Summary
Objective:
We determined whether the primary motor hand area was most frequently located in the precentral gyrus in young patients with intractable focal seizures.
Methods:
Sixty-five patients with focal seizures aged between 5 months and 20 years who underwent a two-stage epilepsy surgery using chronic subdural-EEG monitoring were studied. Pairs of subdural electrodes were electrically stimulated, and the brain region with contralateral hand movement induced by the lowest-intense stimulus was defined as the primary motor hand area.
Results:
Contralateral hand movement was induced without afterdischarges in 50 children but not in the remaining 15 children. The unpaired t-test revealed that failure to induce contralateral hand motor movement was associated with younger age of subjects. Among the 50 patients with a positive motor response, the primary motor hand area was confined to the precentral gyrus in 9 patients, confined to the postcentral gyrus in 24, and located in both the pre- and post-central gyri in the remaining 17. The McNemar's test revealed that the observed frequency of 24 patients showing the primary motor hand area confined to the postcentral gyrus was larger than chance frequency. Logistic regression analysis failed to demonstrate that the observation of the primary motor hand area confined to the postcentral gyrus was associated with the age, the presence of dysplastic lesion or the seizure onset involving the frontal lobe.
Conclusion:
Our study failed to support the traditionally-accepted notion that the primary motor hand area is most frequently located in the precentral gyrus but rather demonstrated that a substantial proportion of young patients had the primary motor hand area in the postcentral gyrus.
Keywords: Motor cortex, Functional cortical mapping, Somatosensory evoked potentials (SEPs), Electrical brain stimulation, Functional MRI (fMRI)
Introduction
Traditionally, the anterior bank of the precentral gyrus, known as Brodmann's Area 4, has been considered to be the primary motor area in humans (Penfield and Boldrey, 1937). To identify the primary motor area, electrical stimulation via subdural electrodes implanted on the brain surface has been clinically utilized for patients undergoing surgical treatment of uncontrolled focal seizures or brain tumors (Uematsu et al., 1992). The cortical region where the lowest-intensity stimulus is capable of inducing a contralateral hand movement is considered to be the primary motor area for the hand, and is generally assumed to be located in the lateral surface of the precentral gyrus (Ropper and Brown, 2005). Thus, the location of the motor hand area is based on the results of electrical stimulation.
Have previous studies of cortical stimulation proven the generally-accepted assumption that the primary motor hand area is most frequently located in the precentral gyrus? Although previous human studies have indicated that the primary motor hand area was frequently identified in the presumed precentral gyrus, some variability in location was also noted (Penfield and Boldrey, 1937; Uematsu et al., 1992; Nii et al., 1996). In those studies, there were no techniques used to exactly coregister the locations of subdural electrodes on the MR image, and estimation of the central sulcus was biased by the results of electrical stimulation (Penfield and Boldrey, 1937).
In the present study of 65 children and young adults with medically-uncontrolled focal epilepsy, we systematically defined the central sulcus using well-established anatomical landmarks (Berger et al., 1990; Yousry et al., 1997; Lehericy et al., 2000; Makela et al., 2001; Sunaert, 2006) delineated on the three-dimensional brain surface MR image as previously described (von Stockhausen et al., 1997; Juhasz et al., 2000). The spatial relationship between the primary hand motor area determined by electrical stimulation and the anatomically-defined precentral gyrus was then determined on each individual's three-dimensional brain surface image. We subsequently tested the validity of the generally-accepted assumption that the primary motor hand area is most frequently located in the precentral gyrus. We also determined the validity of the results of functional motor mapping in the present study by taking into consideration the concurrent findings of somatosensory evoked potentials (SEPs) for the median nerve of the contralateral hand. We finally determined whether the distribution of the primary hand motor area was associated with age, the presence of dysplastic brain lesions or the location of seizure onset.
Methods
Patients
The inclusion criteria of the present study included: (i) age ranging from 5 months to 20 years, (ii) a two-stage epilepsy surgery using chronic subdural EEG recording in Children's Hospital of Michigan, Detroit between January 2001 and August 2006, (iii) functional cortical mapping for the primary hand motor area using electrical stimulation and (iv) subdural electrodes chronically implanted on both the pre- and post-central gyri at least 4 cm above the Sylvian fissure (Nii et al., 1996; Polkey, 2000). The exclusion criteria included: (i) the presence of massive brain malformations (such as large porencephaly, polymicrogyria or hemimegalencephaly) which is known to disorganize the anatomical landmarks for the central sulcus, (ii) history of previous epilepsy surgery, and (iii) the presence of epilepsia partialis continua involving the hand. A total of 81 patients met the inclusion criteria, and 4 of the 81 patients were excluded due to the presence of massive brain malformations and another 12 were excluded due to the history of previous epilepsy surgery. Thus, we studied a consecutive series of 65 patients with a diagnosis of medically-uncontrolled focal seizures (age: 5 months—20 years; 27 females) who met the inclusion criteria and satisfied the exclusion criteria (Supplementary Table 1). The study has been approved by the Institutional Review Board at Wayne State University, and written informed consent was obtained from the parents or guardians of all subjects.
Subdural electrode placement
For chronic subdural EEG recording and subsequent functional cortical mapping, platinum grid electrodes (10 mm intercontact distance, 4 mm diameter; Ad-tech, Racine, WI) were surgically implanted as previously described (Asano et al., 2005). The total number of electrode contacts in each subject ranged from 64 to 128. The placement of intracranial electrodes was guided by the results of scalp video-EEG recording, MRI and interictal glucose metabolism on positron emission tomography (PET). All electrode plates were stitched to adjacent plates and/or the edge of dura mater, to avoid movement of subdural electrodes after placement. In addition, intraoperative pictures were taken with a digital camera before dural closure, to confirm the spatial accuracy of electrode display on three-dimensional brain surface reconstructed from MRI (Asano et al., 2005).
Chronic subdural EEG recording
Chronic subdural EEG recordings were performed for 2−7 days as previously described (Asano et al., 2005). Anti-epileptic medications were discontinued or reduced during subdural EEG monitoring until a sufficient number of habitual seizures were captured. Ictal subdural EEG recordings were visually reviewed and seizure-onset zones were determined by the consensus of two clinical neurophysiologists. Clinical manifestations were assessed using synchronized digital videos with 30 frames per second, and surface EMG recordings from the left and right deltoid muscles were added as needed.
Electrical stimulation protocol
As a component of the clinical management of the patients being evaluated for cortical resection to achieve seizure control, functional cortical mapping using electrical stimulation was performed during the intracranial EEG recording, using a method similar to those described previously (Girvin, 1986; Ojemann et al., 1993; Jayakar and Lesser, 1997; Chitoku et al., 2001). A train of repetitive electrical stimuli were delivered using the Grass S12 constant-current stimulator (Astro-Med, Inc., West Warwick, RI; Jayakar and Lesser, 1997), and clinical responses associated with stimulation were observed by at least two investigators. To minimize the risk of charge accumulation in the participant's body, biphasic stimulus pulses were used (Ojemann et al., 1993) and a single ground lead was placed at the contralateral mastoid by a registered EEG technician. To minimize the risk of stimulation-induced seizures, phenytoin was intravenously loaded prior to the mapping session in patients whose anti-epileptic drugs had been discontinued. To determine the presence of afterdischarges, subdural EEG and video were recorded continuously during the entire mapping session (Jayakar and Lesser, 1997).
All electrodes covering the premotor, precentral, postcentral, and anterior-parietal areas were electrically stimulated using the following stimulation parameters. Pairs of subdural electrodes were stimulated with a 10 s train of repetitive biphasic electrical pulses (Jayakar and Lesser, 1997; Chitoku et al., 2001), where the duration of each biphasic pulse was set to 300 µs (Ojemann et al., 1993; Jayakar and Lesser, 1997), the stimulus intensity was initially set to 6.5 mA, and stimulus frequency was initially set to 10 Hz (Girvin, 1986). When a clinical response was induced, the stimulation was immediately terminated (Chitoku et al., 2001). On the other hand, if neither clinical response nor afterdischarge was induced by a train of stimuli with an intensity of 6.5 mA, the stimulus intensity was increased from 6.5 to 16.5 mA in a stepwise manner by 5 mA until a clinical response or afterdischarge was noted. Once the after-discharge threshold was determined, stimulus intensity above that threshold was no longer utilized (Ojemann et al., 1993). In limited cases where neither clinical response nor afterdischarge could be induced by a train of stimuli with a frequency of 10 Hz and intensity of 16.5 mA, the stimulus frequency was increased from 10 to 20 Hz and 20 to 50 Hz (Ojemann et al., 1993; Jayakar and Lesser, 1997; Chitoku et al., 2001) until a clinical response or afterdischarge was noted. Usage of stimulus frequency up to 50 Hz along with the stimulus intensity of 16.5 mA has been previously validated (Jayakar and Lesser, 1997; Chitoku et al., 2001; Gordon et al., 1990). Stimulus frequency at 10 Hz or higher has been reported to produce facilita-tory effects to elicit positive responses easily (Girvin, 1986; Lesser and Gordon, 2000).
When stimulation of a pair of electrodes across a sulcus induced a clinical response without afterdischarges, each electrode with another neighboring electrode was stimulated to determine whether one, the other or both gyri were responsible for the clinical response (Girvin, 1986; Ojemann et al., 1993; Jayakar and Lesser, 1997; Chitoku et al., 2001). The brain region at which stimulation consistently induced a clinical response was classified as an eloquent area specific to the clinical response. When no clinical response but afterdischarge was observed, or when neither clinical response nor afterdischarge was induced by the maximally-intense stimuli, the brain region was classified as an area not proven eloquent (Chitoku et al., 2001). When both clinical response and afterdischarges were noted, another train of electrical stimuli with the same or 1 mA smaller intensity was given until either clinical response or afterdischarge subsided (Jayakar and Lesser, 1997). Finally, a brain region with a contralateral hand movement (any part of the hand) induced by the lowest-intense stimulation was defined as “the primary motor hand area” in the present study. If a contralateral hand movement was induced only by stimulation of 16.5 mA intensity, a brain region with its movement induced by the stimuli with the lowest frequency was defined as “the primary motor hand area”.
Imaging acquisition protocol
MRI and glucose metabolism PET studies were performed as previously described (Juhasz et al., 2000). MRI scans included a T1-weighted spoiled gradient echo (SPGR) image as well as fluid-attenuated inversion recovery image. Planar X-ray images (lateral and anteroposterior images) were acquired with the subdural electrodes in place for determining the location of the electrodes on the brain surface. Three metallic fiducial markers were placed at anatomically well-defined locations on the patient's head for coregistration of the X-ray with the MRI, as previously described (von Stockhausen et al., 1997; Juhasz et al., 2000).
Image analysis
MRI SPGR and glucose metabolism PET image volumes were coregistered, as previously described (Juhasz et al., 2000). To reconstruct surface views corresponding to the planar X-ray image, three virtual markers were defined in the SPGR MR image volume at the same position as in the planar X-ray image, as previously described (Juhasz et al., 2000). As a result, a surface view was created which corresponds to the planar X-ray image position and where the location of electrodes was directly defined on the brain surface. The accuracy of this procedure was reported previously as 1.24 ± 0.66 mm with a maximal misregistration of 2.7 mm (von Stockhausen et al., 1997). Finally, accurate coregistration of the location of subdural electrodes and the brain surface image was visually confirmed using the intraoperative photograph of brain surface (Asano et al., 2005).
Identification of the central sulcus, precentral gyrus and postcentral gyrus
The central sulcus was determined by the consensus of two investigators according to the anatomical MRI landmarks which have been previously validated (Berger et al., 1990; Yousry et al., 1997; Lehericy et al., 2000; Makela et al., 2001; Sunaert, 2006). The criteria defining the central sulcus included: the omega-shaped sulcus at 4−5 cm above the Sylvian fissure on the horizontal plane (Yousry et al., 1997). If there were potentially two sulci showing the omega shape, the sulcus just posterior to the precentral sulcus which has a junction with the superior frontal sulcus was defined as the central sulcus (Berger et al., 1990; Lehericy et al., 2000; Sunaert, 2006). The sulcus meeting the above criteria was determined on the 3D Tool Software package (Max-Planck-Institut, Cologne, Germany; von Stockhausen et al., 1997), where each patient's three-dimensional surface brain image was visualized simultaneously in the horizontal, coronal and sagittal planes and all views were automatically updated as the cursor was moved to any point in a given section (Germann et al., 2005). Once the central sulcus was determined, the gyrus just anterior to the central sulcus was determined as the precentral gyrus, and the one just posterior to the central sulcus was determined as the postcentral gyrus (Figs. 1 and 2; Yousry et al., 1997; Lehericy et al., 2000; Germann et al., 2005).
Figure 1.
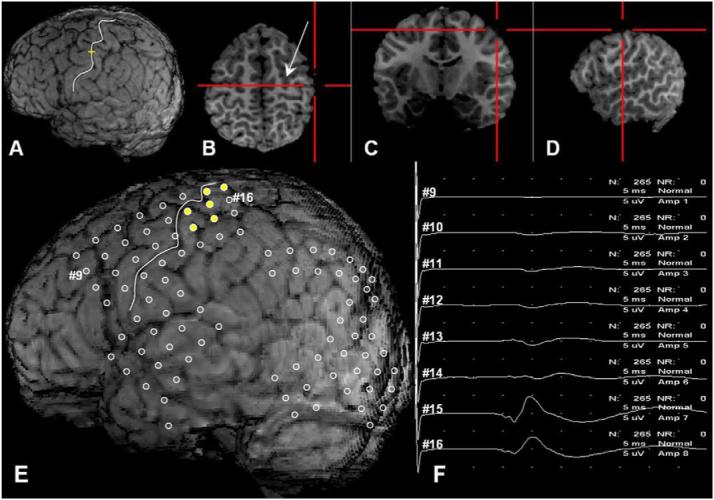
A 7-year-old boy with left occipital lobe epilepsy. The three-dimensional surface brain image (A) was visualized simultaneously in the horizontal (B), coronal (C) and sagittal planes (D). As the red cursor on the horizontal plane (B) was moved to the omega-shaped sulcus which is posterior to the precentral sulcus at the junction with the superior frontal sulcus (arrow), the yellow cursor was moved to the sulcus (white line) on the three-dimensional surface brain image (A). This white line was defined as the central sulcus in this patient. (E) The three-dimensional brain surface image showed the subdural electrodes (circles) placed over the left hemisphere especially on the left occipital region. The primary motor hand area (yellow circles) was confined to the postcentral gyrus. (F) The study of somatosensory evoked potentials (SEPs) for the median nerve revealed that electrode #15 on the postcentral gyrus had the largest N20 amplitude. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
Figure 2.
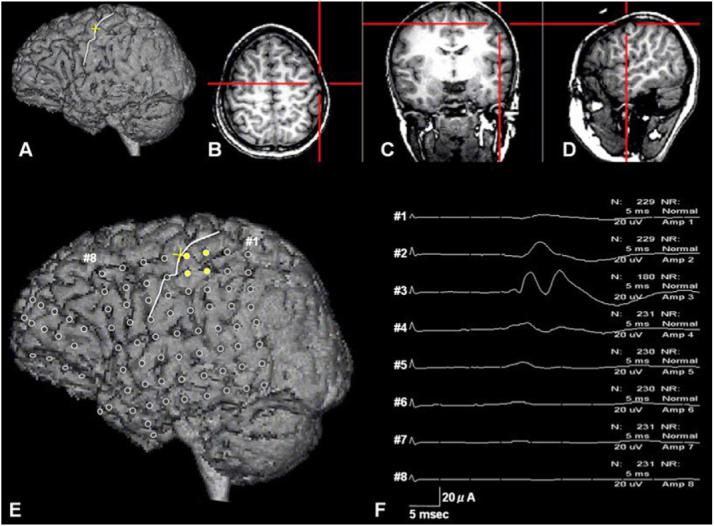
A 9-year-old girl with left temporal lobe epilepsy. The three-dimensional surface brain image (A) was visualized simultaneously in the horizontal (B), coronal (C) and sagittal planes (D). As the red cursor on the horizontal plane (B) was moved to the omega-shaped sulcus, the yellow cursor was moved to the sulcus (white line) on the three-dimensional surface brain image (A). This white line was defined as the central sulcus in this patient. (E) The three-dimensional brain surface image showed the subdural electrodes (circles) placed over the left hemisphere. The primary motor hand area (yellow circles) was confined to the postcentral gyrus. (F) The study of somatosensory evoked potentials (SEPs) for the median nerve revealed that electrode #3 on the postcentral gyrus had the largest N20 amplitude. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of the article.)
Somatosensory evoked potential (SEPs) protocol
As a component of the clinical management of the patients being evaluated for cortical resection to achieve seizure control, SEPs were recorded on chronically-implanted subdural electrodes, using a method similar to those described previously (Allison et al., 1989). A single patient with left temporal lobe epilepsy (Supplementary Table 1) failed to undergo the SEP study, due to malfunction of the equipment. The median nerve contralateral to the presumed epileptogenic foci was stimulated at the wrist with a frequency of 5.1 Hz, a square wave electric impulse of 200 ms, and a current intensity between 5 and 15 mA. Consistent twitching of the thenar muscle was documented throughout the testing. All recordings were performed using a filter band pass of 30−3000 Hz. An electrode either in the inferior temporal region or in the prefrontal region distant from the central lobule served as a reference. Somatosensory evoked responses were obtained using electrodes covering the premotor, precentral, postcentral, and anterior-parietal areas; 200−500 somatosensory evoked responses were averaged on referential and bipolar montages using the Nicolet Viking IV EMG/EP System (Nicolet Biomedical, Madison, WI, USA).
An N20 peak was defined as a reproducible peak between 18 and 22 ms and visually identified by the consensus of two investigators. The N20 amplitude was measured on each electrode, where the N20 amplitude was defined as the height between the highest peak of N20 and the preceding trough peak. The brain region underlying the electrode showing the largest N20 amplitude was identified to estimate the location of ‘the primary sensory area for the median nerve’.
Classification of the primary motor hand area
Based on the location of the primary motor hand area, each patient was classified into one of the following four groups (Supplementary Table 1; Fig. 3). (i) ‘No Motor Response Group’ included those who failed to show a hand motor response elicited by cortical stimulation, (ii) ‘Precentral Gyrus Group’ included those who had the primary motor hand area confined to the precentral gyrus, (iii) ‘Postcentral Gyrus Group’ included those who had the primary motor hand area confined to the postcentral gyrus, and (iv) ‘Pre- & Postcentral Gyri Group’ included those who had the primary motor hand area localized in both pre- and postcentral gyri. Those with an electrode on the central sulcus found to be a part of the primary motor hand area were classified into ‘Pre- & Postcentral Gyri Group’.
Figure 3.
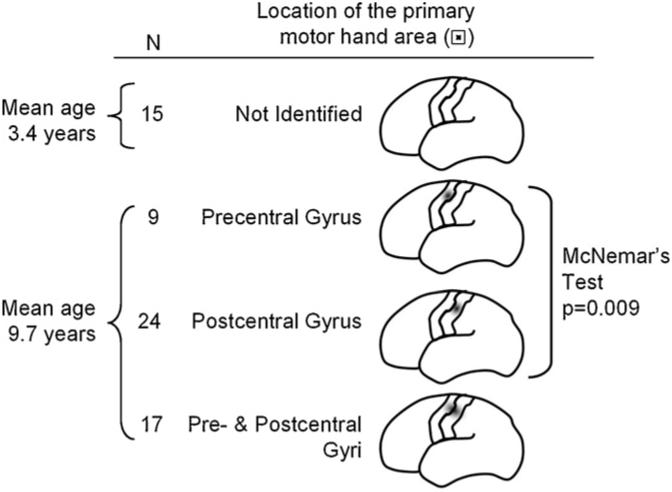
Summary of mapping results. Electrical stimulation failed to induce a contralateral hand movement in 15 patients, whose mean age was 3.4 years. On the other hand, a contralateral hand movement was induced by electrical stimulation in the remaining 50 patients, whose mean age was 9.7 years. The primary motor hand area was confined to the precentral gyrus in 9 patients (18%), confined to the postcentral gyrus in 24 patients (48%), and located to both the pre- and post-central gyri in the remaining 17 patients (34%). McNemar's test revealed that the observed frequency of 24 patients showing the primary motor hand area confined to the postcentral gyrus was significantly larger than chance frequency. It should be noted that the number of patients classified as ‘No Motor Response Group’ or ‘Pre- & Postcentral Gyri Group’ did not affect the test statistics.
Statistical analyses
All statistical analyses were performed using a computer software Statistical Analysis System 9.1 (SAS Institute Inc., Cary, NC). Initially, the unpaired t-test was applied to determine whether the mean age of ‘No Motor Response Group’ differed from that of the remaining aggregate consisting of ‘Precentral Gyrus Group’, ‘Postcentral Gyrus Group’ and ‘Pre- & Postcentral Gyri Group’.
Subsequently, McNemar's test (Woodward, 2005) was applied to a 2 × 2 contingency table (Supplementary Table 2), where each of the four cells contained the number of patients classified as ‘No Motor Response Group’, ‘Precentral Gyrus Group’, ‘Postcentral Gyrus Group’, and ‘Pre- & Postcentral Gyri Group’, respectively. We determined whether the observed frequency of patients classified as ‘Precentral Gyrus Group’ or ‘Postcentral Gyrus Group’ was different from random chance frequency. The statistical null hypothesis was that the number of patients classified as ‘Precentral Gyrus Group’ was same as that of ‘Postcentral Gyrus Group’. The alternative hypothesis was that the number of patients classified as ‘Precentral Gyrus Group’ was different from that of ‘Postcentral Gyrus Group’. It should be noted that the number of patients classified as ‘No Motor Response Group’ or ‘Pre- & Postcentral Gyri Group’ did not affect the test statistics.
Finally, logistic regression analysis was applied to the aggregate consisting of ‘Precentral Gyrus Group’ and ‘Postcentral Gyrus Group’, in order to determine whether (a) age, (b) the presence of dysplastic brain lesions (i.e., dysplasia, cortical tuber or periventricular heterotopia), or (c) seizure onset involving the frontal lobe were associated with the observation of ‘Postcentral Gyrus Group’.
Results
Primary motor hand area
Among the 65 patients, a contralateral hand movement was elicited without after discharges in 50 patients but not in the remaining 15 patients who were classified as ‘No Motor Response Group’ (Fig. 3). The mean age of ‘No Motor Response Group’ was 3.4 years, whereas the mean age of the remaining aggregate was 9.7 years. The unpaired t-test revealed that ‘No Motor Response Group’ was significantly younger than the remaining aggregate (p < 0.001; mean difference: 6.3 years; 95% Confidence interval: 4.2−8.4 years).
Among the 50 patients with a contralateral hand movement elicited by electrical stimulation, 9 patients (18%) were classified as ‘Precentral Gyrus Group’, 24 (48%) as ‘Postcentral Gyrus Group’, and 17 (34%) as ‘Pre- & Postcentral Gyri Group’. None of the patients had the primary motor hand area in the premotor or anterior parietal region. The McNemar's test revealed that the observed frequency of 24 patients classified as ‘Postcentral Gyrus Group’ was larger than random chance frequency (p = 0.009).
The logistic regression was applied to the 33 patients classified as either ‘Precentral Gyrus Group’ or ‘Postcentral Gyrus Group’. Neither the age, the presence of dysplastic lesion nor seizure onset involving the frontal lobe was significantly associated with the chance of a patient classified as ‘Postcentral Gyrus Group’ (age: p = 0.87; Dysplastic lesion: p = 0.72; seizure onset involving the frontal lobe: p = 0.96).
Somatosensory evoked potentials for the median nerve
The largest N20 amplitude was identified in the electrode overlying the postcentral gyrus in 54 patients (84%), the central sulcus in 8 patients (13%), and the precentral gyrus in 2 patients (3%) (Supplementary Table 1). None of the patients had the largest N20 peak outside the precentral−postcentral gyri. Among 49 patients who had a positive motor response and underwent the SEP study, 35 patients (71%) had the largest N20 amplitude in the electrode on the postcentral gyrus (Supplementary Table 1).
Discussion
The major findings in the present study can be summarized into four aspects below. (i) Younger age was associated with more frequent failure to identify the primary motor hand area using electrical stimulation. (ii) A substantial proportion of young patients with focal seizures had the primary motor hand area in the postcentral gyrus. (iii) Neither the age, the presence of dysplastic lesion nor the seizure onset involving the frontal lobe was significantly associated with the identification of the primary motor hand area in the post-central gyrus. (iv) The majority of patients had the primary sensory hand area for the median nerve localized on the postcentral gyrus or the bank of central sulcus.
Younger age associated with failure to elicit a hand motor response
The observation that cortical stimulation more frequently failed to elicit motor responses in younger children has been well described in human studies using electrical stimulation (Wyllie and Awad, 1991), human studies using transcranial magnetic stimulation (TMS) (Muller et al., 1991) as well as a cat study using electrical stimulation (Chakrabarty and Martin, 2000). Studies have shown that the motor threshold is negatively correlated with the age of subjects (Muller et al., 1991; Chitoku et al., 2001). It has been speculated that immature development of myelination in the cortico-spinal tract may be associated with failure to generate motor responses in young children (Muller et al., 1991; Wyllie and Awad, 1991). Thus, failure to elicit motor responses in young children by electrical stimulation does not necessarily indicate the absence of motor function on the brain region electrically stimulated (Wyllie and Awad, 1991; Chitoku et al., 2001).
Validity of the observation that the primary motor hand area is identified more frequently in the postcentral gyrus
The observation that the primary motor hand area was localized in the postcentral gyrus more often than random chance frequency is a novel finding in the present study and apparently inconsistent with the classic Penfield's homunculus (Penfield and Boldrey, 1937) serving as the practical guide to clinicians (Ropper and Brown, 2005). Our findings may not seem all that surprising considering that localization of hand motor function to the postcentral gyrus has been already indicated by previous studies of lesioning, electrical and magnetic stimulation, as well as studies of motor task-induced brain activation using functional MRI (fMRI), PET, magnetoencephalography (MEG) and intracranial EEG recording.
Evidence from lesion studies
Previous studies revealed that a lesion (Xerri et al., 1998) or cooling (Brinkman et al., 1985) confined to the presumed postcentral gyrus resulted in hemiparesis of the contralateral upper extremity in monkeys. A previous human study of epilepsy surgery in extratemporal lobe epilepsy suggested that surgical resection of the postcentral gyrus resulted in more pronounced deficits of the contralateral extremities compared to that after resection of the precentral gyrus (Polkey, 2000). Weakness of the contralateral hand associated with lesioning of the postcentral gyrus was attributed to loss of proprioception by the investigators (Polkey, 2000; Xerri et al., 1998).
Evidence from stimulation studies
Previous studies of electrical stimulation indicated that a subset of patients had the primary motor hand area in the presumed postcentral gyrus. In a classic study of 126 intraoperative cortical stimulation procedures (Penfield and Boldrey, 1937), for example, the investigators estimated the central sulcus according to the results of electrical stimulation and reported that 77 among the 102 finger motor responses were elicited by the stimulation of the presumed precentral gyrus, whereas the remaining 25 responses were elicited by the stimulation of the presumed postcentral gyrus. In a recent report of 33 patients studied using chronically implanted subdural electrodes (Nii et al., 1996), the investigators identified the central sulcus using the anterior commissure-posterior commissure line seen on sagittal MRI combined with the manually-traced outline of the skull seen on X-ray, and reported that the 32 of the 44 hand motor responses were elicited by the stimulation of the presumed precentral gyrus, whereas the remaining 12 responses were elicited by the stimulation of the presumed postcentral gyrus. An animal study demonstrated that contralateral hand movement could be elicited by electrical stimulation of the presumed postcentral gyrus in monkeys with Brodmann areas 4 and 6 ablated (Kennard and McCulloch, 1943).
TMS is a noninvasive technique used to stimulate the primary motor area via a brief magnetic pulse from a coil placed on the scalp. The spatial resolution of TMS is estimated to be approximately 1 cm within the primary motor hand area (Brasil-Neto et al., 1992; Pascual-Leone et al., 1994; Werhahn et al., 1994). It has been speculated that electrical stimulation methods directly stimulate the Betz cells in the motor cortex, whereas TMS methods have the Betz cells indirectly stimulated via interneuronal networks (Pascual-Leone et al., 1994). Previous TMS studies of healthy adult volunteers demonstrated that the TMS-induced current flowing across the central sulcus not in an ‘anterior-to-posterior’ but ‘posterior-to-anterior’ direction optimally activated the motor cortex (Brasil-Neto et al., 1992; Werhahn et al., 1994); this well-replicated observation seems to be consistent with the observation in the present study that stimulation of the postcentral gyrus preferably elicited a contralateral hand motor response in a substantial number of young patients with focal epilepsy.
Evidence from activation studies using fMRI, MEG and intracranial EEG
The observations in the present study using electrical stimulation are consistent with those reported in previous studies using fMRI (Yousry et al., 1997), MEG (Kristeva et al., 1991) and intracranial EEG (Crone et al., 1998). Previous neuroimaging studies using fMRI and [15O]-water PET demonstrated that motor tasks such as finger tapping or hand grasping consistently activated the contralateral post-central gyrus defined by anatomical landmarks, in addition to the precentral, premotor and supplementary motor areas (Yousry et al., 1997). Similarly, previous studies using MEG coregistered to MRI demonstrated that a large-amplitude motor task-evoked magnetic field was localized to the contralateral postcentral gyrus in healthy volunteers (Kristeva et al., 1991). A previous study of adults with focal epilepsy using intracranial EEG recording and each individual's brain surface image demonstrated that gamma-band EEG power was increased in both pre- and postcentral gyri about 200−500 ms after the onset of contralateral fist-clenching (Crone et al., 1998).
Evidence from anatomical studies
Do the previous anatomical studies validate the possible causal role of the postcentral gyrus in hand motor function? A study using the technique of retrograde labeling with horseradish peroxidase in monkeys showed that the precentral gyrus contained approximately 50% of the total corticospinal cells, whereas the postcentral gyrus contained approximately 20% of those cells (Toyoshima and Sakai, 1982). Another study using monkeys showed strong connections between the pre- and postcentral gyri (Jones and Powell, 1969). It is possible that electrical stimulation of the postcentral gyrus secondarily activated the precentral gyrus through such a cortico-cortical connection, although it is difficult to explain the results of the present study solely on the basis of cortico-cortical connections between the postcentral and precentral gyri.
No evidence of the differential effect of age on the location of the primary motor hand area
The present study failed to prove an association between age and the localization of the primary motor hand area in the postcentral gyrus. To our best knowledge, none of the previous studies of functional cortical mapping have indicated a possible differential effect of age on the location of the primary motor hand area. A study of MRI in neonates and infants showed that myelination in the pre- and postcentral gyri occur during a similar period which is earlier than that in the other frontal-parietal regions (Barkovich et al., 1988). Similarly, a study of glucose-metabolism using PET scans in children showed that local cerebral metabolic rates for glucose were equally high in the pre- and postcentral gyri in infants less than 5 weeks old and that glucose metabolism gradually increased in the other neocortical regions (Chugani et al., 1987). A study of diffusion tensor imaging (DTI) in premature newborns showed that fractional anisotropy levels, measurements of the directional diffusivity of water, were quite similar between the precentral and postcentral gyri and that fractional anisotropy levels in the pre and postcentral gyri were lower than those in the superior frontal or superior occipital gyri (Deipolyi et al., 2005). A study using a quantitative DTI fiber tracking technique in premature newborns failed to indicate a difference in the amount of subcortical fibers reaching the posterior limb of the internal capsule between the precentral and postcentral gyri (Berman et al., 2005).
Observations in the present study influenced by dysplastic lesion or seizure onset?
The present study failed to prove an association between localization of the primary motor hand area in the postcentral gyrus and the presence of dysplastic lesion or seizure onset zone in the frontal lobe. Previous human studies indicated that healthy individuals have the ipsilateral uncrossed motor pathway, which plays a less role in motor function, under normal circumstances, compared to the contralateral pyramidal motor pathway (Kobayashi et al., 2003). It has been reported that early brain insults or malformations which involve both pre- and postcentral gyri in one hemisphere often induce brain reorganization so that the contralateral sensory-motor cortex at least partially compensates for loss of motor function from the abnormal hemisphere (Cowan et al., 2003). It has also been reported that patients with epilepsia partialis continua may undergo interhemispheric reorganization of hand motor function (Stoeckel et al., 2002). Since, in the present study, we excluded patients with extensive brain malformations and status epilepsia partialis continua of the hand, the observa- tions of the present study probably cannot be accounted for by the effect of interhemispheric reorganization.
On the other hand, intrahemispheric reorganization of hand motor function from the precentral to postcentral gyrus was hypothesized and tested for in the present study. A previous study of adults with stroke using fMRI demonstrated that a patient with a small infarct limited to the precentral gyrus had the postcentral gyrus activated by finger tapping, whereas another patient with a small infarct in the post-central gyrus had the precentral gyrus activated by tactile stimulation of the finger (Cramer et al., 2000). A now classic study on monkeys with precentral gyrus ablation demonstrated that contralateral hand movement could be elicited by electrical stimulation of the presumed postcentral gyrus (Kennard and McCulloch, 1943). Yet, the logistic regression analysis in the present study failed to demonstrate that primary motor hand area confined to the postcentral gyrus was associated with the presence of congenital dys-plastic lesion or the seizure onset zone involving frontal lobe.
Correlation between SEP and cortical stimulation
The analysis of median nerve SEPs in the present study revealed that the largest N20 amplitude was identified in the electrode overlying the postcentral gyrus in 54 patients (84%), the central sulcus in 8 patients (13%), and the pre-central gyrus in 2 patients (3%). This finding indicated that ‘the primary sensory hand area’ estimated by N20 amplitude was mostly located in the postcentral gyrus in our subjects; this observation is quite consistent with observations from previous studies using intracranial recording (Allison et al., 1989) and MEG (Kawamura et al., 1997). These studies localized the generator of N20 peaks on the postcentral gyrus in the majority of patients, but on the precentral gyrus in only a small subset of patients (Allison et al., 1989; Kawamura et al., 1997). Consistency in the SEP findings between the present and previous studies indicates that subdural electrodes and the three-dimensional surface brain image were accurately coregistered, and also validates the method identifying the central sulcus using the anatomical landmarks. In the present study, furthermore, at least 70% of the patients showing a positive motor response on cortical stimulation exhibited an overlap of primary motor and sensory areas in the postcentral gyrus. A previous study of cortical stimulation has shown that such an overlap of motor and sensory functions in the postcentral gyrus may be seen in a subset of patients (Nii et al., 1996).
Future direction
In the present study, we analyzed hand movement as a whole, and not for each individual finger, partially because the intercontact distance of platinum grid electrodes was 10 mm, which is not small enough to assess movement in each finger. Usage of a different type of subdural electrodes with a smaller intercontact distance and a smaller disk diameter may further localize the primary motor areas for various body parts including the face and leg in more detail. Our observation that the primary hand motor cortex was localized to the postcentral gyrus more frequently than to the precentral gyrus needs to be replicated in a similar or different population including adults with and without epilepsy. Such studies may determine whether the primary hand motor cortex localized in the postcentral gyrus is an observation specific to children with focal seizures.
Acknowledgements
This work was supported in part by NIH grants NS47550 (to E.A.). We are grateful to Carol Pawlak, R. EEG/EP. T. and Ruth Roeder, R.N., M.S., and the staff of the Division of Electroneurodiagnostics at Children's Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above. We also appreciate Brenda Gillespie, Ph.D. in the School of Public Health at University of Michigan for her advice on the statistical analysis.
Appendix A. Supplementary dataSupplementary
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.eplepsyres. 2007.07.007.
Supplementary Material
References
- Allison T, McCarthy G, Wood CC, Darcey TM, Spencer DD, Williamson PD. Human cortical potentials evoked by stimulation of the median nerve. I. Cytoarchitectonic areas generating short-latency activity. J. Neurophysiol. 1989;62:694–710. doi: 10.1152/jn.1989.62.3.694. [DOI] [PubMed] [Google Scholar]
- Asano E, Juhasz C, Shah A, Muzik O, Chugani DC, Shah J, Sood S, Chugani HT. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia. 2005;46:1086–1097. doi: 10.1111/j.1528-1167.2005.05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkovich AJ, Kjos BO, Jackson DE, Jr., Norman D. Normal maturation of the neonatal and infant brain: MR imaging at 1.5 T. Radiology. 1988;166:173–180. doi: 10.1148/radiology.166.1.3336675. [DOI] [PubMed] [Google Scholar]
- Berger MS, Cohen WA, Ojemann GA. Correlation of motor cortex brain mapping data with magnetic resonance imaging. J. Neurosurg. 1990;72:383–387. doi: 10.3171/jns.1990.72.3.0383. [DOI] [PubMed] [Google Scholar]
- Berman JI, Mukherjee P, Partridge SC, Miller SP, Ferriero DM, Barkovich AJ, Vigneron DB, Henry RG. Quantitative diffusion tensor MRI fiber tractography of sensorimotor white matter development in premature infants. Neuroimage. 2005;27:862–871. doi: 10.1016/j.neuroimage.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, McShane LM, Fuhr P, Hallett M, Cohen LG. Topographic mapping of the human motor cortex with magnetic stimulation: factors affecting accuracy and reproducibility. Electroencephalogr. Clin. Neurophysiol. 1992;85:9–16. doi: 10.1016/0168-5597(92)90095-s. [DOI] [PubMed] [Google Scholar]
- Brinkman J, Colebatch JG, Porter R, York DH. Responses of precentral cells during cooling of post-central cortex in conscious monkeys. J. Physiol. 1985;368:611–625. doi: 10.1113/jphysiol.1985.sp015879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S, Martin JH. Postnatal development of the motor representation in primary motor cortex. J. Neurophysiol. 2000;84:2582–2594. doi: 10.1152/jn.2000.84.5.2582. [DOI] [PubMed] [Google Scholar]
- Chitoku S, Otsubo H, Harada Y, Jay V, Rutka JT, Weiss SK, Abdoll M, Snead OC., III Extraoperative cortical stimulation of motor function in children. Pediatr. Neurol. 2001;24:344–350. doi: 10.1016/s0887-8994(01)00264-8. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain functional development. Ann. Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Cowan F, Rutherford M, Groenendaal F, Eken P, Mercuri E, Bydder GM, Meiners LC, Dubowitz LM, de Vries LS. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet. 2003;361:736–742. doi: 10.1016/S0140-6736(03)12658-X. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Moore CI, Finklestein SP, Rosen BR. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31:668–671. doi: 10.1161/01.str.31.3.668. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Deipolyi AR, Mukherjee P, Gill K, Henry RG, Partridge SC, Veeraraghavan S, Jin H, Lu Y, Miller SP, Ferriero DM, Vigneron DB, Barkovich AJ. Comparing microstructural and macrostructural development of the cerebral cortex in premature newborns: diffusion tensor imaging versus cortical gyration. Neuroimage. 2005;27:579–586. doi: 10.1016/j.neuroimage.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Germann J, Robbins S, Halsband U, Petrides M. Pre-central sulcal complex of the human brain: morphology and statistical probability maps. J. Comp. Neurol. 2005;493:334–356. doi: 10.1002/cne.20820. [DOI] [PubMed] [Google Scholar]
- Girvin JP. Neurosurgical considerations and general methods for craniotomy under local anesthesia. Int. Anesthesiol. Clin. 1986;24:89–114. doi: 10.1097/00004311-198602430-00010. [DOI] [PubMed] [Google Scholar]
- Gordon B, Lesser RP, Rance NE, Hart J, Jr., Webber R, Uematsu S, Fisher RS. Parameters for direct cortical electrical stimulation in the human: histopathologic confirmation. Electroencephalogr. Clin. Neurophysiol. 1990;75:371–377. doi: 10.1016/0013-4694(90)90082-u. [DOI] [PubMed] [Google Scholar]
- Jayakar P, Lesser RP. Extraoperative methods. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. Lippincott-Raven; Philadelphia: 1997. pp. 1785–1793. [Google Scholar]
- Jones EG, Powell TP. Connexions of the somatic sensory cortex of the rhesus monkey. I. Ipsilateral cortical connexions. Brain. 1969;92:477–502. doi: 10.1093/brain/92.3.477. [DOI] [PubMed] [Google Scholar]
- Juhasz C, Chugani DC, Muzik O, Watson C, Shah J, Shah A, Chugani HT. Is epileptogenic cortex truly hypometabolic on interictal positron emission tomography? Ann. Neurol. 2000;48:88–96. [PubMed] [Google Scholar]
- Kawamura T, Nakasato N, Seki K, Kanno A, Fujita S, Fujiwara S, Yoshimoto T. Neuromagnetic evidence of pre- and post-central cortical sources of somatosensory evoked responses. Electroencephalogr. Clin. Neurophysiol. 1997;104:101–102. doi: 10.1016/0168-5597(95)00217-0. [DOI] [PubMed] [Google Scholar]
- Kennard MA, McCulloch WS. Motor respnses to timulation of ceebral cortex in the absence of areas 4 and 6 (Macaca mulatta) J. Neurophysiol. 1943;6:181–189. [Google Scholar]
- Kobayashi M, Hutchinson S, Schlaug G, Pascual-Leone A. Ipsilateral motor cortex activation on functional magnetic resonance imaging during unilateral hand movements is related to interhemispheric interactions. Neuroimage. 2003;20:2259–2270. doi: 10.1016/s1053-8119(03)00220-9. [DOI] [PubMed] [Google Scholar]
- Kristeva R, Cheyne D, Deecke L. Neuromagnetic fields accompanying unilateral and bilateral voluntary movements: topography and analysis of cortical sources. Electroencephalogr. Clin. Neurophysiol. 1991;81:284–298. doi: 10.1016/0168-5597(91)90015-p. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Duffau H, Cornu P, Capelle L, Pidoux B, Carpen-tier A, Auliac S, Clemenceau S, Sichez JP, Bitar A, Valery CA, van Effenterre R, Faillot T, Srour A, Fohanno D, Philippon J, Le Bihan D, Marsault C. Correspondence between functional magnetic resonance imaging somatotopy and individual brain anatomy of the central region: comparison with intraoperative stimulation in patients with brain tumors. J. Neurosurg. 2000;92:589–598. doi: 10.3171/jns.2000.92.4.0589. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Gordon B. Methodologic considerations in cortical. Electrical stimulation in adults. In: Lüders HO, Noachtar S, editors. Epileptic Seizures: Pathophysiology and Clinical Semi-ology. Churchill Livingstone; New York: 2000. pp. 153–165. [Google Scholar]
- Makela JP, Kirveskari E, Seppa M, Hamalainen M, Forss N, Avikainen S, Salonen O, Salenius S, Kovala T, Randell T, Jaaskelainen J, Hari R. Three-dimensional integration of brain anatomy and function to facilitate intraoperative navigation around the sensorimotor strip. Hum. Brain Mapp. 2001;12:180–192. doi: 10.1002/1097-0193(200103)12:3<180::AID-HBM1014>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K, Homberg V, Lenard HG. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalogr. Clin. Neurophysiol. 1991;81:63–70. doi: 10.1016/0168-5597(91)90105-7. [DOI] [PubMed] [Google Scholar]
- Nii Y, Uematsu S, Lesser RP, Gordon B. Does the central sulcus divide motor and sensory functions? Cortical mapping of human hand areas as revealed by electrical stimulation through subdural grid electrodes. Neurology. 1996;46:360–367. doi: 10.1212/wnl.46.2.360. [DOI] [PubMed] [Google Scholar]
- Ojemann GA, Sutherling WW, Lesser RP, Dinner DS, Jayakar P, Saint-Hilaire JM. Cortical stimulation. In: Engel J Jr., editor. Surgical Treatment of the Epilepsies. second ed Raven Press; New York: 1993. pp. 399–414. [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- Polkey CE. Physical complications of epilepsy surgery. In: Oxbury J, Polkey C, Duchowny M, editors. Intractable Focal Epilepsy. W.B. Saunders; London: 2000. pp. 784–794. [Google Scholar]
- Ropper AH, Brown RH. Motor paralysis. In: Ropper AH, Brown RH, editors. Adams and Victor's Principles of Neurology. eighth ed McGraw-Hill; New York: 2005. pp. 39–54. [Google Scholar]
- Stoeckel MC, Kleinschmidt A, Ebner A, Witte OW, Seitz RJ. Reorganization of motor representation in a patient with epilepsia partialis continua as shown by [O15]-labeled butanol positron emission tomography and functional magnetic resonance imaging. J. Neuroimaging. 2002;12:276–281. [PubMed] [Google Scholar]
- Sunaert S. Presurgical planning for tumor resectioning. J. Magn. Reson. Imaging. 2006;23:887–905. doi: 10.1002/jmri.20582. [DOI] [PubMed] [Google Scholar]
- Toyoshima K, Sakai H. Exact cortical extent of the origin of the corticospinal tract (CST) and the quantitative contribution to the CST in different cytoarchitectonic areas. A study with horseradish peroxidase in the monkey. J. Hirnforsch. 1982;23:257–269. [PubMed] [Google Scholar]
- Uematsu S, Lesser R, Fisher RS, Gordon B, Hara K, Krauss GL, Vining EP, Webber RW. Motor and sensory cortex in humans: topography studied with chronic subdural stimulation. Neurosurgery. 1992;31:59–71. doi: 10.1227/00006123-199207000-00009. [DOI] [PubMed] [Google Scholar]
- von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U. A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage. 1997;5:S514. Abstract. [Google Scholar]
- Werhahn KJ, Fong JK, Meyer BU, Priori A, Rothwell JC, Day BL, Thompson PD. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr. Clin. Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Woodward M. Epidemiology: Study Design and Data Analysis (Texts in Statistical Science) second ed Chapman and Hall/CRC; Washington, D.C.: 2005. [Google Scholar]
- Wyllie E, Awad I. Invasive neurophysiologic techniques in the evaluation for epilepsy surgery in children. In: Luders HO, editor. Epilepsy Surgery. Raven Press; New York: 1991. pp. 409–412. [Google Scholar]
- Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J. Neurophysiol. 1998;79:2119–2148. doi: 10.1152/jn.1998.79.4.2119. [DOI] [PubMed] [Google Scholar]
- Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


