Abstract
Cervical stimulation induces two daily rhythmic prolactin surges, nocturnal and diurnal, which persist for several days. We have shown that a bolus injection of oxytocin initiates a similar prolactin rhythm, which persists despite low levels of oxytocin following injection. This suggests that oxytocin may trigger the cervical stimulated-induced rhythmic prolactin surges. To investigate this hypothesis, we infused an oxytocin antagonist that does not cross the blood brain barrier for 24h before and after cervical stimulation and measured serum prolactin. We also measured dopaminergic neuronal activity, since mathematical modeling predicted that this activity would be low in the presence of the oxytocin antagonist. We thus tested this hypothesis by measuring dopaminergic neuronal activity in the tuberoinfundibular, periventricular hypophyseal, and tuberohypophyseal dopaminergic neurons. Infusion of oxytocin antagonist before cervical stimulation abolished prolactin surges and infusion of oxytocin antagonist after cervical stimulation abolished the diurnal and significantly decreased the nocturnal surges of prolactin. The rhythmic prolactin surges returned after the clearance of the oxytocin antagonist. Hypothalamic dopaminergic activity was elevated in anti-phase with prolactin surges and the anti-phase elevation was abolished by the oxytocin antagonist in the tuberoinfundibular and tuberohypophyseal dopaminergic neurons, consistent with the mathematical model. These findings suggest that oxytocin is a physiologically relevant prolactin-releasing factor. However, the cervical stimulated-induced prolactin surges are maintained even in the absence of oxytocin actions at the lactotroph which strongly suggests the maintenance of prolactin surges are not dependent upon oxytocin actions at the pituitary gland.
INTRODUCTION
In response to mating, prolactin (PRL) is secreted from lactotrophs in the anterior pituitary gland in two daily surges during the first half of pregnancy; a nocturnal surge peaking at 0300 h and a diurnal surge at 1700 h (1,2). These PRL surges are partially responsible for inhibition of cyclic ovarian activity and the promotion of luteal function and development (3). The two daily rhythmic PRL surges are reproducible in the absence of ovaries (2,4). In ovariectomized (OVX) rats, the rhythmic PRL surges persist for 10-12 days following brief electrical stimulation of the uterine cervix. Due to the persistence of the PRL surges, it has been suggested that there is a “memory” present that allows the surges to occur for several days without additional stimuli and this “memory” has been suggested to reside in the hypothalamus (4,5).
Dopamine (DA) acts on lactotrophs to inhibit PRL secretion. Release of this inhibitory tone is required for PRL secretion, and PRL, in turn, up regulates the activity of dopaminergic neurons, by enhancing tyrosine hydroxylase activity (6,7). DA is released from three subpopulations of hypothalamic dopaminergic neurons, designated as tuberoinfundibular (TIDA) and tuberohypophyseal dopaminergic (THDA) neurons located throughout the arcuate nucleus, and the periventricular hypophyseal dopaminergic (PHDA) neurons located in the periventricular nucleus. The TIDA axons terminate on a fenestrated capillary bed in the external zone of the median eminence, THDA axons terminate on short portal vessels in the neural lobe and intermediate lobe, and PHDA axons terminate solely on short portal vessels in the intermediate lobe. DA supply reaches lactotrophs in the anterior lobe of the pituitary gland from each of these regions via these long or short portal vessels (8).
Oxytocin (OT), a neurohormone classically known for its role in parturition and milk let down, and PRL are both released in response to the suckling response and mating (9,10). There is evidence that OT plays a physiological role by acting at the lactotroph. There are OT receptors on lactotrophs in the anterior pituitary gland (11-13) and OT reaches the lactotroph via long and short portal vessels (14). Immunoneutralization of OT attenuates the surge of PRL on proestrous day (15),and inhibition of OT abolishes this surge (16) as well as suckling-induced PRL increase (17). It is known that cervical stimulation produces an immediate surge of OT in rats (18), sheep (19), pigs (20), and humans (21) and is followed by rhythmic PRL secretion in rats (22). We have found that OT stimulates the secretory activity of the lactotrophs (23) and that a single injection of OT initiates rhythmic PRL surges in OVX rats similar to those seen in OVX-cervically stimulated rats (24). These results together give a foundation for OT's physiological control of PRL secretion.
The known interactions between DA and PRL, and the suggested role of OT, were previously illustrated by our laboratory with a mathematical model (25). According to this model, cervical stimulation induces a surge of OT and results in a long-lasting inhibition of DA neuronal activity. The reduction in DA tone, along with the direct stimulatory influence of OT on lactotrophs, facilitates rhythmic PRL secretion. The continued interaction between DA neurons and lactotrophs leads to a rhythmic secretory PRL pattern for several days. Thus, we suggest that this mechanism is the basis of the “memory” for the PRL rhythm that resides in the hypothalamus, while the secretion of PRL surges is due to actions of DA and OT on the lactotroph.
The aim of this study is to test two hypotheses. The first is that the direct stimulatory action of OT on lactotrophs is necessary for rhythmic PRL secretion in OVX-cervically stimulated rats. The second is that the cervically stimulated-induced “memory” of the PRL rhythm resides in the hypothalamus, and is therefore not affected by actions of OT on lactotrophs. We use an OT antagonist that does not cross the blood-brain barrier to remove the direct stimulatory effects of OT on lactotrophs. According to our hypotheses, the cervically stimulated-induced “memory” should still be triggered, even though PRL surges do not occur due to the inhibitory effects of the OT antagonist on the lactotrophs. However, when the OT antagonist has cleared from the circulation, we expect rhythmic PRL secretion to occur, since the “memory” was activated by cervical stimulation and should (according to our second hypothesis) be unaffected by the OT antagonist that acts in the pituitary. If hypothalamic DAergic neurons are involved in PRL surges, we predict that DAergic neuronal activity will be high when PRL secretion is low. That is, DAergic activity will be elevated in anti-phase with the PRL surges. Because we predict PRL surges to be abolished in the presence of the OT antagonist, we also expect DA neuronal activity to be low due to the lack of stimulation from PRL. That is, both PRL secretion levels and DA neuronal activity will be low in the presence of OT antagonist. We tested the model predictions using measurements of serum PRL levels and the activity of DA neurons that project to the median eminence, intermediate lobe and neural lobe.
MATERIALS AND METHODS
Mathematical Model
We use a mathematical model to help with the experimental design. This was described in detail in a previous publication (25), but will be briefly outlined here. The results of experiments described herein have helped to clarify the role of OT. In addition, we add an inhibitory link from lactotrophs to hypothalamic OT neurons. Thus, some aspects of the earlier model have been modified.
The essential components of the model are illustrated in Fig. 1. This is a mean field model, where the activity level of each cell population is described by a single variable. Four cell populations are included: hypothalamic DAergic neurons (DA), OTergic neurons of the paraventricular nucleus (OT), vasoactive intestinal polypeptide neurons of the suprachiasmatic nucleus (VIP), and pituitary lactotrophs (PRL). Differential equations for the dynamics of each of these variables are briefly developed below.
Figure 1.
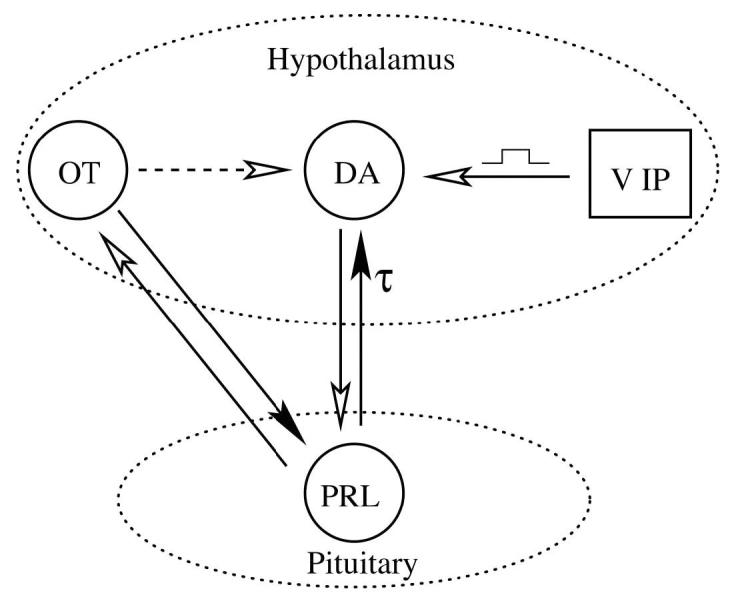
Connections within the network model. A filled arrow represents a stimulatory influence, while an open arrow represents an inhibitory influence. The connection from lactotrophs to DAergic neurons is delayed by τ = 3 h. The dashed arrow represents an indirect inhibitory connection. VIP input to DAergic neurons is applied periodically in the morning of each day for 3 h. A version of this model was described in an earlier publication (25).
The DAergic neurons are stimulated by PRL, but with a time delay (26). The stimulatory term is , where Td represents constant stimulatory drive, PRLτ represents PRL secretory activity delayed by τ = 3 h, and kp is the strength of the PRL feedback. The inhibitory terms include a term for first order decay of DA neuron activity, −q DA , and inhibition due to VIP synaptic input from the suprachiasmatic nucleus, −rνVIP · DA . Here q is a decay or clearance rate and rν is the strength of the VIP inhibition. , The differential equation for DA neuron activity (DA) is:
| (1) |
The differential equation for PRL secretory activity (PRL) is:
| (2) |
The lactotrophs are subject to inhibition by DA and stimulation by OT. The first term in (2) includes both of these effects, with OT in the numerator and DA in the denominator of the expression. The strength of these feedback effects is determined by the parameters ν o and kd. Also in the numerator is a constant stimulatory drive factor T p . Finally, there is a first-order decay of lactotroph activity, −q PRL . An OT antagonist is simulated by setting ν o = 0 .
We postulate that cervical stimulation (CS) activates OT neurons of the paraventricular nucleus (PVN). We further postulate that these or another population of OT neurons (such as those in the supraoptic nucleus) trigger the PRL rhythm by indirectly inhibiting hypothalamic DA neurons. The motivation for this mechanism is the focus of an earlier publication [see (25)]. The DA neuron inhibition is achieved in the model by setting Td = 0 .
The two sources of OT are designated OTcs (the first pulse of OT induced by CS) and OTPVN (the subsequent pulses of OT on the following days) The total OT level is OT = OTcs + OTPVN . The first component of OT released as a direct result of cervical stimulation and is transient in nature:
| (3) |
where CS = 1 for 2 h immediately following cervical stimulation, otherwise CS = 0 . The other OT source is due to the activity of OTergic neurons of the paraventricular nucleus. There is evidence that PRL has a rapid inhibitory influence on the electrical activity of these neurons (27). We therefore include this inhibitory action in the differential equation for OTPVN. The OTPVN differential equation is:
| (4) |
where To = 0 prior to cervical stimulation. The rapid inhibitory action of PRL is achieved by placing PRL in the denominator of the first term. Note that this is a rapid PRL effect, not a delayed PRL effect as was used in Eq. 1.
Under normal lighting conditions the activity of VIPergic neurons of the suprachiasmatic nucleus is high during the morning and low the rest of the day (28-30). We therefore model their activity as a square pulse that is elevated for three hours during the morning (VIP = 2 ) and is 0 for the rest of the day. This VIP is input to the DA equation (1).
Parameter values for this mean field model were set to produce the PRL rhythm and are given in Table 1. The equations were solved numerically using the 4th-order Runge-Kutta method implemented in the XPPAUT software package (31). The variables are in arbitrary units (except time, in hours), so curves are presented in normalized form.
Table 1.
Parameter values used in the model.
| Tp=1 | kd=1 | q=0.5 | Td=10 |
| kp=0.3 | τ = 3 h | rv=2 | νo = 1 |
| Pcs=1000 | νcs = 0.0002 | rn=0.2 | To = 3 |
| ko = 1 |
Animals
Adult female Sprague-Dawley rats (200–250 g; Charles River, Raleigh, NC) were kept in standard rat cages under a 12:12-h light-dark cycle (lights on at 0600), with water and rat chow available ad libitum. All animals were bilaterally ovariectomized under Halothane anesthesia. Animal procedures were approved by the Florida State University Animal Care and Use Committee.
Cervical Stimulation
The uterine cervix was stimulated with an electrode constructed from a Teflon rod (diameter, 5 mm), with two platinum wires protruding from the tip. Each rat was stimulated twice, the first time at 1700 h and the second time on the following morning at 0900 h; times which mimic normal mating on proestrus evening and the morning of estrus. Stimulations were applied as two consecutive trains of electric current of 10-s duration (rectangular pulses, 1 ms of 25 V at 200 Hz). This procedure has been shown to yield the highest success rate in initiating two daily PRL surges that are characteristic of mated rats (32).
Jugular vein catheter implantation and OT antagonist infusion
Polyurethane catheter tubing (Micro-Renathane; Braintree Scientific, Braintree, MA) was inserted into the jugular vein as the rats were anesthetized with Halothane. Blood was collected in 200 μL volumes every 2 hours over a period of 24 h. Blood loss during sampling was compensated by sterile saline replacement. Serum samples were stored at −40 C until analysis for PRL concentration. The tubing extending from the jugular vein, was filled with heparinized saline (50 U/mL), fitted subcutaneously and exteriorized at the back of the animal's neck. To alleviate stress, extensions were connected at least 4 hours prior to the first blood collection.
The selective OT antagonist desGly-NH2-d(CH2)5[D-Tyr2,Thr4]OVT was generously provided by Dr. Maurice Manning, Toledo, Ohio (33). The OT antagonist was dissolved in sterile saline and infused at 0.5μg/Kg·min via osmotic pumps (Alzet® Osmotic pumps, model AP-2001D, rate=8 μL /h, duration=24 h; Braintree Scientific, Braintree, MA). The osmotic pumps were filled with the OT antagonist or sterile saline and infused intravenously using the jugular vein catheter implantation described above. The osmotic mini pump was implanted subcutaneously and connected to the end of the tubing All infusions persisted for 24 h.
Preparation of the median eminence, intermediate lobe, and neural lobe
Rats were sacrificed at 0900 h, 1200 h, and 1700 h. The median eminence, intermediate lobe, and neural lobe was separated and stored in vials with 200μL of homogenization buffer (0.15 N perchloric acid, 50μM EGTA, 13.6 nM dihydroxybenzylamine) at −80 C until the day of assay.
Radioimmunoassay
Serum concentrations of PRL were estimated in duplicate by the rat PRL RIA kit as previously described (22). Rat PRL RP-3 standard was supplied by Dr. Albert Parlow through the National Hormone and Pituitary Program (Torrance, CA). To prevent interassay variation, all samples were assayed in the same RIA. The lower limit of detection for PRL was 0.10 ng/mL. The intra-assay coefficient of variation was 5%.
DA and dihydroxyphenylacetic acid (DOPAC) measurement by high performance liquid chromatography coupled to electrochemical detection (HPLC-EC)
DOPAC:DA ratio was measured as an index of DA neuronal activity. DA is synthesized and metabolized to DOPAC by monoamine oxidase on the outer membrane of the mitochondria before or after release and reuptake of DA. The presence of DOPAC in axon terminals of the median eminence, neural lobe, and intermediate lobe is indicative of the activity of DA neurons of the TIDA, THDA, and PHDA, respectively (34,35). HPLC-EC is a well established procedure in our laboratory (36,37). Median eminence, neural lobe and intermediate lobe samples were thawed, homogenized, and sonicated in 1.5 N perchloric acid and 50 μM EGTA. The samples were centrifuged (20 min at 8000×g). The supernatant was filtered through a 0.2mm nylon microfiltration unit (Osmonics, Livermore, CA.), and then placed into autosampler vials. The concentration of DA and DOPAC was measured using HPLC-EC. Twenty microliters of each sample was injected by an autosampler (Model 542 Autosampler, ESA, Inc., Chelmesford, MA). Mobile phase consisted of 75 mM sodium dihydrogen phosphate monohydrate (EM Science, Gibbstown, NJ), 1.7 mM 1-octane sulfonic acid (Fisher Scientific), 100 mL/L triethylamine (Aldrich, Milwaukee, WI), 25 μM EDTA (Fisher Scientific), 6% Acetonitrile (EM Science), titrated to pH 3.0 with phosphoric acid (Fisher Scientific), was delivered by a dual piston pump (LC-20AD Shimadzu Co. Analytical & Measuring Instruments Division, Kyoto, Japan) at 600μL/min. Water was purified on a Milli-Q system (Millipore, Bedford, MA) to 18 MΩ resistance and further polished with a Sep-Pak mini-column (Millipore). Catecholamines were separated on a reverse phase C18 column (MD-150, Dimensions 150×3 mm, particle size 3μm, ESA, Chelmsford, MA), oxidized on a conditioning cell (E: +300 mV, ESA 5010 Conditioning Cell, ESA) and then reduced on a dual channel analytical cell (E1: −65 mV, E2 : −225 mV, ESA 5011 High Sensitivity Analytical Cell, ESA). The change in current on the second analytical electrode was measured by a coulometric detector (ESA Coulochem II, ESA) and recorded using EZStart 7.3 SP1 (Shimadzu, Kyoto, Japan). DA and DOPAC were identified on the basis of their peak retention times. The amount of catecholamine or internal standard, dihydroxybenzylamine, in all sample peaks was estimated by comparison to the area under each peak for known amounts of each. Recovery of dihydroxybenzylamine as the internal standard corrected for any loss of sample. The sensitivity of the assay was 6 pg of DA.
Protein Assay
In order to insure the accuracy of each dissection, the amount of protein in each sample was measured using a micro-modified form of the Pierce Bichinchoninic Acid (BCA) Protein Assay Kit (Pierce, Rockford, Ill., USA). Tissue homogenate (10 μL) was aliquoted in duplicate into 96-well plates (Corning, Corning, N.Y., USA) with 200 μL of BCA solution and incubated at room temperature for 20 min. The absorbance of each well was measured at 562 nm by a micro-plate spectrophotometer (Molecular Devices, Palo Alto, Calif., USA). Unknowns were compared against standards of bovine serum albumin. Assay sensitivity was 100μg/mL. There was no significant difference in amount of protein within each dissected group of the median eminence, intermediate lobe, and neural lobe (data not shown).
Data analysis
All values are expressed as means ± SE. Two-way analysis of variance (treatment × time) was used for the comparison of differences between treatment groups, followed by post hoc Bonferroni comparison. One-way analysis of variance was used for comparison of differences within treatment groups, followed by post hoc Bonferroni comparison. Statistical analyses were performed and graphs were created using GraphPad Prism 3.0 (GraphPad Software, San Diego, CA). Differences were considered significant at the level of P ≤ 0.05.
EXPERIMENTAL DESIGN
Experiment 1. Effects of OT antagonist on initiation of cervical stimulation-induced rhythmic PRL secretion
Ten days after OVX, rats were infused with OT antagonist or saline through the jugular vein via osmotic pumps beginning at 1300 h, 4 h prior to the first cervical stimulation (day 0), and continuing for 24 h (1300 h, Day 1). Blood sampling was begun 6 hours later (1900 h, Day 1) and continued every two hours for 24 h through 1900 h on Day 2.
Experiment 2. Effects of OT antagonist on maintenance of cervical stimulation-induced rhythmic PRL secretion
Ten days after OVX, rats were cervically stimulated and infused with OT antagonist for 24 h through the jugular vein via osmotic pumps beginning 4 h (1300 h) after the second cervical stimulation on Day 1 and continuing through 1300 h on Day 2. Blood sampling was begun at 1900 h on Day 1 and continued every two hours for 24 h through 1900 h on Day 2.
Experiment 3. Return of rhythmic PRL secretion after clearance of OT antagonist
Ten days after OVX, rats were infused with OT antagonist or saline for 24 hours through the jugular vein via osmotic pumps beginning at 1300 h on Day 0 (4 hours before cervical stimulation) and continuing through 1300 h on Day 1. Blood was collected beginning 36 hours later (1900 h, Day 2) and continued every two hours for 24 h through 1900 h on Day 3.
Experiment 4. Activity of dopaminergic neurons during OT antagonist treatment
Ten days following OVX, rats were treated as described below and sacrificed at 0900 h, 1200 h, or 1700 h and the median eminence, intermediate lobe, and neural lobe were rapidly dissected. The animals were divided into three groups: 1. OVX: animals were ovariectomized and 10 days later sacrificed. 2. OVX-cervically stimulated: OVX animals were cervically stimulated (as previously described) and sacrificed. 3. OVX-cervically stimulated/OT antagonist: OVX animals were cervically stimulated then infused with OT antagonist (as previously described in experiment 1) and sacrificed on Day 2.
RESULTS
Modeling Predicts that an OT Antagonist will Suppress the PRL Rhythm but not the Memory of Cervical Stimulation
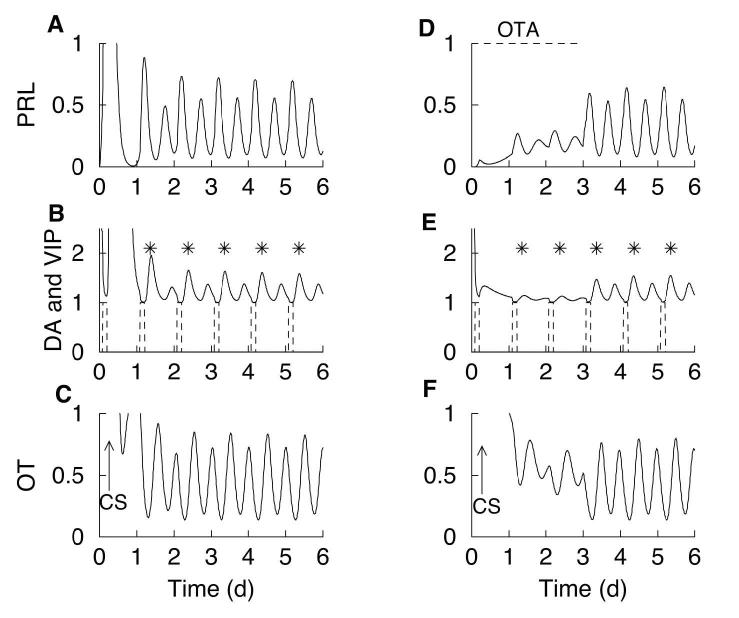
In (25), we developed a mathematical model for the initiation, maintenance, and termination of the PRL rhythm. As described in Methods, we have modified the model to include the rapid inhibitory influence of PRL on the activity of OT neurons that is described in (27). We have also increased the role that the direct stimulatory action of OT has on lactotrophs, so that now this action is necessary (but not sufficient) for a PRL surge. In this model (illustrated in Fig. 1), cervical stimulation increases OT release from the hypothalamus into the pituitary gland. OT within the hypothalamus acts on a population of interneurons that inhibit hypothalamic DAergic neurons, triggering the PRL rhythm. The rhythm, once triggered, results from the interaction between PRL (which stimulates DA neurons) and DA (which inhibits PRL). This is shown in Fig. 2 (A,B). The OT surge (panel C) induced by cervical stimulation inhibits DAergic neurons causing a surge in PRL (panel A). Since PRL stimulates DAergic neurons with a time delay (τ), an increase of DAergic neuronal activity occurs following the PRL surges (panel B). This inhibits lactotrophs, so PRL levels decline. The decline in PRL levels causes a delayed decline in DA, which allows PRL secretion to increase. In this way, a circadian PRL-DA rhythm is established, with DA surges occurring between surges of PRL. The DA surges occur at noon, and are marked with asterisks in Fig. 2B. The phase of the oscillation is set by VIP input from the suprachiasmatic nucleus. In the model, there is a VIP surge each morning for 3 h, prior to and after cervical stimulation (Fig. 2B, E, dashed curve). Each VIP surge lowers DA, as is evident in Fig. 2E.
Figure 2.
Model simulation. (A) Cervical stimulation on day 0 induces a circadian PRL rhythm. (B) DAergic neuronal activity peaks at noon (asterisks) and is out of phase with the PRL surges. VIP surges (dashed) occur every morning for 3 h and have an inhibitory action on DAergic neurons. The DAergic neuronal activity time course has been translated upward by one unit for clarity. (C) Cervical stimulation results in a surge in OT that triggers PRL surges. Cervical stimulation also activates OTergic neurons in the paraventricular nucleus, which provide stimulatory drive to the lactotrophs. The lactotrophs feedback onto and inhibit the OTergic neurons, producing an OT rhythm. (D-F) Same variables, but with an OT antagonist present through day 3 (simulated by setting ν o = 0 ). Once the OT antagonist is removed, DAergic neurons interact with lactotrophs to produce a circadian rhythm in PRL and DA, with DAergic neuronal activity peaking at noon (asterisks).
The trigger for the secretory PRL rhythm in Fig. 2(A-C) is the cervical stimulation-induced OT surge. One result of cervical stimulation is that hypothalamic OT neurons are activated, and remain activated after the surge has subsided (panel C). This provides stimulatory drive to lactotrophs, which acts in conjunction with reduced inhibitory DAergic input to allow the circadian PRL-DA rhythm to emerge. The OT level itself exhibits oscillations, due to the rapid negative feedback from PRL.
The model suggests that the “memory” induced by cervical stimulation is in the hypothalamus, but the secretory PRL rhythm itself involves the interaction of hypothalamic DAergic neurons with lactotrophs. Thus, it should be possible to suppress PRL surges induced by cervical stimulation by inhibiting the lactotrophs, while not interfering with the “memory” of cervical stimulation. To demonstrate this with the model, we simulate the application of an OT antagonist that does not cross the blood-brain barrier. This will eliminate the direct stimulatory influence of OT on the lactotrophs, without affecting the influence of OT in the hypothalamus. The results of this computer simulation are shown in Fig. 2 (D-F). The OT antagonist is simulated by setting ν o = 0 in Eq. (2), so that the direct influence of OT on lactotrophs is removed. The simulated OT antagonist is applied until day 3 (dashed curve, panel D). The cervical stimulation is given on day 0. As before, cervical stimulation induces a surge of OT followed by activation of hypothalamic OT neurons, so that OT peaks and then falls to a lower oscillatory level (panel F). Also as before, the OT surge inhibits the DAergic neurons (panel E). Although the OT neuronal activity is elevated, the OT receptors on lactotrophs are antagonized by the OT antagonist. Without the direct stimulatory influence of OT, a reduction of the DA tone is insufficient to allow significant lactotroph activation and PRL release. Thus, the PRL level remains low following cervical stimulation, showing only small fluctuations. The DA level also lacks a rhythm, and in particular there are no surges in DA neuronal activity at noon (asterisks) as there were in the case of no OT antagonist (Fig. 2 (DF)). This prediction is somewhat counterintuitive, since one typically associates low PRL secretion with an elevated level of DA tone. However, in the model simulation, OT antagonist suppresses the PRL rhythm and, indirectly, the noontime DAergic neuronal activity elevations.
Although the PRL rhythm does not occur when OT antagonist is present in the model, the “memory” mechanism that responds to cervical stimulation has been activated. This is seen in Fig. 2 (D-F) as a reduction in DA tone and an elevation in OTergic neuronal activity. Therefore, when the OT antagonist is removed at the end of day 3, disinhibiting the OT receptors on the lactotrophs, the secretory PRL rhythm begins almost immediately (panel D). The rhythm in DAergic neuronal activity begins at the same time, peaking at noon (asterisks). When the PRL surges begin, they occur at the right time of the day (early morning and late afternoon). This is because the timing of the pulses is influenced by the daily VIP pulses, which are not effected by the OT antagonist.
In summary, the model makes several predictions:
(1) The cervical stimulation-induced secretory PRL rhythm should be suppressed while the OT antagonist is present, but should resume soon after the OT antagonist leaves the system,
(2) When the PRL rhythm begins, the PRL surges should occur at the right time of day (early morning and late afternoon),
(3) In animals subject to cervical stimulation but without the OT antagonist, the DA activity peaks at around noon. If, however, the cervical stimulation-induced secretory PRL rhythm is suppressed by an OT antagonist, the DA activity will be low at noon. When the OT antagonist leaves the system and the secretory PRL rhythm begins, one should observe DA activity peaks at around noon.
These predictions were tested in the following studies.
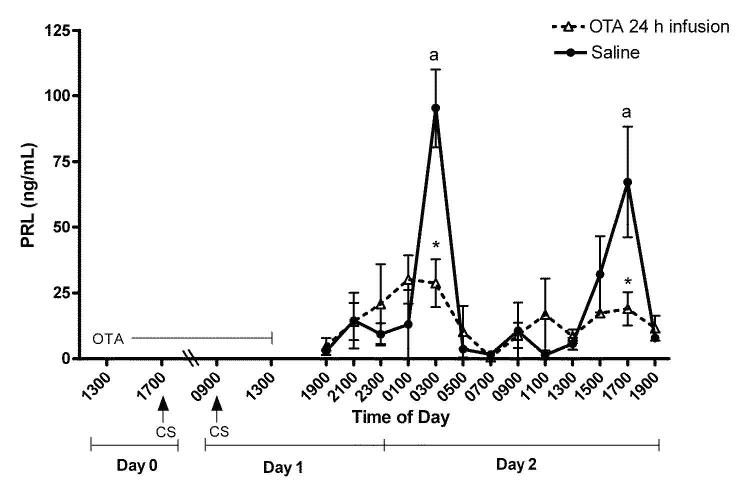
Experiment 1. Prevention of the induction PRL surges by cervical stimulation during infusion of OT antagonist
Figure 3 shows plasma PRL levels obtained every 2 h on Day 2 from rats exposed to an OT antagonist for 24 h prior to and during cervical stimulation on Days 0 and 1. In saline-infused OVX-cervically stimulated rats, PRL levels were elevated at 0300 h (P < 0.01) and 1700 h (P < 0.05). These are time points at which the nocturnal and diurnal surges occur. The OT antagonist infusion prevented the induction of PRL surges by cervical stimulation. These data support our model prediction (Fig 2) that the OT antagonist abolishes PRL surges induced by cervical stimulation, leaving only small, non-significant variations of PRL levels throughout the day.
Figure 3.
Rhythmic secretion of PRL on Day 2 after 24h infusion of the OT antagonist (dotted line) or saline (solid line) in OVX rats before/during cervical stimulation. Infusion of the OT antagonist 24h before cervical stimulation prevented initiation of PRL surges. Values are expressed as mean ng/mL of PRL ± SE (n=3-10 serial samples/point). *Significantly lower PRL levels than corresponding times in saline infused animals (P < 0.05). There were no significant elevation in PRL secretion at any time in the OT antagonist-treated group. aStatistical difference from all other time points within the saline infused animals.
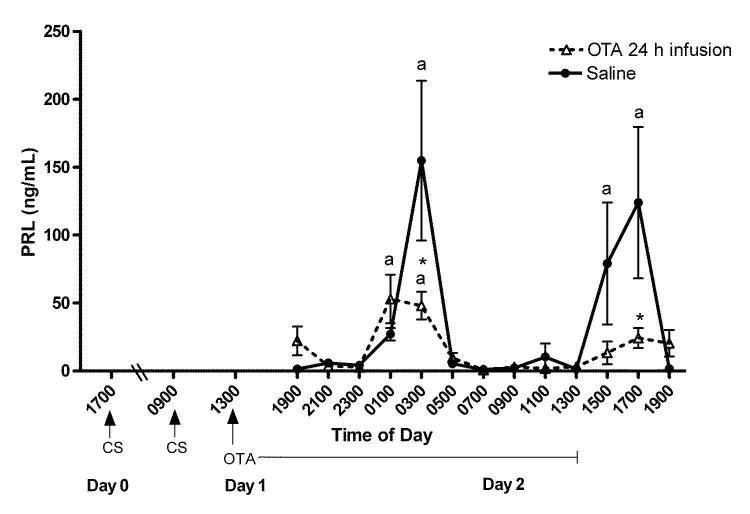
Experiment 2. Decrease of the nocturnal surge and abolition of the diurnal surge of PRL by an OT antagonist infusion after cervical stimulation
Figure 4 shows the pattern of plasma PRL secretion during a 24 h period, beginning at 1900 h on day 2 after cervical stimulation. In the saline-infused OVX-cervically stimulated rats, PRL levels were elevated at 0300 h (P < 0.01) and 1700 h (P < 0.05). These are time points at which the nocturnal and diurnal surges occur. In the OT antagonist- infused rats, PRL levels were elevated at only 0100 h and 0300 h (P < 0.05), representing only the time of the nocturnal surge. PRL levels did not increase at the times corresponding to the diurnal surge. However, the concentration of PRL in the OT antagonist-treated rats was significantly lower than that of saline infused rats at 0300 h and 1700 h (P < 0.05), the times of both the nocturnal and diurnal PRL surges. Together, these data show that the OT antagonist infused after cervical stimulation decreases the nocturnal and abolishes the diurnal PRL surges. Thus, the PRL rhythm, induced by cervical stimulation, was diminished and later eliminated by the OT antagonist.
Figure 4.
Rhythmic secretion of PRL when the OT antagonist infusion (dotted line) or saline infusion (solid line) began after cervical stimulation in OVX rats. The OT antagonist decreases the nocturnal and abolishes the diurnal surges of PRL when infused after cervical stimulation. Values are expressed as mean ng/ml of PRL ± SE (n=3-10 serial samples). *Significantly lower PRL levels than corresponding times in saline infused animals (P < 0.05). aStatistical difference from all other time points within the OT antagonist or saline infused animals.
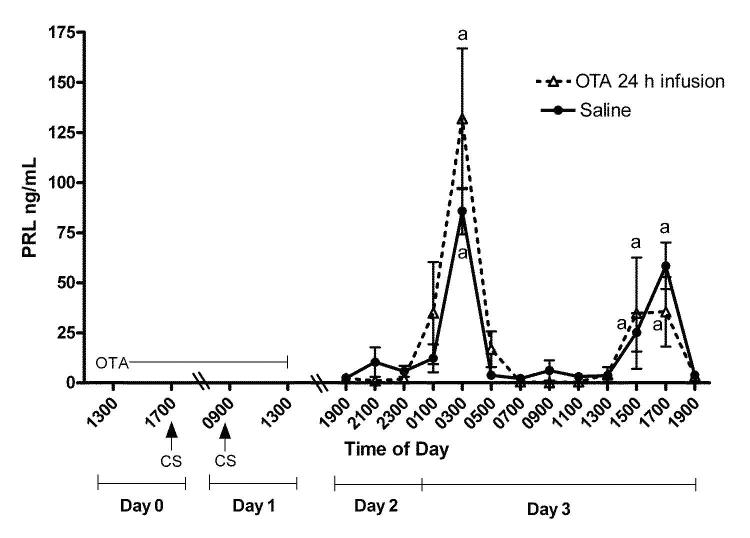
Experiment 3. Return of rhythmic PRL secretion after treatment with an OT antagonist
Figure 5 shows plasma PRL levels obtained every 2 h on Day 3 from rats exposed to the OT antagonist for 24 h prior to and during cervical stimulation on Days 0 and 1. In the saline-infused OVX-cervically stimulated rats, PRL levels were elevated at 0300 h (P < 0.01) and 1700 h (P < 0.05). These are time points at which the nocturnal and diurnal surges occur. In OVX-cervically stimulated rats infused with an OT antagonist, PRL levels were also significantly elevated at 0300 h and 1700 h (P < 0.05) on day 3. The PRL surges observed in the OT antagonist and saline treated animals were equivalent in timing and magnitude, suggesting clearance of the OT antagonist. These findings support our model prediction (Fig 2) that the OT antagonist will not interfere with the triggering of the “memory” of cervical stimulation, so that once the OT antagonist is cleared from the system the rhythmic PRL surges will appear. This suggests that the cervical stimulation-induced “memory” resides at a location other than the pituitary, most likely in the hypothalamus.
Figure 5.
Rhythmic secretion of PRL returns two days after cessation of the OT antagonist infusion. OVX-cervically stimulated rats were infused with the OT antagonist (dotted line) or saline (solid line) for 24 h as in Figure 3. Blood samples were obtained for 24 h beginning 30 h after cessation of infusion. Values are expressed as mean ng/ml of PRL ± SE (n=3-10 serial samples). There were no differences in serum concentrations of PRL within any time point between animals infused with saline or OT antagonist. aStatistical difference from all other time points within the saline or OT antagonist infused animals.
Experiment 4. Activity of dopaminergic neurons during the OT antagonist treatment
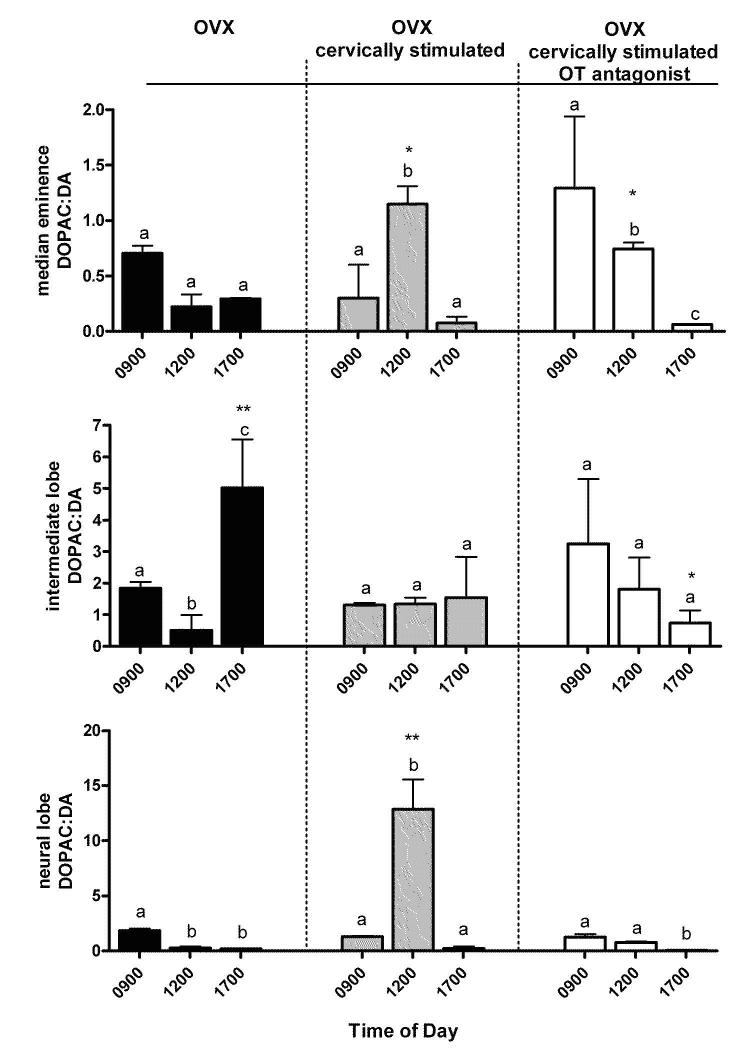
The data in Figure 6 represent the DOPAC:DA ratio, an index of DA neuronal activity (34,35). Animals were either OVX, OVX-cervically stimulated or OVX-cervically stimulated and treated with an OT antagonist. The latter group was exposed to an OT antagonist for 24 h prior to and during cervical stimulation on Days 0 and 1. In the OVX-cervically stimulated group, the DOPAC:DA ratio in the median eminence and neural lobe was significantly elevated at 1200h (P < 0.05 and P < 0.001, respectively). This elevation was not observed in the intermediate lobe (Fig 6). These data indicate that DA neuronal activity was increased at 1200 h in anti-phase with PRL secretion in TIDA and THDA neurons but not in the PHDA neurons. In the OT antagonist/cervically stimulated group, the DOPAC:DA ratio in the median eminence was elevated at 0900h and decreased at 1700h (P < 0.05), and in the neural lobe was elevated at 0900 h and decreased at 1700 h (P < 0.05). The presence of the OT antagonist disrupted the anti-phasic DA neuronal activity. In addition, the OT antagonist eliminated the increase in the DOPAC:DA ratio at 1200 h in the cervically stimulated group in the neural lobe (P < 0.01). Together, these data support our model prediction (Fig 2) that cervical stimulation induces an increase in DOPAC:DA ratio at 1200 h in anti-phase with PRL secretion in TIDA and THDA neurons and that this increase is abolished by OT antagonism.
Figure 6.
DOPAC:DA ratio in the median eminence, intermediate lobe, and neural lobe indicating DA neuronal activity of the TIDA, PHDA, THDA, respectively of OVX, OVX-cervically stimulated, and OVX-cervically stimulated/OT antagonist treated rats at 0900 h, 1200 h, and 1700 h. Cervical stimulation induces an increase in DA neuronal activity in the TIDA and THDA neurons at 1200 h (P < 0.05), in anti-phase with PRL surges. The presence of the OT antagonist disrupts the anti-phasic DA neuronal activity. Values are expressed as mean DOPAC:DA ± SE (n=3-6). Astericks represent significant differences within anatomical area at the corresponding times between OVX, OVX-cervically stimulated, and OVX-cervically stimulated/OT antagonist (*P<0.05, **P<0.01). Dissimilar letters represent statistical differences between times within each treatment group.
DISCUSSION
In the present study, we have taken parallel, but differing approaches to describe the role of OT and DA in control of mating-induced PRL secretion. First, we have developed mathematical models to predict their respective roles and then tested the predictions with the use of an OT antagonist, which does not cross the blood brain barrier. We have shown that, in the presence of the OT antagonist, rhythmic cervical stimulation-induced PRL secretion is prevented or abolished and, after the probable clearance of the OT antagonist, rhythmic PRL secretion returns. Thus, the “memory” of cervical stimulation was maintained even though the rhythmic PRL surges were inhibited by the OT antagonist. This further supports that the “memory”, which is not affected by the OT antagonist, is not in the pituitary gland. In response to cervical stimulation hypothalamic DAgeric neuronal activity is elevated in antiphase with rhythmic PRL surges, the OT antagonist disrupts this cervical stimulation-induced elevation.
We hypothesize that OT is necessary for PRL surges in OVX-cervically stimulated rats by directly acting at the lactotroph. We predict, using mathematical modeling, that in the presence of an OT antagonist, cervical stimulation-induced PRL surges would be abolished with only small variations in PRL secretion. To investigate our hypothesis, we infused an OT antagonist for 24h before cervical stimulation and began blood collections after the OT antagonist infusion in OVX rats. As predicted by our model, the OT antagonist blocked the initiation of cervical stimulation-induced PRL surges. To further investigate our hypothesis, we infused an OT antagonist for 24h after cervical stimulation and began blood collection during the OT antagonist infusion. We found the OT antagonist decreased the nocturnal and abolished the diurnal surges of PRL. These findings support our predictions and previous suggestions of OT's stimulatory role on lactotrophs (23) and the effects of the OT antagonist on PRL surges in OVX-cervically stimulated rats, previously demonstrated in our laboratory (38). We attribute the decrease and not abolition of the nocturnal surge in animals infused with an OT antagonist after cervical stimulation to the shorter period of time between the beginning of the OT antagonist infusion and blood collections, thus not permitting the OT antagonist to exert its full effect.
We hypothesize that the “memory” of cervical stimulation is independent of OT actions at the lactotroph. Therefore, even in the presence of the OT antagonist, cervical stimulation should impose a “memory” for rhythmic PRL secretion in the hypothalamus and after the OT antagonist is cleared, rhythmic PRL secretion should begin. To investigate our hypothesis, an OT antagonist was infused before cervical stimulation for 24 h in OVX rats and blood collections began 30 hours after ending the infusion. As we predicted (Fig 2), rhythmic PRL secretion returned on Day 3, suggesting the clearance of the OT antagonist. To further investigate our hypothesis, an OT antagonist was infused after cervical stimulation for 24 h in OVX rats and blood collections began 5 days later. Rhythmic PRL secretion also returns on Day 6, after the antagonist was presumably cleared (data not shown). These findings support our hypothesis that OT actions at the lactotroph are essential for the occurrence of PRL surges, but are not involved in the cervical stimulation-induced “memory”.
We have previously found that VIP neurons in the SCN are involved in the control of rhythmic activity of DA neurons in the hypothalamus (25). In the present study, we also hypothesized that VIPergic neurons, controlling the time of day of the PRL surges, would not be affected by an OT antagonist; therefore after the OT antagonist clears, the PRL surges would return at the time of day of the nocturnal and diurnal PRL surges. As shown in Fig. 5, the PRL surges return at the same time of day as the nocturnal and diurnal surges in control animals. This supports our hypothesis that the timing of the PRL surges is controlled from outside of the pituitary, most likely by VIP neurons of the SCN.
In our model, rhythmic PRL secretion is produced by interaction with hypothalamic DA neurons, such that DAergic neuronal activity peaks at noon, in anti-phase with the PRL surges. Model simulations suggested if the OT antagonist inhibits the PRL surges, then it will also inhibit the noontime peak in DAergic neuronal activity, since this peak is due to the stimulatory effects of PRL. To investigate our hypothesis, we determined neuroendocrine DA neuronal activity (TIDA, PHDA, THDA) by measuring the DOPAC:DA ratio in the median eminence, intermediate lobe, neural lobe, in OVX, OVX-cervically stimulated, and the OT antagonist/cervically stimulated groups. As our model predicted, DA neuronal activity of OVX-cervically stimulated rats was elevated at 1200 h, in anti-phase with PRL surges, in the TIDA and THDA neurons and not elevated at 1200 h in the PHDA neurons (Fig 6). Therefore, this suggests that the DAergic neurons in the arcuate nucleus are involved in the cervically stimulated-induced PRL surges and not DAergic neurons in the periventricular nucleus. The presence of the OT antagonist disrupts the anti-phasic DA neuronal activity. Because hypothalamic DAergic neurons display a spontaneous rhythmic pattern of activity in unstimulated OVX rats (39-41), the low levels of DA neuronal activity observed at 1700 h might be the expression of an endogenous circadian rhythm of these neurons in OVX-cervically stimulated and OVX-cervically stimulated rats treated with OT antagonist. Interestingly, the OT antagonist did not affect the expression of this rhythm. Yet, even with low DAergic activity, a PRL surge is not seen in either the OVX rats (data not shown) or OVX cervically stimulated rat under OT antagonist treatment, confirming our hypothesis that the PRL surge is due to the combination of a decrease in DA neuronal activity and the actions of a PRL releasing factor. Our results suggest that OT is the PRL releasing factor required for the cervical stimulation-induced PRL surges.
In our mathematical model we have predicted that cervical stimulation triggers the PRL rhythm by indirectly inhibiting hypothalamic DA neurons. The evidence for this is indirect and based on our previous finding that an OT injection initiates a long-lasting circadian PRL rhythm (24) and a parallel modeling study (25) showing that partial inhibition of DA neurons is the only way to initiate and maintain the PRL rhythm. In the modeling study, we postulated a population of bistable OT-sensitive interneurons that are switched from the “off” to the “on” state by the OT bolus, and which innervate and inhibit DA neurons (which themselves don't have OT receptors). This is but one potential mechanism for transducing the OT bolus into a sustained PRL rhythm. However there is no direct evidence for these OT-sensitive interneurons. We are also investigating additional mechanisms in which cervical stimulation triggers rhythmic PRL surges by exploring other parts of the brain that project to the hypothalamus and are activated by cervical stimulation. In turn, these brain regions may also be involved in the sustained PRL rhythm.
Taken together, this study confirms the stimulatory role of OT on lactotrophs in the production of PRL surges in response to cervical stimulation.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical support and suggestions of Cheryl Fitch-Pye. We thank Albert Parlow, Ph.D. and NHPP for supplying the rat PRL radioimmunoassay reagents and Maurice Manning, Ph.D., D.Sc. from the Biochemistry and Cancer Biology Department at Medical College of Ohio for his generous supply of the OT antagonist. This work was supported by grants NIH DK-43200 to M.E.F.and NIH DA-19356 to R.B. and M.E.F..
NIH Statement: Financial support from the National Institutes of Health Grants DA-19356 to R. Bertram, M. Egli, and M. E. Freeman and DK-43200 to M. E. Freeman.
Footnotes
Disclosure statement: The authors of this manuscript have nothing to disclose.
Publisher's Disclaimer: This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.”
Reference List
- 1.Butcher RL, Fugo NW, Collins WE. Semicircadian rhythm in plasma levels of prolactin during early gestation in the rat. Endocrinology. 1972;90:1125–1127. doi: 10.1210/endo-90-4-1125. [DOI] [PubMed] [Google Scholar]
- 2.Smith MS, Neill JD. Termination at midpregnancy of the two daily surges of plasma prolactin initiated by mating in the rat. Endocrinology. 1976;98:696–701. doi: 10.1210/endo-98-3-696. [DOI] [PubMed] [Google Scholar]
- 3.Soares MJ, Faria TN, Roby KF, Deb S. Pregnancy and the Prolactin Family of Hormones - Coordination of Anterior-Pituitary, Uterine, and Placental Expression. Endocr Rev. 1991;12:402–423. doi: 10.1210/edrv-12-4-402. [DOI] [PubMed] [Google Scholar]
- 4.Freeman ME, Smith MS, Nazian SJ, Neill JD. Ovarian and hypothalamic control of the daily surges of prolactin secretion during pseudopregnancy in the rat. Endocrinology. 1974;94:875–882. doi: 10.1210/endo-94-3-875. [DOI] [PubMed] [Google Scholar]
- 5.Freeman ME, Banks JA. Hypothalamic sites which control the surges of prolactin secretion induced by cervical stimulation. Endocrinology. 1980;106:668–673. doi: 10.1210/endo-106-3-668. [DOI] [PubMed] [Google Scholar]
- 6.Hentschel K, Moore KE, Lookingland KJ. Effects of prolactin on expression of Fos-related antigens in tyrosine hydroxylase-immunoreactive neurons in subdivisions of the arcuate nucleus. Brain Res. 2000;857:110–118. doi: 10.1016/s0006-8993(99)02362-8. [DOI] [PubMed] [Google Scholar]
- 7.Moore KE, Demarest KT, Johnston CA. Influence of Prolactin on Dopaminergic Neuronal Systems in the Hypothalamus. Fed Proc. 1980;39:2912–2916. [PubMed] [Google Scholar]
- 8.Ben Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22:724–763. doi: 10.1210/edrv.22.6.0451. [DOI] [PubMed] [Google Scholar]
- 9.Erskine MS. Prolactin-Release After Mating and Genitosensory Stimulation in Females. Endocr Rev. 1995;16:508–528. doi: 10.1210/edrv-16-4-508. [DOI] [PubMed] [Google Scholar]
- 10.Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- 11.Chadio SE, Antoni FA. Characterization of oxytocin receptors in rat adenohypophysis using a radioiodinated receptor antagonist peptide. J Endocrinol. 1989;122:465–470. doi: 10.1677/joe.0.1220465. [DOI] [PubMed] [Google Scholar]
- 12.Morel G, Chabot JG, Dubois PM. Ultrastructural Evidence for Oxytocin in the Rat Anterior-Pituitary Gland. Acta Endocrinologica. 1988;117:307–314. doi: 10.1530/acta.0.1170307. [DOI] [PubMed] [Google Scholar]
- 13.Breton C, Pechoux C, Morel G, Zingg HH. Oxytocin Receptor Messenger-Ribonucleic-Acid - Characterization, Regulation, and Cellular-Localization in the Rat Pituitary-Gland. Endocrinology. 1995;136:2928–2936. doi: 10.1210/endo.136.7.7540544. [DOI] [PubMed] [Google Scholar]
- 14.Samson WK, Schell DA. Oxytocin and the anterior pituitary gland. Adv Exp Med Biol. 1995;395:355–364. [PubMed] [Google Scholar]
- 15.Sarkar DK. Immunoneutralization of Oxytocin Attenuates Preovulatory Prolactin Secretion During Proestrus in the Rat. Neuroendocrinology. 1988;48:214–216. doi: 10.1159/000125012. [DOI] [PubMed] [Google Scholar]
- 16.Johnston CA, Negro-Vilar A. Role of oxytocin on prolactin secretion during proestrus and in different physiological or pharmacological paradigms. Endocrinology. 1988;122:341–350. doi: 10.1210/endo-122-1-341. [DOI] [PubMed] [Google Scholar]
- 17.Samson WK, Lumpkin MD, McCann SM. Evidence for a physiological role for oxytocin in the control of prolactin secretion. Endocrinology. 1986;119:554–560. doi: 10.1210/endo-119-2-554. [DOI] [PubMed] [Google Scholar]
- 18.Moos F, Richard P. Level of Oxytocin Release Induced by Vaginal Dilatation (Ferguson Reflex) and Vagal-Stimulation (Vago-Pituitary Reflex) in Lactating Rats. J Physiol (Paris) 1975;70:307–314. [PubMed] [Google Scholar]
- 19.Kendrick KM, Keverne EB, Hinton MR, Goode JA. Cerebrospinal-Fluid and Plasma-Concentrations of Oxytocin and Vasopressin During Parturition and Vaginocervical Stimulation in the Sheep. Brain Res Bull. 1991;26:803–807. doi: 10.1016/0361-9230(91)90178-m. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert CL, Boulton MI, Forsling ML, Goode JA, McGrath TJ. Restricting maternal space during parturition in the pig. Effects on oxytocin, vasopressin and cortisol secretion following vagino-cervical stimulation and administration of naloxone. Animal Reproduction Science. 1997;46:245–259. doi: 10.1016/s0378-4320(96)01596-5. [DOI] [PubMed] [Google Scholar]
- 21.Ferguson J. A study of the motility of the intact uterus at term. Surg Gynecol & Obstet. 1941;73:359–366. [Google Scholar]
- 22.Freeman ME, Sterman JR. Ovarian steroid modulation of prolactin surges in cervically stimulated ovariectomized rats. Endocrinology. 1978;102:1915–1920. doi: 10.1210/endo-102-6-1915. [DOI] [PubMed] [Google Scholar]
- 23.Egli M, Bertram R, Sellix MT, Freeman ME. Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology. 2004;145:3386–3394. doi: 10.1210/en.2003-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME. Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab. 2006;290:E566–E572. doi: 10.1152/ajpendo.00427.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertram R, Egli M, Toporikova N, Freeman ME. A mathematical model for the mating-induced prolactin rhythm of female rats. Am J Physiol Endocrinol Metab. 2006;290:E573–E582. doi: 10.1152/ajpendo.00428.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeMaria JE, Lerant AA, Freeman ME. Prolactin activates all three populations of hypothalamic neuroendocrine dopaminergic neurons in ovariectomized rats. Brain Res. 1999;837:236–241. doi: 10.1016/s0006-8993(99)01667-4. [DOI] [PubMed] [Google Scholar]
- 27.Kokay IC, Bull PM, Davis RL, Ludwig M, Grattan DR. Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. American Journal of Physiology-Regulatory Integrative and Comparative Physiology. 2006;290:R1216–R1225. doi: 10.1152/ajpregu.00730.2005. [DOI] [PubMed] [Google Scholar]
- 28.Gerhold LM, Sellix MT, Freeman ME. Antagonism of vasoactive intestinal peptide mRNA in the suprachiasmatic nucleus disrupts the rhythm of FRAs expression in neuroendocrine dopaminergic neurons. J Comp Neurol. 2002;450:135–143. doi: 10.1002/cne.10307. [DOI] [PubMed] [Google Scholar]
- 29.Okamoto S, Okamura H, Miyake M, Takahashi Y, Takagi S, Akagi Y, Fukui K, Okamoto H, Ibata Y. A Diurnal-Variation of Vasoactive-Intestinal-Peptide (Vip) Messenger-Rna Under A Daily Light-Dark Cycle in the Rat Suprachiasmatic Nucleus. Histochemistry. 1991;95:525–528. doi: 10.1007/BF00315750. [DOI] [PubMed] [Google Scholar]
- 30.Shinohara K, Tominaga K, Isobe Y, Inouye SIT. Photic Regulation of Peptides Located in the Ventrolateral Subdivision of the Suprachiasmatic Nucleus of the Rat - Daily Variations of Vasoactive Intestinal Polypeptide, Gastrin-Releasing Peptide, and Neuropeptide-y. J Neurosci. 1993;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ermentrout GB. Stimulating, Analyzing, and Animating Dynamical Systems: A Guide to XPPAUT for Researchers and Students. SIAM; Philadelphia, PA: 2002. [Google Scholar]
- 32.Gorospe WC, Freeman ME. The effects of various methods of cervical stimulation on continuation of prolactin surges in rats. Proc Soc Exp Biol Med. 1981;167:78–82. doi: 10.3181/00379727-167-41128. [DOI] [PubMed] [Google Scholar]
- 33.Manning M, Miteva K, Pancheva S, Stoev S, Wo NC, Chan WY. Design and synthesis of highly selective in vitro and in vivo uterine receptor antagonists of oxytocin: comparisons with Atosiban. Int J Pept Protein Res. 1995;46:244–252. doi: 10.1111/j.1399-3011.1995.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 34.Annunziato L, Weiner RI. Characteristics of Dopamine Uptake and 3,4-Dihydroxyphenylacetic Acid (Dopac) Formation in the Dopaminergic Terminals of the Neurointermediate Lobe of the Pituitary-Gland. Neuroendocrinology. 1980;31:8–12. doi: 10.1159/000123043. [DOI] [PubMed] [Google Scholar]
- 35.Lookingland KJ, Jarry HD, Moore KE. The Metabolism of Dopamine in the Median-Eminence Reflects the Activity of Tuberoinfundibular Neurons. Brain Res. 1987;419:303–310. doi: 10.1016/0006-8993(87)90597-x. [DOI] [PubMed] [Google Scholar]
- 36.DeMaria JE, Zelena D, Vecsernyes M, Nagy GM, Freeman ME. The effect of neurointermediate lobe denervation on hypothalamic neuroendocrine dopaminergic neurons. Brain Res. 1998;806:89–94. doi: 10.1016/s0006-8993(98)00740-9. [DOI] [PubMed] [Google Scholar]
- 37.Sellix MT, Egli M, Henderson RP, Freeman ME. Ovarian steroid hormones modulate circadian rhythms of neuroendocrine dopaminergic neuronal activity. Brain Res. 2004;1005:164–181. doi: 10.1016/j.brainres.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 38.Arey BJ, Freeman ME. Oxytocin, Vasoactive-Intestinal Peptide, and Serotonin Regulate the Mating-Induced Surges of Prolactin Secretion in the Rat. Endocrinology. 1990;126:279–284. doi: 10.1210/endo-126-1-279. [DOI] [PubMed] [Google Scholar]
- 39.DeMaria JE, Livingstone JD, Freeman ME. Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons. Brain Research. 2000;879:139–147. doi: 10.1016/s0006-8993(00)02763-3. [DOI] [PubMed] [Google Scholar]
- 40.Mai LM, Shieh KR, Pan JT. Circadian Changes of Serum Prolactin Levels and Tuberoinfundibular Dopaminergic Neuron Activities in Ovariectomized Rats Treated with Or Without Estrogen - the Role of the Suprachiasmatic Nuclei. Neuroendocrinology. 1994;60:520–526. doi: 10.1159/000126789. [DOI] [PubMed] [Google Scholar]
- 41.Sellix MT, Freeman ME. Circadian rhythms of neuroendocrine dopaminergic neuronal activity in ovariectomized rats. Neuroendocrinology. 2003;77:59–70. doi: 10.1159/000068334. [DOI] [PubMed] [Google Scholar]