160_at | 2.73 | Slc6a9 | Solute carrier family 6 (neurotransmitter transporter, glycine), member 9 | |
| 92648_at | 2.24 | Stxbp3 | Syntaxin binding protein 3 | |
| 92582_at | 1.91 | Slc1a7 | Solute carrier family 1 (neutral amino acid transporter), member 5 | |
| 104706_at | 1.55 | Pex7 | Peroxisome biogenesis factor 7 | |
| Electron transport | ||||
| 92587_at | 2.17 | Fdx1 | Ferredoxin 1 | |
| 93844_at | 1.74 | Qpc-pending | Ubiquinol-cytochrome c reductase binding protein | |
| Ion transport | ||||
| 102786_at | 2.11 | Clcn3 | Chloride channel 3 | |
| 104704_at | 0.45 | Clcn4-2 | Chloride channel 4-2 | |
| Apoptosis | ||||
| 160412_at | 2.41 | Pdcd2 | Programmed cell death 2 | |
| Other | ||||
| 97914_at | 2.88 | Hspa9a | Heat shock protein, A | |
| 94643_at | 2.25 | Taa1 | Poliovirus receptor | |
| 160530_at | 2.12 | Ghitm | Growth hormone inducible transmembrane protein | |
| 104033_at | 2.1 | Mgea6 | Meningioma expressed antigen 6 | |
| 98451_at | 1.94 | Dnajb10 | DnaJ (Hsp40) homolog, subfamily B, member 10 | |
| 160230_at | 1.94 | Cox17 | Cytochrome c oxidase, subunit XVII assembly protein homolog (yeast) | |
| 97269_f_at | 1.91 | Kcp2-pending | Keratinocytes associated protein 3 | |
| 160176_at | 1.86 | Hirip5 | Histone cell cycle regulation defective interacting protein 5 | |
| 93385_at | 1.84 | Nthl1 | nth (endonuclease III)-like 1 | |
| 97268_i_at | 1.78 | Kcp2-pending | Keratinocytes associated protein 2 | |
| 101947_at | 1.77 | Nakap95-pending | A kinase (PRKA) anchor protein 8-like | |
| 160092_at | 1.71 | Ifrd1 | Interferon-related developmental regulator 1 | |
| 95109_at | 1.67 | Nol5a | Nucleolar protein 5A | |
| Other | ||||
| 97204_s_at | 1.61 | Dnajd1 | DnaJ (Hsp40) homolog, subfamily D, member 1 | |
| 94346_at | 1.61 | Wtap | Wilms’ tumour 1-associating protein | |
| 94455_at | 0.59 | Lsm3 | LSM3 homolog, U6 small nuclear RNA associated | |
| 96254_at | 0.51 | Dnajb1 | DnaJ (Hsp40) homolog, subfamily B, member 1 | |
| 103598_at | 0.46 | Dhx9 | DEAH (Asp-Glu-Ala-His) box polypeptide 9 | |
| Unknown | ||||
| 104116_at | 11.8 | D5Ertd593e | Unknown | |
| 104363_at | 2.75 | AI747712 | Unknown | |
| 103739_at | 2.68 | 1110017N23Rik | Unknown | |
| 94340_at | 2.62 | 1110004L07Rik | Unknown | |
| 95636_at | 2.34 | 0610010K14Rik | Unknown | |
| 96539_at | 2.31 | 9330147J08Rik | Unknown | |
| 104513_at | 2.28 | 2410004N09Rik | Unknown | |
| 95634_at | 2.07 | 0610010K14Rik | Unknown | |
| 103773_at | 2.27 | 1110020K19Rik | Unknown | |
| 97919_at | 2.12 | 1110021E09Rik | Unknown | |
| 160279_at | 2.01 | AA408582 | Unknown | |
| 97118_at | 1.95 | AI159700 | Unknown | |
| 98089_at | 1.88 | 1190005P17Rik | Unknown | |
| 102056_f_at | 1.83 | 2610002J02Rik | Unknown | |
| 97308_at | 1.71 | 5730466P16Rik | Unknown | |
| 94366_at | 1.7 | 2310079N02Rik | Unknown | |
| 95592_at | 1.6 | 1110019N10Rik | Unknown | |
| 103312_f_at | 1.56 | C79684 | Unknown | |
| 104045_at | 1.53 | 2610102M01Rik | Unknown | |
| 94830_at | 0.5 | 8030460C05Rik | Unknown |
Interestingly, it was observed that ER-localized H-RasV12 (M1) induced the largest effect in the cell transcriptome (438 up-regulated, 486 down-regulated, Appendix A). The analysis of this transcriptome using the Ingenuity program revealed an interactive network nucleated around the proteins p53, NFκB, c-Jun, Caspase-3 and cEBP (Fig. 2D), a result that is in agreement with previous results indicating the implication of ER-specific H-RasV12 signaling in cell survival and apoptosis. The analysis of the transcriptome of Golgi-localized H-RasV12 (KDEL) indicated a deregulation of 669 genes (317 up-regulated, 352 down-regulated) (Fig. 2A). Of those, only 4 genes exhibited a Golgi-specific regulation (Table 2). One of those loci encoded the tyrosine phosphatase receptor κ (PTPκ), a putative tumor suppressor gene [17]. Three additional genes were shared by the ER- and Golgi-dependent H-RasV12 transcriptomes (Fig. 2B, Supplementary Table S2). Taken together, these results indicate that H-RasV12, when located in endomembranes, promotes a very small fraction of specific transcriptomal changes (9 genes).
Table 2.
Genes specifically regulated from Golgi complex (KDEL) and from Endoplasmic Reticulum (M1) Endoplasmic Reticulum (M1)
| ID probe set | R-fold | Gene symbol | Functional class Gene name |
|---|---|---|---|
| KDEL | |||
| Signal transduction | |||
| 160760_at | 1.8 | Ptprk | Protein tyrosine phosphatase, receptor type, K |
| Apoptosis | |||
| 95094_g_at | 1.75 | Ccar1 | Cell division cycle and apoptosis regulator 1 |
| Other | |||
| 102712_at | 3.47 | Saa3 | Serum amyloid A 3 |
| Unknown | |||
| 100073_at | 1.63 | 2510005D08Rik | Unknown |
| M1 | |||
| Metabolism | |||
| 95430_f_at | 0.67 | Spg21 | Spastic paraplegia 21 homolog |
| Other | |||
| 160196_at | 0.66 | Smap1 | Stromal membrane-associated protein 1 |
As comparative control, we included in our studies a cell line expressing untethered H-RasV12 (V12). The transcriptomal changes induced by this “ubiquitously localized” H-RasV12 protein included a total of 699 genes (Fig. 2A). 51 of those genes were specifically regulated by this H-Ras version and not shared by the site-specific H-Ras GTPases (Fig. 2B). The gene for Caveolin-1, exhibiting a pronounced down-regulation, was among them (Table 3). In addition, a number of loci deregulated by untethered H-RasV12 were shared with the transcriptomes of other site-specific H-Ras proteins. For example, 61 genes were shared between untethered and DM-localized H-RasV12 GTPases. Remarkably, 28 of those genes exhibited an antagonistic regulation by these two proteins. Untethered H-RasV12 also deregulated 16 genes that were also targeted by Golgi-localized H-RasV12, 13 of those genes displayed opposite regulation (Fig. 2B Supplementary Table S2) (see Supplementary Text, Section II). Overall, these results clearly indicate that the subcellular localization heavily conditions the genetic program orchestrated by H-RasV12 in mouse fibroblasts.
Table 3.
Genes specifically regulated by ubiquitous H-RasV12
| ID probe set | R-fold | Gene symbol | Functional class Gene name |
|---|---|---|---|
| Cell adhesion | |||
| 160319_at | 2.7 | Sparcl1 | SPARC-like 1 (mast9, hevin) |
| 101110_at | 2.47 | Col6a3 | Procollagen, type VI, alpha 3 |
| 95661_at | 2.19 | Cd9 | CD9 antigen |
| 162193_f_at | 2.13 | Itgb7 | Integrin beta 7 |
| 95706_at | 1.73 | Lgals3 | Lectin, galactose binding, soluble 3 |
| 98331_at | 0.55 | Col3a1 | Procollagen, type III, alpha 1 |
| 100153_at | 0.36 | Ncam1 | Neural cell adhesion molecule 1 |
| Regulation of transcription | |||
| 102867_at | 2.23 | Tead4 | TEA domain family member 4 |
| 104701_at | 1.83 | Bhlhb2 | Basic helix-loop-helix domain containing, class B2 |
| 93008_at | 0.55 | Lsm4 | LSM4 homolog, U6 small nuclear RNA associated |
| 95049_at | 0.55 | Snrpd2 | Small nuclear ribonucleoprotein D2 |
| 96711_at | 0.49 | Znrd1 | Zinc ribbon domain containing, 1 |
| Cytoskeleton | |||
| 160417_at | 1.73 | Kif5b | Kinesin family member 5B |
| Protein biosynthesis | |||
| 92628_at | 0.48 | Rpl36 | Ribosomal protein L36 |
| Proteol and peptidolysis | |||
| 96061_at | 1.97 | Usp14 | Ubiquitin specific protease 14 |
| Metabolism | |||
| 96790_f_at | 2.38 | Galm | Galactose mutarotase |
| 103392_at | 2.34 | Adcy7 | Adenylate cyclase 7 |
| 95758_at | 2.12 | Scd2 | Stearoyl-Coenzyme A desaturase 2 |
| 160737_at | 2.02 | Lss | Lanosterol synthase |
| 98931_at | 1.82 | Gns | Glucosamine (N-acetyl)-6-sulfatase |
| 102381_at | 1.74 | Acsl4 | Acyl-CoA synthetase long-chain family member 4 |
| 94276_at | 1.7 | Hsd17b12 | Hydroxysteroid (17-beta) dehydrogenase 12 protein |
| 99148_at | 0.63 | Fh1 | Fumarate hydratase 1 |
| 93984_at | 0.6 | Atpi | ATPase inhibitor |
| 97907_at | 0.56 | Lsm7 | LSM7 homolog, U6 small nuclear RNA associated |
| 101084_f_at | 0.55 | Dpm3 | Dolichyl-phosphate mannosyltransferase polypeptide 3 |
| 101990_at | 0.46 | Ldh2 | Lactate dehydrogenase 2, B chain |
| Transport | |||
| 93738_at | 2.6 | Slc2a1 | Solute carrier family 2 (facilitated glucose transporter), member 1 |
| 95625_at | 2.36 | Slc6a8 | Solute carrier family 6 (neurotransmitter transporter, creatine), member 8 |
| 94807_at | 1.92 | Slc25a1 | Solute carrier family 25, member 1 |
| 104565_at | 0.48 | Ap4s1 | Adaptor-related protein complex AP-4, sigma 1 |
| 160280_at | 0.44 | Cav | Caveolin, caveolae protein |
| Ion transport | |||
| 102861_at | 4.92 | Slc22a1l | Solute carrier family 22 (organic cation transporter), member 18 |
| 160529_r_at | 1.93 | Vdac2 | Voltage-dependent anion channel 2 |
| 103459_at | 1.83 | Slc39a6 | Solute carrier family 39 (metal ion transporter), member 6 |
| Proton transport | |||
| 160202_at | 2.74 | Atp6ip2 | ATPase, H+ transporting, lysosomal accessory protein 2 |
| 95745_g_at | 2.05 | Atp6v1a1 | ATPase, H+ transporting, V1 subunit A, isoform 1 |
| Other | |||
| 98045_s_at | 3.17 | Dab2 | Disabled homolog 2 |
| 92802_s_at | 2.07 | Plp | Proteolipid protein (myelin) |
| 95456_r_at | 0.6 | Shfdg1 | Split hand/foot deleted gene 1 |
| 160550_i_at | 0.59 | Magoh | Mago-nashi homolog, proliferation-associated |
| 96004_at | 0.58 | Sri | Sorcin |
| 98992_at | 0.55 | Eppb9 | Endothelial precursor protein B9 |
| 104078_g_at | 0.51 | Eso3 | Eso3 protein |
| Unknown | |||
| 100477_at | 2.7 | C630002M10Rik | Unknown |
| 95288_i_at | 1.87 | C330012F17Rik | Unknown |
| 160569_at | 0.51 | 2310008M10Rik | Unknown |
3.3. Identification of participants in Ras-induced transformation
Finally, we utilized the information obtained from the site-specific Ras genetic profiles, to acquire further insights into the process of cellular transformation. Our previous studies have revealed that the signals generated by RasV12 at lipid rafts, DM and ER induced transformation, whereas signals from the Golgi did not [14]. Thus, we reasoned that the elements in common among the transcriptomes resulting from transforming signals should be of special relevance for the upbringing of malignant growth. We found that only 31 genes were common to Ras transforming signals. 14 genes were down-regulated, including those encoding Connective Tissue Growth Factor (CTGF), PDGF receptor β and DLK1. 17 genes were up-regulated (For example, Epiregulin, R-PTPε, STK2/SLK, WIP and p120-GAP (Table 4). Many of these proteins have been previously linked to cellular transformation and/or malignant processes (see Supplementary Text, Section III).
Table 4.
Genes specifically regulated by Ras transforming signals
| ID probe set | R-fold | Gene symbol | Functional class Gene name | |||
|---|---|---|---|---|---|---|
| M1 | LCK | CD8 | V12 | |||
| Growth factor | ||||||
| 103556_at | 1.63 | 1.8 | 1.53 | 2.71 | Angptl2 | Angiopoietin-like 2 |
| 98802_at | 1.94 | 2.3 | 2.39 | 3.8 | Ereg | Epiregulin |
| 93294_at | 0.4 | 0.16 | 0.14 | 0.13 | Ctgf | Connective tissue growth factor |
| 96712_at | 0.68 | 0.49 | 0.58 | 0.53 | Smoc1 | SPARC related modular calcium binding 1 |
| Cell adhesion | ||||||
| 104614_at | 1.67 | 1.36 | 2.06 | 2.74 | Gpc1 | Glypican 1 |
| 101130_at | 0.7 | 0.41 | 0.72 | 0.37 | Col1a2 | Procollagen, type I, alpha 2 |
| 101560_at | 0.66 | 0.62 | 0.5 | 0.56 | Emb | Embigin |
| Signal transduction | ||||||
| 101932_at | 1.8 | 1.65 | 1.32 | 2.18 | Ptpre | Protein tyrosine phosphatase, receptor type, E |
| 99467_at | 1.57 | 2.22 | 1.81 | 2.54 | Rasa1 | RAS p21 GAP |
| 93874_s_at | 0.7 | 0.42 | 0.58 | 0.44 | Il11ra2 | Interleukin 11 receptor, alpha chain 2 |
| 160867_at | 0.74 | 0.74 | 0.7 | 0.49 | Pdgfrb | Platelet derived growth factor receptor, beta |
| 99964_at | 0.67 | 0.5 | 0.5 | 047 | Vdr | Vitamin D receptor |
| Regulation of transcription | ||||||
| 95297_at | 0.57 | 0.46 | 0.61 | 0.65 | Hoxa1 | Homeobox A1 protein |
| Cytoskeleton | ||||||
| 160106_at | 1.32 | 1.36 | 1.59 | 2.17 | Capg | Capping protein (actin filament), gelsolin-like |
| 100084_at | 1.3 | 1.32 | 1.29 | 2.47 | Vil2 | Villin 2 |
| 104602_at | 1.62 | 1.66 | 1.8 | 2.51 | Waspip | Wiskott-Aldrich syndrome protein interacting protein (WIP) |
| 94835_f_at | 0.65 | 0.51 | 0.29 | 0.57 | Tubb2 | Tubulin, beta 2 |
| Proteol and peptidolysis | ||||||
| 99643_f_at | 1.46 | 1.67 | 1.83 | 2.48 | Cpe | Carboxypeptidase E |
| 94831_at | 1.19 | 1.29 | 1.58 | 2.13 | Ctsb | Cathepsin B |
| Metabolism | ||||||
| 98984_f_at | 1.46 | 1.67 | 1.67 | 3.53 | Gpd2 | Glycerol phosphate dehydrogenase 2, mitochondrial |
| 104406_at | 1.29 | 1.7 | 2.53 | 1.29 | Ptges | Prostaglandin E synthase |
| 94057_g_at | 1.53 | 1.42 | 1.52 | 1.29 | Ptges | Stearoyl-Coenzyme A desaturase 1 |
| 92335_at | 0.63 | 0.52 | 0.58 | 0.38 | Ltbp2 | Latent transforming growth factor beta binding protein 2 |
| Electron transport | ||||||
| 103850_at | 0.68 | 0.58 | 0.58 | 0.48 | Loxl | Lysyl oxidase-like 1 |
| Other | ||||||
| 93833_s_at | 1.34 | 1.52 | 2.2 | 1.3 | Hist1h2bl | Histone 1, H2bl |
| 99489_at | 1.36 | 1.45 | 1.48 | 2.4 | Osp94 | Osmotic stress protein |
| 96130_at | 1.35 | 1.5 | 2.4 | 2.34 | Stk2 | STE20-like kinase |
| 101975_at | 0.86 | 063 | 057 | 014 | Dlk1 | Delta-like 1 homolog (DLK1) |
| Other | ||||||
| 101876_s_at | 0.72 | 0.47 | 0.53 | 0.44 | H2-T17 | Histocompatibility 2, T region locus 17 |
| 94224_s_at | 0.67 | 0.7 | 0.77 | 0.33 | Ifi205 | Interferon activated gene 205 |
| Unknown | ||||||
| 92398_at | 1.56 | 1.61 | 2.11 | 1.98 | BC026744 | Unknown |
The gene encoding for Vitamin D receptor (VDR) was one of the down-regulated genes shared by all Ras transforming signals, so we tested the relevance of its attenuated expression for transformation. By RT-PCR, we verified that VDR mRNA levels were markedly diminished in cells expressing transforming RasV12 constructs, even to greater levels than those revealed by the chips (Fig. 3A). Next, we tested whether increasing the levels of VDR could impact on Ras transforming potential. To this end, we assayed the ability of the site-specific RasV12 proteins to generate foci in NIH3T3 cells when cotransfected with ectopic VDR. It was found that VDR significantly diminished Ras-induced transformation. Interestingly, VDR inhibitory effect varied depending on the site from which the Ras transforming signal emanated. Whereas VDR abrogated transformation by lipid raft-targeted H-RasV12 almost completely, its negative effects on the transformation induced by RasV12 at the ER or at DM were modest, ranging between 25% and 30% (Fig. 3B). p120-GAP emerged as one of the up-regulated genes common to the Ras transforming signals. We confirmed by immunoblotting that this was indeed the case (Fig. 3C) and then tested the necessity for p120-GAP overexpression in Ras-induced transformation. Cotransfection of ectopic p120-GAP with the site-specific H-RasV12 constructs enhanced the number of transformed foci in all cases (Fig. 3D). This demonstrated that p120-GAP overexpression facilitated RasV12-induced transformation.
Fig. 3. Identification of participants in Ras-induced transformation.
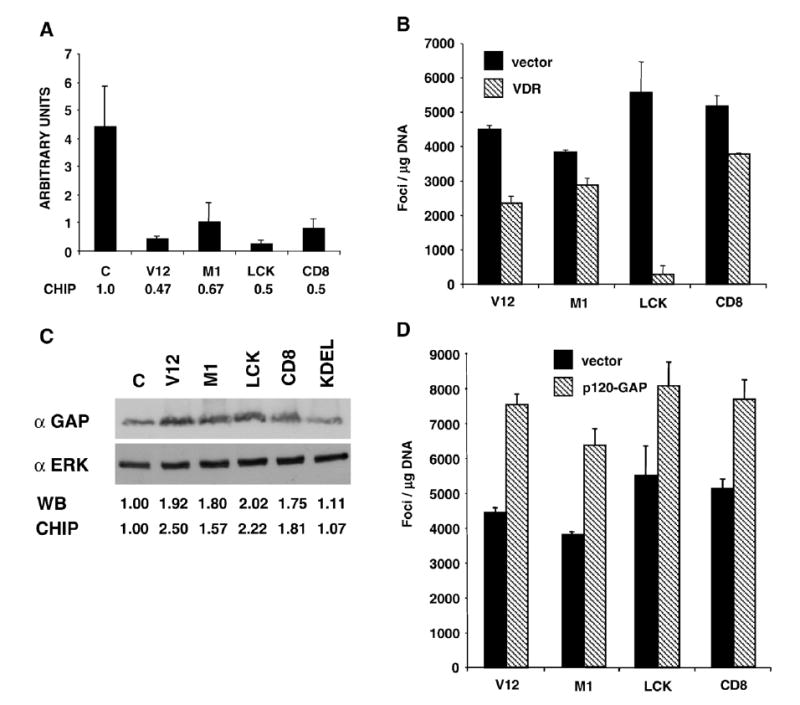
A) RT-PCR determination of the VDR mRNA expression levels in the parental control (c) and in cell lines expressing the indicated site-specific H-RasV12 constructs. Bars show average±SEM of three independent experiments. Figures show the expression levels relative to those in the parental cell line. B) VDR overexpression impairs Ras-induced transformation. Number of transformed foci in NIH3T3 cells transfected with the Ras constructs, plus either an empty vector or with a VDR-encoding plasmid. C) p120-GAP expression is up-regulated by Ras transforming signals. P120-GAP expression levels in parental (c) and the cell lines expressing the site-specific Ras constructs. Immunoblotting for ERK is shown as a loading control. Figures show the levels of expression relative to those in the parental cell line (c), in the immunoblots and those revealed by the gene arrays. D) p120-GAP overexpression potentiates Ras-induced transformation. Number of transformed foci in NIH3T3 cells transfected with the Ras constructs plus vector or a p120-GAP expressing construct. B and E) Bars show average±SEM of five independent experiments.
4. Discussion
We have used Affymetrix microarrays to acquire a genome-wide view of the gene expression profiles induced by site-specific Ras signals in murine NIH3T3 fibroblasts, the level of overlap among them and the singularities of the genetic outputs resulting from Ras activation at the different membrane compartments where it resides. We have applied highly stringent and restrictive parameters of significance for the processing and selection of the data and we have utilized algorithms that allowed us to minimize the background noise and to maximize the statistical significance of the data. Our results show that H-RasV12 signals regulate the expression of 1074 genes in total. A figure of similar magnitude to those documented for homologous models such as rat fibroblasts (1257 genes) [3] and MEFs (815 genes) [8]. Qualitative and quantitative analyses of the transcriptomal patterns resulting from site-specific Ras activations, unveil that, in general, there is a significant overlap among the genetic programs orchestrated from the different Ras compartments. Most genes could be regulated from several localizations. Furthermore, the regulation of 329 genes, nearly a third, including many genes essential for proliferation and survival, was common to all Ras compartments. Our analysis showing that the functional classes in which the genes were grouped, are represented to similar extents in all the transcriptomes, further highlighting the genotypic overlap among the different Ras signals.
On the other hand, our results also confirm the presence of unique transcriptomal signatures associated to each of the sublocalization-specific Ras signals. The signal generated at the DM is the one that controls the greatest number of genes specifically. Interestingly, 98% of these genes exhibit up-regulation. The cellular functions and biological outputs specifically orchestrated by Ras from the DM are unknown, but the fact that other subsignals are equally competent in the regulation of proliferation and transformation [14] suggests that the switch-on of this unique genetic program would be largely unrelated to these events. Interestingly, a significant proportion of the DM-specific genes are involved in angiogenesis. Contrarily to DM, the transcriptomal profile regulated by Ras at lipid rafts is the least numerous, since it deregulated a total number of 442 genes. Most interestingly, not a single gene is specifically regulated from this sublocalization. This observation is in full agreement and provides a plausible explanation for the fact that H-Ras/N-Ras double knock-out mice, completely devoid of Ras isoforms at lipid rafts, are viable and exhibit a normal phenotype [14,18].
Our results demonstrate that the total number of genes regulated by Ras from endomembranes and from PM sublocalizations is similar. Furthermore, all the functional classes were regulated alike by the endomembrane and the PM-localized Ras pools. Contrarily, there are dramatic differences regarding the number of genes specifically regulated from the inner and outer membrane systems: whereas 121 genes are exclusively regulated by Ras at the PM, only 9 genes are distinctive for Ras endomembrane signals. This result, however, should not lead to the assumption that Ras endomembrane signals are less important. Our previous results have demonstrated that Ras signals emanating from the ER are unique in their role of generating antiapoptotic responses [14]. The only two genes specifically regulated by ER signals, Spg1 and SMAP1, are associated with severe medical conditions, but they show no evident connection with cell survival. A deeper analysis of the ER transcriptome with the Ingenuity program has unveiled the regulation of a complex network of genes functionally related to apoptosis and survival. Thus, it is conceivable that not only qualitative changes, but also quantitative differences, even small ones, in the regulation of common genes, can account for abrupt variations in biological outputs.
From the Golgi, H-RasV12 is incapable of generating transforming signals [14]. Only four genes are specifically regulated from this compartment, including R-PTPκ . This locus is frequently deleted in tumors and has been attributed a putative tumor suppressor function [17]. R-PTPκ down-modulates the EGFr and the β-catenin pathways, essential for cellular proliferation and transformation [19,20]. Thus, one possibility is that the inability of Ras Golgi signals for inducing transformation is a consequence of the up-regulation of R-PTPκ . Another possibility is that Ras at the Golgi cannot regulate genes essential for transformation. In this respect, we have identified 31 genes exclusive for the transforming signals emanating from different Ras pools. Many of these genes have been previously linked to cellular transformation and/or malignant processes (see Supplementary Text, Section III).
We have analyzed in further depth the role in transformation of two of these genes. The VDR gene is down-regulated by Ras transforming signals, in agreement with previous studies reporting the instability of VDR mRNA in Ras-transformed cells [21]. We demonstrate that overexpression of VDR can abrogate Ras-induced transformation. Similar effects have been shown for other nuclear receptors, such as the thyroid hormone receptor [22]. Interestingly, the effect of VDR is dependent on localization: whereas VDR blocks the transformation induced by lipid rafts signals almost completely, its effects on the transformation orchestrated from DM are modest. The mechanisms underlying in these sublocalization-related effects are completely unknown. Surprisingly, the gene encoding for p120-GAP is up-regulated by Ras transforming signals. We demonstrate that p120-GAP over-expression enhances RasV12-induced transformation. It is known that the loss of wild-type Ras alleles promotes transformation by oncogenic Ras [23-25]. Thus, it can be envisioned that the upregulation p120-GAP could achieve similar suppressive effects over wild-type Ras putative antioncogenic effects. Alternatively, it is possible that RasGAP could play some GAP-independent effector functions in the Ras pathway.
We included in our studies “untargeted” H-RasV12 as a reference. Theoretically, the transcriptome resulting from “global” Ras activation should be similar to the sum of the site-specific gene profiles. However, it must be noticed that: a) The targeted Ras proteins are fixed to their compartments, whereas untargeted Ras is free to translocate between localizations and the cytoplasm [26]. This could cause differences in signal intensities and therefore impact on the resulting transcriptomes. b) Some genes are regulated antagonistically from different localizations. Thus, they could be under-represented or even absent in the “global” Ras transcriptome. c) Our targeted Ras constructs do not cover all the possible Ras localizations. Ras is also present in mitochondria [27,28], endosomes [13] and it cannot be discarded that also at PM and endomembrane microdomains that escape the tethers used herein. Our analyses identify 51 genes uniquely regulated by untargeted Ras. Maybe these genes are regulated from sublocalizations undisclosed by our tethered Ras constructs. Alternatively, their regulation could require the input from several sublocalizations. Thus, they would be silent under the single signals generated by our targeted proteins.
Overall, our analyses demonstrate that although there is significant overlapping among the transcriptomes resulting from Ras site-specific signals, there also exists a defined genetic signature on the transcriptional events regulated by Ras from each of its localizations. This adds further support to the notion of the subcellular microenvironment as a key regulator of Ras functions at all levels. As such, physiological and pathological conditions that affect Ras subcellular distribution, for example by altering the composition of cellular membranes, or by shifting Ras palmitoylation/depalmitoylation balance, can have a profound impact on the genetic program switched-on by Ras and thereby on the resultant biological outcomes. Thus far, the biological functions regulated by each of the Ras subsignals are largely unknown and under current investigation.
5. Conclusions
Using micro-array technology, we have identified the transcriptional program of the conventional H-RasV12 oncoprotein as well as of versions of H-RasV12 that have been placed in specific subcellular compartments (lipid rafts, disordered plasma membrane, endoplasmic reticulum and Golgi apparatus) via the attachment of subcellular localization targeting signals.
We have found that, although sharing a significant overlap in their respective transcriptomes, the different subcellular localizations of H-RasV12 are linked to the engagement of specific transcriptional programs.
Disordered membrane-localized H-Ras elicits the largest pool of specifically regulated genes when compared to the rest of H-RasV12 mutants used. Instead, lipid raff-localized H-RasV12 does not trigger any specific gene.
Plasma-membrane and endomembrane-localized H-RasV12 proteins trigger specifically a total number of 121 and 9 genes, respectively.
By comparing the transcriptomes of transforming and non-transforming H-RasV12 mutants, we have identified 31 genes that are distinctively associated to the cell transformation program. The involvement of two of those transformation-associated genes in H-RasV12 transformation was demonstrated using cell biology techniques.
Acknowledgments
We are indebted to Dr A. Aranda for providing reagents. PC’s work is supported by grants from the Spanish Ministry of Education and Science (MES) (BFU2005-00777 and GEN2003-20239-C06-03), the EU Sixth Framework Program under the SIMAP project, and the Red Temática de Investigación Cooperativa en Cáncer (RTICC) (RD06/0020/0105). Fondo de Investigaciones Sanitarias (FIS), Carlos III Institute, Spanish Ministry of Health. XRB’s work is supported by grants from the US National Cancer Institute/NIH (5R01-CA73735-10), the MES (SAF2006-01789 and GEN2003-20239-C06-01), the Castilla-León Autonomous Government (SA053A05), and the RTICC (RD06/0020/0001). F.N. was partially supported by a fellowship by the Ernst Schering Foundation. LA, FC, and IMB are Spanish Ministry of Education predoctoral fellows. All Spanish funding is co-sponsored by the European Union.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at 10.1016/j.cellsig.2007.06.025.
References
- 1.Crespo P, Leon J. Cell Mol Life Sci. 2000;57:1613. doi: 10.1007/PL00000645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bos JL. Cancer Res. 1989;49:4682. [PubMed] [Google Scholar]
- 3.Zuber J, Tchernitsa OI, Hinzmann B, Schmitz AC, Grips M, Hellriegel M, Sers C, Rosenthal A, Schafer R. Nat Genet. 2000;24(2):144. doi: 10.1038/72799. [DOI] [PubMed] [Google Scholar]
- 4.Brem R, Certa U, Neeb M, Nair AP, Moroni C. Oncogene. 2001;20(22):2854. doi: 10.1038/sj.onc.1204403. [DOI] [PubMed] [Google Scholar]
- 5.Sers C, Tchernitsa OI, Zuber J, Diatchenko L, Zhumabayeva B, Desai S, Htun S, Hyder K, Wiechen K, Agoulnik A, Scharff KM, Siebert PD, Schafer R. Adv Enzyme Regul. 2002;42:63. doi: 10.1016/s0065-2571(01)00024-3. [DOI] [PubMed] [Google Scholar]
- 6.Croonquist PA, Linden MA, Zhao F, Van Ness BG. Blood. 2003;102(7):2581. doi: 10.1182/blood-2003-04-1227. [DOI] [PubMed] [Google Scholar]
- 7.Ohnami S, Aoki K, Yoshida K, Ohnami S, Hatanaka K, Suzuki K, Sasaki H, Yoshida T. Biochem Biophys Res Commun. 2003;309(4):798. doi: 10.1016/j.bbrc.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 8.Vasseur S, Malicet C, Calvo EL, Labrie C, Berthezene P, Dagorn JC, Iovanna JL. Mol Cancer. 2003;2:19. doi: 10.1186/1476-4598-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweet-Cordero A, Tseng GC, You H, Douglass M, Huey B, Albertson D, Jacks T. Genes Chromosomes Cancer. 2006;45(4):338. doi: 10.1002/gcc.20296. [DOI] [PubMed] [Google Scholar]
- 10.Castellano E, De Las Rivas J, Guerrero C, Santos E. Oncogene. 2007;26(6):917. doi: 10.1038/sj.onc.1209845. [DOI] [PubMed] [Google Scholar]
- 11.Rocks O, Peyker A, Bastiaens PI. Curr Opin Cell Biol. 2006;18(4):351. doi: 10.1016/j.ceb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Chiu VK, Bivona T, Hach A, Sajous JB, Silletti J, Wiener H, Johnson RL, Cox AD, Philips MR. Nat Cell Biol. 2002;4:343. doi: 10.1038/ncb783. [DOI] [PubMed] [Google Scholar]
- 13.Roy S, Wyse B, Hancock JF. Mol Cell Biol. 2002;22:5128. doi: 10.1128/MCB.22.14.5128-5140.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matallanas D, Sanz-Moreno V, Arozarena I, Calvo F, Agudo-Ibanez L, Santos E, Berciano MT, Crespo P. Mol Cell Biol. 2006;26(1):100. doi: 10.1128/MCB.26.1.100-116.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berenjeno IM, Nunez F, Bustelo XR. Oncogene. 2007;26:4295. doi: 10.1038/sj.onc.1210194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arozarena I, Matallanas D, Berciano MT, Sanz-Moreno V, Calvo F, Munoz MT, Egea G, Lafarga M, Crespo P. Mol Cell Biol. 2004;24(4):1516. doi: 10.1128/MCB.24.4.1516-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakamura M, Kishi M, Sakaki T, Hashimoto H, Nakase H, Shimada K, Ishida E, Konishi N. Cancer Res. 2003;63(4):737. [PubMed] [Google Scholar]
- 18.Esteban LM, Vicario-Arbejon C, Fernandez-Salguero P, Fernandez-Melarde A, Swaminathan N, Yienger K, Lopez E, McKay R, Ward JM, Pellicer A, Santos E. Mol Cell Biol. 2001;21:1444. doi: 10.1128/MCB.21.5.1444-1452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs M, Muller T, Lerch MM, Ullrich A. J Biol Chem. 1996;271(28):16712. doi: 10.1074/jbc.271.28.16712. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Tan LJ, Grachtchouk V, Voorhees JJ, Fisher GJ. J Biol Chem. 2005;280(52):42694. doi: 10.1074/jbc.M507722200. [DOI] [PubMed] [Google Scholar]
- 21.Rozenchan PB, Folgueira MA, Katayama ML, Snitcovsky IM, Brentani MM. J Steroid Biochem Mol Biol. 2004;92(1–2):89. doi: 10.1016/j.jsbmb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Silva S, Aranda A. Mol Cell Biol. 2004;24(17):7514. doi: 10.1128/MCB.24.17.7514-7523.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bremner R, Balmain A. Cell. 1990;61:407. doi: 10.1016/0092-8674(90)90523-h. [DOI] [PubMed] [Google Scholar]
- 24.Finney RE, Bishop JM. Science. 1993;260:1524. doi: 10.1126/science.8502998. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Z, Wang Y, Vikis HG, Johnson L, Liu G, Li J, Anderson MW, Sills RC, Hong HL, Devereux TR, Jacks T, Guam K, You M. Nat Genet. 2001;29:25. doi: 10.1038/ng721. [DOI] [PubMed] [Google Scholar]
- 26.Rocks O, Peyker A, Kahms M, Verveer PJ, Koerner C, Lumbierres M, Kuhlmann J, Waldmann H, Wittinghofer A, Bastiaens PI. Science. 2005;307(5716):1746. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 27.Rebollo A, Perez-Sala D, Martinez-Arias C. Oncogene. 1999;18:4930. doi: 10.1038/sj.onc.1202875. [DOI] [PubMed] [Google Scholar]
- 28.Matallanas D, Arozarena I, Berciano MT, Aaronson DS, Pellicer A, Lafarga M, Crespo P. J Biol Chem. 2003;278:4572. doi: 10.1074/jbc.M209807200. [DOI] [PubMed] [Google Scholar]


