Abstract
Exposure to trichloroethylene (TCE, an environmental toxicant) reduced oocyte fertilizability in the rat. In vivo, TCE may be metabolized by cytochrome P450 dependent oxidation or glutathione conjugation in the liver or kidneys, respectively. Cytochrome P450 dependent oxidation is the higher affinity pathway. The primary isoform of cytochrome P450 to metabolize TCE in the liver, cytochrome P450 2E1, is present in the rodent ovary. Ovarian metabolism of TCE by the oxidative pathway and the production of reactive oxygen species may occur given the presence of the metabolizing enzyme. The objectives of this study were to define the sensitive interval of oocyte growth to TCE exposure, and to determine if TCE exposure resulted in the formation of ovarian protein carbonyls, an indicator of oxidative damage. Rats were exposed to TCE in drinking water (0.45% TCE (v/v) in 3% Tween) or 3% Tween (vehicle-control) during three 4–5 day intervals of oocyte development preceding ovulation. Oocytes from TCE-exposed females were less fertilizable compared with vehicle-control oocytes. Immunohistochemical labeling of ovaries and Western blotting of ovarian proteins demonstrated TCE treatment induced a greater incidence of protein carbonyls compared with vehicle controls. Protein carbonyl formation in the ovary is consistent with TCE metabolism by the cytochrome P450 pathway. Oxidative damage following ovarian TCE metabolism or the presence of TCE metabolites may contribute to reduced oocyte fertilizability. In summary, these results indicate maturing oocytes are susceptible to very short in vivo exposures to TCE.
Keywords: Ovary, In vitro fertilization, Oocyte, Trichloroethylene metabolism
INTRODUCTION
Trichloroethylene (TCE), a chlorinated hydrocarbon, is primarily used as a solvent for degreasing metal parts; approximately 85% of the TCE produced in the United States is used for cleaning metal [1]. Although TCE is not naturally found in the environment, it is an environmental toxicant due to production, use, and disposal. Trichloroethylene is detectable in underground water sources, surface water, and the air. Degradation rates for TCE vary in the environment based on the physical state of TCE, and the half-life of TCE in groundwater ranges from 10.7 months to 4.5 years depending upon the concentration of TCE [2]. Volatilization of TCE from surface water produces TCE vapor in the air, which has a half-life of approximately 7 days [3]. Some removal of TCE from the air also occurs with precipitation because of the moderate solubility of TCE in water, 1.1 g/L [4]. Trichloroethylene has been detected in the air throughout the United States with levels of TCE being about 3 times higher in urban areas compared with rural areas. Analysis of measurements taken in 1998 from 115 monitors in 14 different states indicated TCE levels in the air ranged between 0.01 μg/m3 and 3.90 μg/m3 with a mean of 0.88 μg/m3 [4].
Trichloroethylene is most frequently detected at high levels in persons exposed to TCE through occupational degreasing operations, silk screening, taxidermy, and electronic cleaning. The general population is likely to have low levels of exposure to TCE via the environment through inhalation of ambient air, ingestion of drinking water, and/or transdermal absorption. Commercial products such as wood stains, varnishes, finishes, lubricants, adhesives, typewriter correction fluid, paint removers, and cleaners that contain TCE may also contribute to exposure of the general population at a low to moderate level.
Studies in rats and mice have substantiated the adverse effects of TCE on male reproduction at the gamete level [5, 6]. There is some conflict as to whether the effects are specific to reproduction or a result of general systemic toxicity due to the high levels of TCE typically used in toxicology studies [7]. In studies where no significant decreases in body weight were observed, it has been suggested that an effect on reproduction is not confounded by systemic toxicity [7]. In a study published in 2003 by DuTeaux et al., male rats received 0.205 –0.293 g TCE/kg body weight/day via drinking water in the 0.2% TCE-treatment group, and 0.410 – 0.585 g TCE/kg body weight/day via drinking water in the 0.4% TCE-treatment group [5, 8]. There was no effect on final body weights as a result of TCE-treatment [5], suggesting systemic toxicity did not confound reproductive parameters. Histological changes observed in the efferent ductule epithelium of male rats exposed to TCE, oxidized proteins found in the head and midpiece of sperm, and the decreased ability of sperm to fertilize untreated oocytes were attributed to TCE metabolites formed by the cytochrome P450 dependent oxidative pathway within the efferent ducts [5]. In mice, TCE exposure leads to impairment of sperm fertilizing ability [6]. Trichloroethylene-treated mice and control mice had no significant differences in body weight, which suggests a lack of confounding effects on reproduction from systemic toxicity. Thus, there is some evidence suggesting TCE exposure may affect male gamete quality.
Decreased oocyte fertilizability, apparently independent of hormones, ovulatory concerns and weight, occurred in female rats after in vivo exposure to TCE [9]. In vitro exposure of mouse oocytes to three metabolites of TCE, trichloroactetic acid, dichloroacetic acid, and trichloroethanol, but not to TCE itself also reduced fertilization rates [10]. Taken together, these observations suggest metabolites of TCE produced in the ovary or elsewhere are responsible for the effects on oocytes.
Trichloroethylene metabolism occurs primarily in the liver and kidneys, although it may occur in other tissues. The two known pathways responsible for TCE metabolism are oxidation by cytochrome P450 and conjugation with glutathione [11]. The oxidative pathway is the higher affinity pathway [12], and metabolizes most of the TCE when pathway elements are present or until the pathway is saturated. Enzymes involved in both metabolic pathways are present in the ovary [13–15] which is consistent with the possibility that the ovary participates in TCE metabolism and production of bioactive TCE metabolites. The purpose of this study was to determine if there is a specific interval of sensitivity to TCE during the two weeks of oocyte growth preceding ovulation and to determine if protein oxidation was a potential mechanism for altered oocyte fertilizability.
MATERIALS AND METHODS
Animals
Simonson albino rats, a Sprague-Dawley derived strain, were used in this study. Female rats between 28–45 days old, and male rats approximately 100 days old, came from the Department of Animal Science breeding colony at the University of California, Davis. All animals were housed under a 14L: 10D light cycle in a temperature (70 ± 2°F) and humidity (40–70%) controlled facility. Rats had ad libitum access to Purina Formulab 5008 rat chow (St. Louis, MO, USA). Between weaning (21 days of age) and treatment with TCE, rats had ad libitum access to deionized water. The University of California, Davis Animal Use and Care Administrative Advisory Committee approved all animal use.
Chemicals and Antibodies
Trichloroethylene (ACS reagent ≥ 99.5%), 2,4-dinitrophenylhydrazine ((DNPH), reagent grade 97%), and rabbit anti-dinitrophenyl (DNP) antibody were purchased from Sigma-Aldrich (St. Louis, MO, USA). Media components for in vitro fertilization (IVF) were cell-culture tested or molecular biology grade and obtained from Fisher Scientific (Pittsburgh, PA, USA) or Sigma-Aldrich. Water was obtained from a Milli-Q Synthesis system (Millipore, Bedford, MA, USA). Chorionic gonadotropin was obtained from Intervet, Inc. (Millsboro, DE, USA). Pregnant mare serum gonadotropin was obtained from Sioux Biochemical, Inc. (Sioux Center, IA, USA). Trifluoroacetic acid and a bicinchoninic acid (BCA) protein assay was purchased from Pierce Biotechnology, Inc. (Rockford, IL, USA). Citrisolv was acquired from Fisher Scientific. Antigen Unmasking Solution, Vector Blue Alkaline Phosphatase Substrate Kit III, and Vectastain ABC-AP kit were obtained from Vector Laboratories (Burlingame, CA, USA). Cover Safe mounting medium was acquired from American Master Tech Scientific, Inc. (Lodi, CA, USA). The secondary antibody, peroxidase-conjugated donkey anti-rabbit IgG was purchased from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Western Lightning Western Blot Chemiluminescence Reagent Plus was obtained from Perkin Elmer Life Sciences, Inc. (Wellesley, MA, USA).
Chemical Treatment
Drinking water was made with water from a Milli-Q Synthesis system and contained 0.45% TCE (v/v) in 3% Tween for TCE-treated rats, and 3% Tween for vehicle-control rats [9]. Tween was used as a vehicle to dissolve TCE in drinking water. Each female in the TCE-exposure group received an estimated 0.66 g TCE/kg body weight/day based on the average water consumption of 10 mL/100 g body weight [8]. A computer simulation model suggests this is a slightly higher effective concentration of TCE than the previously reported highest industrial concentration [16, 17] and 2-week exposure to this level of TCE is known to reduce oocyte fertilizability [9]. Female rats were exposed to TCE or to vehicle for 4, 5, or 14 days for IVF and immunohistochemistry trials. Rats received TCE-water on days 1 to 5, days 6 to 10, or days 11 to 14 of the 2-week interval preceding ovulation (oocytes collected the morning of day 15). During the 2-week period, when females were not receiving drinking water containing TCE (days 6 to 14, days 1 to 5 and 11 to 14, and days 1 to 10), they received water containing Tween (vehicle). Females providing tissue for Western blot analysis received 0.45% TCE (v/v) in 3% Tween or vehicle alone for 5 days similar to the day 11–14 treatment without the additional exposure to Tween. Ovaries recovered from females exposed to TCE for the two or three previous days provided tissue for microsome isolation. Drinking water was freshly prepared for each treatment group, stored in tightly capped bottles, and protected from light exposure. Glass water bottles, fit with rubber and stainless steel stoppers, were refilled every 24–48 hours to minimize headspace and volatilization of TCE.
Gamete Retrieval and In Vitro Fertilization
Gamete retrieval and IVF were performed as previously described [9, 18]. Briefly, one male rat, 100 days or older, was used as a semen donor for all females in a replicate of IVF. The male was sacrificed by CO2 asphyxiation followed by cervical dislocation in a warm room (30–32°C) on the morning the oocytes were collected from females. Cauda epididymides and the attached vas deferens were dissected using sterile surgical instruments. Each epididymis and vas deferens was rinsed in warm fertilization media (94.19 mM NaCl, 4.75 mM KCl, 1.71 mM CaCl2, 1.17 mM KH2PO4, 1.17 mM MgSO4, 25.1 mM NaHCO3, 26 mM Na lactate, 0.5 mM Na pyruvate, 5.5 mM glucose, 50 μg gentamycin sulfate/ml, 4 mg bovine serum albumin (BSA)/ml, with 20 mM [4-(2-hydroxyethyl)-1-piperazine]ethanesulfonic acid (HEPES)) to remove fat and hair. Sperm were flushed retrogradely through a vertical incision in the epididymis with a 23 gauge needle inserted into the vas deferens. Collected spermatozoa were evaluated with phase contrast optics. One ml of 7 × 106 sperm/ml was prepared in fertilization media and allowed to capacitate in a 37°C, 5% CO2 incubator for 3 hours.
Female rats in each replicate were induced to ovulate by intraperitoneal (i.p.) injection of pregnant mare serum gonadotropin (PMSG, 15 IU) on day 12, followed 48 hours later by an i.p injection of human chorionic gonadotropin (hCG, 15 IU) [9, 19]. Female rats were sacrificed by CO2 asphyxiation followed by cervical dislocation 16 to 18 hours after receiving hCG. Oviducts were removed and cumulus masses were released into warm saline – BSA (0.9% NaCl, 1 mg BSA/ml). Cumulus masses and zonae pellucidae were removed with hyaluronidase (1 mg/ml saline – BSA) and acid Tyrode’s [20], respectively and oocytes were rinsed with saline – BSA. Oocytes were then grouped in clusters of 10 and placed in 100 μl drops of pre-equilibrated fertilization media under water-saturated dimethylpolysiloxane (DMPS; Sigma-Aldrich). Each group of oocytes was incubated with 10 μl of 7×106 capacitated rat sperm/ml.
After 20 hours of incubation at 37°C in 5% CO2 in air, oocytes were rinsed through 3 drops of fertilization media to remove loosely bound sperm. Oocytes and associated sperm were then stained with Hoescht 33342 (Sigma-Aldrich, 0.08 mg/ml fertilization media) for 10 minutes. Oocytes were rinsed with fertilization media, placed on slides, compressed with a coverslip resting on vaseline-paraffin, and sealed with nail polish. Oocytes were examined by fluorescence microscopy (400× magnification), and the percentage of oocytes fertilized (decondensed sperm heads) and the percentage of oocytes binding sperm were calculated for each treatment in each replicate.
Tissue Preparation
Ovaries were collected from females that received TCE on days 1 to 5, days 6 to 10, and days 11 to 14 on the morning of day 15 along with ovaries from females that received vehicle water and TCE water for 2 weeks. Tissue was fixed with 4% paraformaldehyde in phosphate buffered saline (PBS). Ovaries were rinsed in PBS then dehydrated with 30%, 50%, and 70% ethanol solutions prior to paraffin embedding (Tissue-Tek VIP5, Sakura Finetek USA, Torrance, CA). Paraffin-embedded ovarian tissue was cut in 5 μm sections and allowed to dry on glass slides.
Immunochemical Detection of Oxidized Protein in the Rat Ovary
Ovarian tissue sections were deparaffinized with Citrisolv and rehydrated in graded ethanols. Subsequently, heat-induced antigen retrieval was performed using Antigen Unmasking Solution. The tissue was rehydrated in PBS (pH 7.4) and underwent an endogenous peroxidase quench with 0.3% H2O2 in methanol. Protein carbonyls were derivatized using a solution of 5 mM 2,4-DNPH in PBS (pH 6.3) for 45 minutes [5]. Ovarian sections were then rinsed 4 times with PBS, treated with blocking solution (Vectastain ABC-AP kit) for 20 minutes, and incubated with rabbit anti-DNP antibody (1:800 in PBS containing 2% normal goat serum). Additional sections of tissue from the same ovaries were incubated with normal rabbit serum (1:800 in PBS containing 2% normal goat serum) to serve as controls for the primary antibody. Following four rinses with PBS, ovarian sections were incubated with biotinylated secondary antibody (Vectastain ABC-AP kit) for 30 minutes. Sections were rinsed with PBS, then incubated with alkaline phosphatase substrate solution (Vectastain ABC-AP kit), and rinsed with PBS again. All slides were coverslipped with Cover Safe mounting medium. Ovarian sections were examined for evidence of protein carbonyls using an Olympus BH-2 trinocular phase contrast microscope (Olympus America, Inc., Melville, NY, USA) and images were taken using a QImaging MicroPublisher 3.3 RTV camera and QCapture Pro software (Burnaby, BC, Canada).
Protein oxidation was quantitatively assessed using Western blots. Ovaries from rats treated with TCE for 5 days were collected, weighed, and immediately immersed in 1 ml of 0.1 M phosphate buffer (pH 7.0) on ice. Tissue was homogenized using a glass tube and Teflon pestle for approximately 2 minutes until the tissue was completely ground. The suspension was centrifuged at 10,000 × g in a Fisher Scientific Micro-Centrifuge Model 59A for 20 minutes. The supernate was collected and stored at −20°C until use. Protein concentration was assessed using the BCA protein assay. Protein carbonyls were derivatized by incubating 25 μg of ovarian protein with 10 mM 2,4-DNPH in 10% trifluoroacetic acid for 15 minutes [21]. Nonderivatized aliquots from each sample were incubated with 10% trifluoroacetic acid to serve as negative controls. After 15 minutes, all solutions were neutralized with 2 M Tris in 30% glycerol [21]. Derivatized and non-derivatized ovarian protein samples were solubilized with 0.9% SDS (w/v), separated on a 12% SDS-polyacrylamide gel, and electroblotted to 0.45μm polyvinylidene fluoride (PVDF) membrane with a wet transfer tank (Hoefer, Inc, San Francisco, CA, USA). Membranes were blocked with 1% BSA, 1% normal donkey serum in PBS-T (PBS containing 0.5% Tween-20, pH 7.4). Subsequently, membranes were incubated with rabbit anti-DNP antibody (1:24,000 in PBS-T containing 1% normal donkey serum). After rinsing with PBS-T, membranes were incubated with peroxidase-conjugated donkey anti-rabbit IgG (1:30,000 in PBS-T containing 1% normal donkey serum). Immunoreactive bands were visualized using chemiluminescence reagent and x-ray film.
Cytochrome P4502E1 activity
Microsomes were prepared from rat ovaries previously frozen in 1 ml 0.1 M phosphate buffer (pH 7.0) as described by Forkert et al. [22] except isolation buffer contained 0.1 mM AEBSF protease inhibitor. Activity of p-nitrophenol hydroxylase was used as an assay of cytochrome P4502E1 activity [22, 23]. In vitro inhibition was assessed following a 60 minute incubation with 5 mM TCE or buffer alone [24]. Ovarian microsomes were prepared from ovaries from rats previously treated with TCE for 2 or 3 days and their respective vehicle controls and analyzed for p-nitrophenol hydroxylase activity.
Experimental Design and Data Analysis
Effects on oocyte fertilizability were assessed by IVF with each interval of exposure evaluated in three separate replicates. Each replicate consisted of six or seven female rats and one untreated male rat. Two females were treated with vehicle water for 2 weeks to serve as negative controls, two or three females were treated with TCE water for 4 or 5 days (days 1–5, days 6–10, or days 11–14) to examine potential critical intervals of exposure, and two females were treated with TCE water for 2 weeks to serve as positive controls. In each replicate, oocytes from females that received the same treatment were pooled. The number of females that ovulated was recorded for each treatment in each replicate. Immunohistochemical localization of protein carbonyls within the ovary were evaluated in three replicates. Each replicate included 1 female per treatment group, for a total of 5 females per replicate; treatments included TCE water on days 1–5, TCE water on days 6–10, TCE water on days 11–14, plus the negative control (vehicle water for 2 weeks) and the positive control (TCE water for 2 weeks). Protein carbonyl formation following TCE exposure was quantitatively assessed on Western blots. Ovaries were recovered and weighed prior to homogenization. Eight females were used in four replicates that consisted of ovaries from one rat treated with TCE for 5 days and one vehicle-control littermate.
In vitro fertilization data, ovarian weight data, and p-nitrophenyl hydroxylase activity were subjected to analysis of variance (ANOVA) using SAS (SAS Statistical Programs, Cary, NC, USA). Treatment was a fixed factor, pooled females within a treatment and replicate were random. The percentage of oocytes fertilized and percentage of oocytes with bound sperm were weighted by the number of oocytes evaluated. The percentage of females that ovulated was analyzed before and after transformation (arc sine square root) to improve normality. P values associated with the transformed data are presented.
RESULTS
Effect of Female Exposure to Trichloroethylene on Oocyte Fertilizability
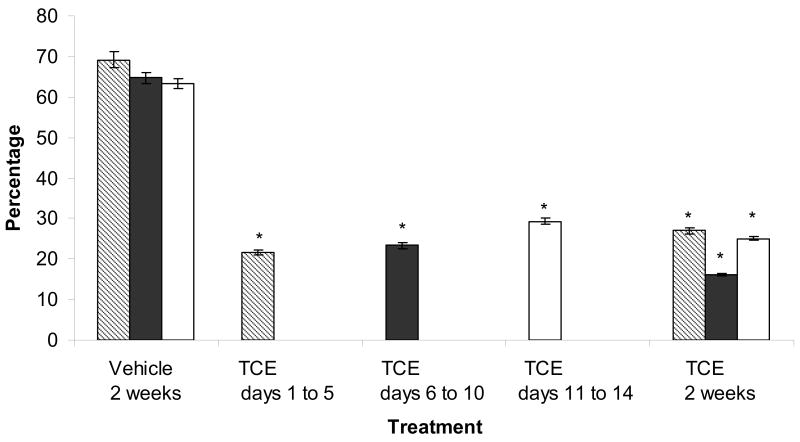
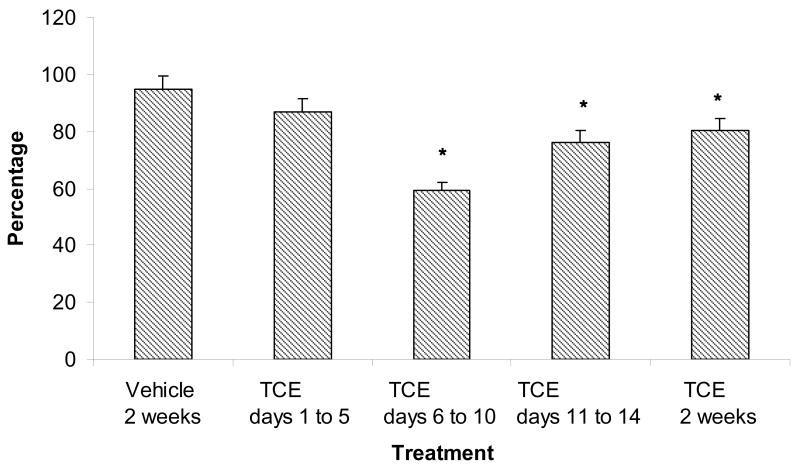
In vivo TCE exposure of females reduced the fertilizability of zona-free oocytes regardless of the interval of TCE exposure. Oocytes from rats exposed to TCE for 2 weeks were consistently less fertilizable than oocytes from vehicle-control females (23% vs. 66%, respectively, P<0.05, Fig. 1A). Females that were treated for shorter intervals within the 2-week period had fertilization rates similar to oocytes from females that received TCE for 2 weeks; 21.7%, 23.3%, and 29.3% for oocytes from females exposed to TCE on days 1 to 5, days 6 to 10, and days 11 to 14, respectively. These oocytes were significantly less fertilizable than oocytes from vehicle-control females, but not different from oocytes from females exposed to TCE for 2 weeks. Oocytes from females exposed to TCE on days 6 to 10, days 11 to 14, and for 2 weeks were also less able to bind sperm compared with oocytes from vehicle controls (P<0.05, Fig. 1B).
Figure 1.
(A) Decreased fertilizability of oocytes from TCE-exposed (0.45% TCE (v/v) in 3% Tween) rats compared with oocytes from vehicle-control (3% Tween) rats. Fertilization was assessed by the presence of decondensed sperm heads. Values represent the least squares means from three replicates. * indicates P<0.05 compared with the vehicle control. (B) Oocytes with bound sperm. Bars represent the percentage of total oocytes (fertilized and unfertilized) that bound sperm. Oocytes from females treated with TCE on days 6 to 10, days 11 to 14 and for 2 weeks bound fewer sperm compared with vehicle-controls. * indicates P<0.05 compared with the vehicle control.
TCE Exposure, Ovulation, and the Ovary
TCE water and vehicle water removal from the drinking water bottles (an estimate of consumption) were consistent with reported average consumption for rats of this size [8]. Liver weights did not differ significantly after 3 days of exposure (P>0.10); 5.68 grams vs. 5.18 grams for TCE treatment and vehicle controls, respectively. The percentage of females responding to hormonal induction of ovulation was not altered by exposure to TCE (P>0.10, SEM=15, Table 1). On the rare occasion, females that did not ovulate showed evidence of previous ovulation (corpora lutea), which may account for the lack of cumulus masses recovered from the oviducts of those females. The mean ovarian weights were similar for TCE-treated females and vehicle controls (0.062 grams compared with 0.071 grams, P>0.25, SEM=0.008). Thus, TCE-exposure did not affect ovarian development.
Table 1.
Reproductive parameters of female rats after exposure to trichloroethylene
| Vehicle 2 weeksa | TCE days 1 to 5 | TCE days 6 to 10 | TCE days 11 to 14 | TCE 2 weeksa | |
|---|---|---|---|---|---|
| Total number of females | 18 | 8 | 8 | 8 | 18 |
| Percentage of total females ovulating | 78 | 87.5b | 100b | 100b | 78b |
A positive and negative control were used for each IVF replicate (9 total replicates).
Percentage of females ovulating in each TCE-treatment group are not significantly different compared with vehicle control (P>0.10, SEM=15).
Evidence of TCE Metabolism in the Ovaries of Treated Females
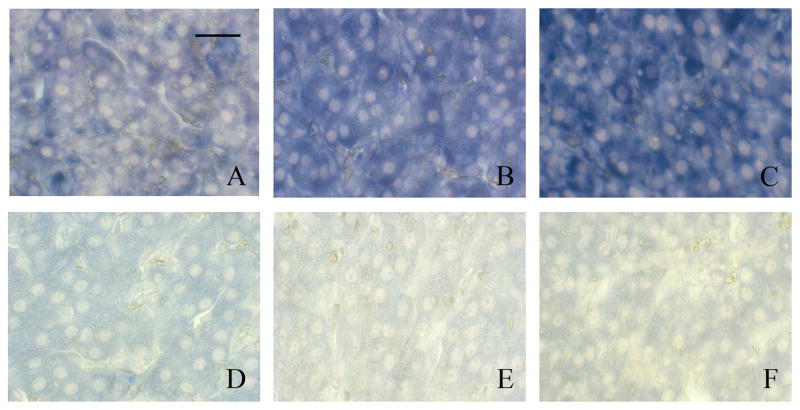
The ovarian tissue from TCE-exposed and vehicle-treated rats did not exhibit any gross histological changes following treatment. However, increased protein carbonyls (an indication of oxidatively modified proteins) were detected immunohistochemically in the ovaries of females exposed to TCE water for 2 weeks compared with females that received vehicle water for 2 weeks (Fig. 2). Granulosa cells from females that received TCE had noticeably more labeling for protein carbonyls (Fig. 2A) compared with granulosa cells in ovaries from vehicle-control rats (Fig. 2B). The immunolabeling negative controls, sections of ovary from TCE-exposed rats and vehicle-control rats that were incubated with normal rabbit serum instead of rabbit anti-DNP antibody, were comparable with no detectable protein carbonyls (Fig. 2C, Fig. 2D).
Figure 2.
Immunohistochemical labeling of protein carbonyls following TCE exposure in rat ovarian tissue. Bar represents 50 μm. (A) Ovarian tissue from a female given drinking water containing 0.45% TCE (v/v) in 3% Tween for 2 weeks. Granulosa cells are prominently labeled with blue substrate indicating the presence of protein carbonyls. Oocytes are not labeled. (B) Ovarian tissue from a female that received 3% Tween vehicle in drinking water for 2 weeks. Granulosa cells and oocytes show limited and no labeling, respectively. Ovarian tissues from the same females (C) and (D) incubated with normal rabbit serum in place of the primary antibody (rabbit anti-DNP). Neither tissues have positive signal indicating negligible background labeling. Pictures are representative of 3 replicates.
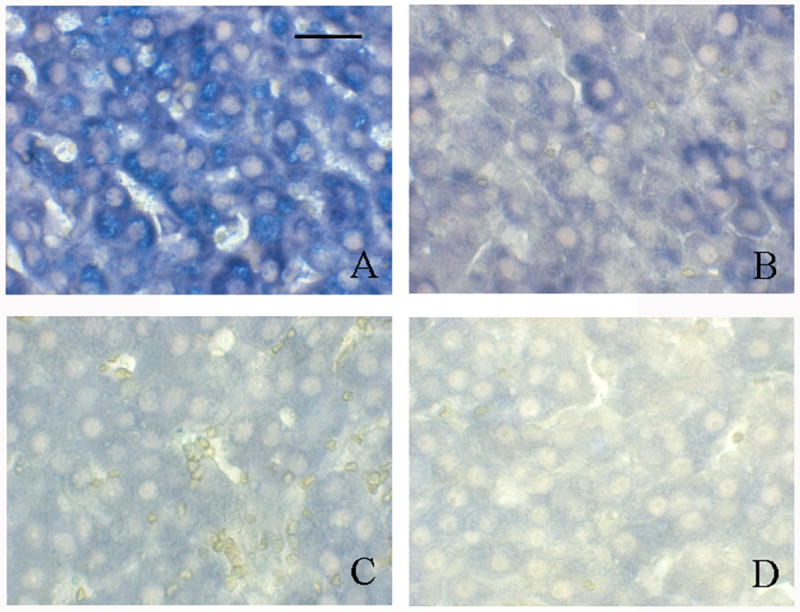
Protein carbonyls were present in all ovarian tissues exposed to TCE for 4 to 5 days within the two week treatment interval. The granulosa cells were positive for immunolabeling of protein carbonyls and the oocytes appeared unlabeled. Although immunohistochemistry is not quantitative, comparatively small amounts of oxidation product were visible in the granulosa cells on day 15 following exposure of females to TCE on days 1 to 5 of the 2-week treatment interval (Fig. 3A). More intense labeling of protein carbonyls was visible on day 15 following TCE exposure on days 6 to 10 (Fig. 3B), and the most oxidation product was present following treatment with TCE on days 11 to 14 (Fig. 3C). Hence, less intense labeling of protein carbonyls were observed in tissues given a recovery period between treatment and histological examination. Ovarian tissue from females exposed to TCE water on days 1 to 5 and 6 to 10 were less intensely labeled for protein carbonyls compared with ovarian tissue from females that received TCE water for 2 weeks (Fig. 3A, 3B, and 2A). Ovarian tissue from females exposed to TCE water on days 11 to 14 had similar labeling of protein carbonyls to ovarian tissue from females that received TCE water for 2 weeks (Fig. 3C and 2A). Sections of ovary from the TCE-treated and vehicle-control rats that were incubated with normal rabbit serum, instead of rabbit anti-DNP antibody, served as negative controls (Fig. 3D, Fig. 3E, Fig 3F).
Figure 3.
Immunohistochemical detection of protein carbonyls following TCE exposure on days 1 to 5, days 6 to 10, and days 11 to 14 of a 2-week treatment interval. Bar represents 50 μm. (A) Ovarian tissue from a female treated with 0.45% TCE (v/v) in 3% Tween on days 1 to 5. Granulosa cells are slightly labeled indicating the presence of protein carbonyls. (B) Ovarian tissue from a female exposed to TCE on days 6 to 10. Granulosa cells are more intensely labeled compared with (A), indicating increased presence of protein carbonyls. (C) Ovarian tissue from a female exposed to TCE on days 11 to 14. Granulosa cells are more intensely labeled compared with (A) and (B), indicating increased presence of protein carbonyls. (D), (E), and (F) Ovarian tissues from the same females represented in (A), (B), and (C) respectively incubated with normal rabbit serum in place of the primary antibody (rabbit anti-DNP). None of the tissues have positive signal indicating negligible background labeling. Pictures representative of 3 replicates.
Quantitative Assessment of Oxidatively Modified Ovarian Protein from Treated Females
The increased presence of oxidized protein in ovarian tissue following TCE exposure was confirmed using Western blotting with the same rabbit anti-DNP antibody that was used for immunohistochemistry (Fig. 4). Increased protein carbonyl formation was visible in the derivatized samples from TCE-treated females (Fig. 4, lane 2) compared with derivatized samples from female rats treated with vehicle (Fig. 4, lane 4). No immunoreactive oxidized proteins were detected in non-derivatized TCE and vehicle samples (Fig. 4, lanes 1 and 3 respectively). Duplicate blots stained with Coomassie blue indicated equal loading of protein in each lane (results not shown).
Figure 4.
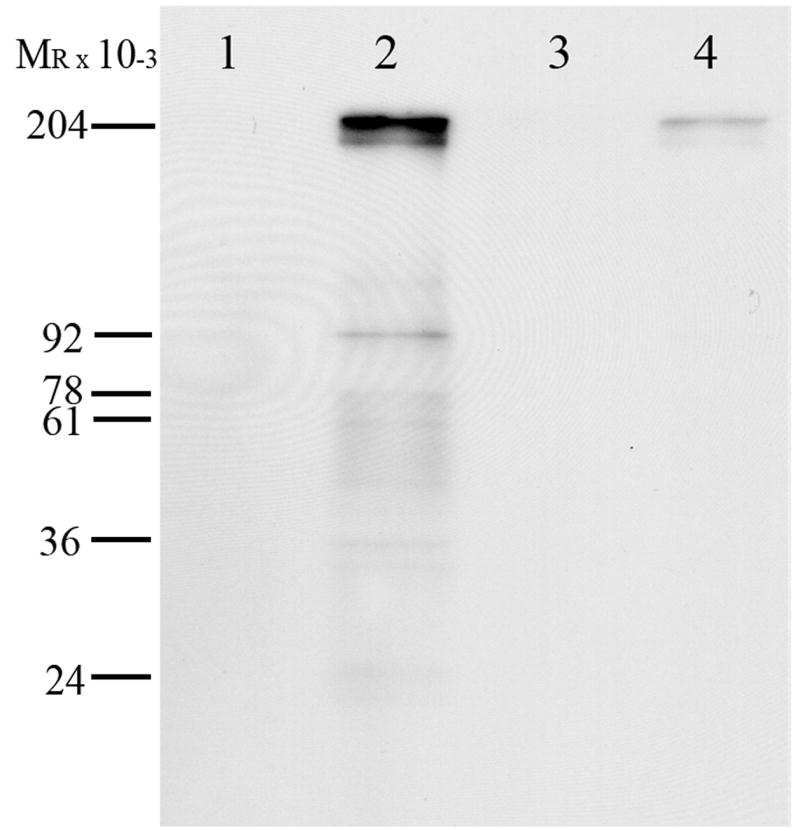
Western blot analysis of protein carbonyls in the ovaries from rats receiving TCE in drinking water for 5 days or vehicle water. Lane 1 – non-derivatized ovarian protein from TCE-treated rat, lane 2 – derivatized ovarian protein from TCE-treated rat, lane 3 – non-derivatized ovarian protein from vehicle-control rat, and lane 4 – derivatized ovarian protein from vehicle-control rat. Protein carbonyls are present in derivatized ovarian protein from TCE-treated rats (lane 2). Vehicle-control rats had much fainter labeling indicating less protein carbonyls (lane 4). The antibody to the derivatization agent for protein carbonyls exhibited no background binding as it did not label the non-derivatized ovarian protein (lanes 1 and 3, respectively). Blot representative of 4 replicates.
Cytochrome P4502E1 activity
In vitro incubation with TCE reduced p-nitrophenyl hydroxylase activity (cytochrome P4502E1) in three microsomal preparations to 54% of control activity (P<0.05). The p-nitrophenol hydroxylase activity following 2 days of in vivo exposure averaged 530 pmoles/ng protein compared with 299 pmoles/ng protein in ovaries from vehicle controls (P<0.20, SEM=136). Values were very similar following 3 days of in vivo exposure (225 vs. 234 pmoles/ng protein for microsomes from vehicle control and treated animals respectively, P>0.25, SEM=60).
DISCUSSION
The goals of this study were two-fold: (1) to determine if a short interval of in vivo TCE-exposure during oocyte growth decreased oocyte fertilizability, and (2) to determine if protein oxidation was a potential mechanism contributing to reduced oocyte fertilizability. In vitro fertilization results demonstrated a short interval of 4 or 5 days of exposure to TCE is sufficient to reduce the fertilizability of oocytes from TCE-treated females. Our laboratory has previously suggested that IVF is an important bioassay which can be used to evaluate in vivo toxicant effects on female gamete quality [18]. Other techniques of assessing oocyte fertility, such as in vivo mating trials, are insensitive because the normal rat ejaculate has approximately ten-fold more sperm than needed for maximum fertilization [25, 26]. Studies by Smith et al. have suggested a more fertile male may compensate for a less fertile female [27, 28]. The same holds true in vitro since increasing concentrations of sperm lead to greater percentages of fertilized oocytes [18].
In these studies, females were dosed via drinking water since earlier studies noted adverse effects of oral gavage [18]. The Tween vehicle is effective in increasing solubility of TCE in the drinking water, but does not affect reproductive parameters or weight in this strain of rats [9, 18]. Negligible differences in liver weights between TCE-treated rats and vehicle-control rats suggest systemic toxicity is not a confounding factor affecting oocyte fertilizability.
The TCE-treatment interval was shortened from 2 weeks to 4 or 5 days in the present study to investigate which stage of oocyte growth and maturation (early, middle, or late) was perturbed by exposure to TCE. These intervals would be expected to vary in relative importance of RNA synthesis, protein synthesis, and redistribution of cellular organelles. Originally, a 2-week TCE treatment was chosen because it covers the interval of massive oocyte growth in the rodent [29]. The extensive cytoplasmic growth and nuclear changes that occur during oocyte maturation [29–31] suggested it might be a period of time when oocytes are particularly sensitive to some toxicants. The age of treatment was chosen because a majority of females have not yet cycled, but the females are readily responsive to the induced ovulation regimen of PMSG and hCG [9]. As expected, the vast majority of females (>90%) had not cycled prior to induced ovulation at 42–45 days.
Granulosa cells including the cumulus cells (the granulosa cells immediately surrounding the oocyte) exhibited a greater incidence of protein carbonyls in the ovaries of TCE-treated rats compared with the same cells from vehicle-control rats. Current results along with cytochrome P4502E1 localization in the granulosa cells [13] are consistent with ovarian TCE metabolism by the cytochrome P450 dependent oxidative pathway [11]. Protein carbonyl content is the most commonly used indicator of oxidative modification of proteins [32–36]. Metabolism of some environmental pollutants, such as TCE, may result in oxidative stress which could lead to the formation of reactive oxygen species (ROS) within the body. Covalent modification of a protein induced either directly by ROS or indirectly by reaction with secondary by-products of oxidative stress can result in protein oxidation [35]. Reactive oxygen species, such as TCE-epoxide, can oxidize amino acid residues on proteins, which leads to the formation of protein carbonyls [32]. Such oxidation is not specific to a single protein. Protein carbonyls are stable products which can be assessed in the laboratory and assays for the detection of protein carbonyls involve derivatization of the carbonyl group with 2,4-DNPH. Derivatization results in the formation of a hydrazone product which can be labeled for visualization by immunoassays, spectrophotometric assays or by radioactive labeling [32, 33, 37, 38].
The granulosa cells of ovaries 10 days after TCE-exposure had subtle labeling for protein carbonyls, which is comparable with labeling of the granulosa cells of ovaries from vehicle-control rats. The amount of protein carbonyls in these tissues may represent a baseline amount of oxidative damage. Normal metabolism can produce reactive oxygen and reactive nitrogen species, which can cause oxidative damage [32]. The less intense staining for protein carbonyls in ovaries from rats exposed to TCE on days 1 to 5 or days 6 to 10 compared with days 11 to 14 may be due to tissue repair as these females received vehicle for several days prior to collection of ovaries on the morning of day 15. The time between TCE-exposure and tissue collection could have allowed the oxidized proteins to be removed from the tissue by proteolysis [35]. Alternatively, the ovary in the final stages of hormone-induced maturation may be the most sensitive to oxidative damage. As a parent compound, TCE does not appear to have adverse effects on oocyte fertilizability following in vitro exposure [10]. In vitro fertilization data suggests the TCE metabolites formed via the oxidative pathway elicit toxic effects on oocytes [10]. These metabolites could bind to proteins forming protein adducts, thus altering the original protein and function.
Oxidative modification of proteins can inhibit normal protein function [35] by loss of catalytic or structural integrity thereby interrupting normal pathways [39]. The inhibition of p-nitrophenyl hydroxylase activity following in vitro exposure is similar to that reported by Lilly and coworkers. Reductions in p-nitrophenol hydroxylase activity were reported 4 h following in vivo exposure in lung microsomes [22] and increased p-nitrophenyl hydroxylase activity was observed in hepatic microsomes 3 h following in vivo exposure [40]. Elevated activity continued to be observed for at least 24 h at the highest dose in this later study but a lower dose that exhibited elevated activity at 3, 6, and 12 h no longer exhibited elevated activity at 16 or 24 h. Hence the arithmetically increased activities we observed during continuous treatment for 2 days and the similar activities observed after 3 days on ovarian microsomal preparations are not surprising.
Given that oxidative modification of protein alters function, granulosa cells may be less able to aid in the growth and maturation of oocytes after exposure to TCE. This may partially explain why oocytes from TCE-exposed females are less fertilizable. Earlier studies reported females exposed to TCE had decreased reproductive performance, and increased incidences of poor pregnancy outcomes [41, 42]; however, it is unclear in these studies whether the TCE metabolites that caused the adverse effects were formed in the reproductive tract. A study conducted by Manson and coworkers noted that the cytochrome P450 oxidized TCE metabolites, trichloroacetic acid and trichloroethanol, were present in the ovaries and uteri of rats, but they did not determine where these metabolites were formed [42].
Female gametogenesis is difficult to examine given the numerous factors that can perturb its success. Several toxicants affect male gametogenesis independently of the endocrine system, but female gametogenesis and oocyte quality are much more difficult to study partially because mammalian female gametes are maintained internally rather than being expelled. The current study adds to previous work by demonstrating that short in vivo exposure to TCE significantly reduces fertility, whereas the females were exposed to TCE for the duration of oocyte growth and maturation in the previous study. Interestingly, oocytes were sensitive to TCE exposure during each third of postnatal oocyte growth and maturation. Although, oocytes from females exposed to TCE on days 6 to 10 and days 11 to 14 were slightly less able to bind sperm compared with vehicle-controls, oocytes from females exposed to TCE on days 1 to 5 were as effective at binding sperm as vehicle-control oocytes. The inability of oocytes from females exposed to TCE on days 6 to 10 and 11 to 14 to bind sperm suggests oocyte plasma membrane proteins have been altered. This result supports earlier work suggesting fertilizability of oocytes from females exposed to TCE was hampered by changes to the oocyte plasma membrane (decreased ability to bind sperm plasma membrane proteins) [9]. Combined with the consistent reduction in fertilizability in oocytes from all exposed females, this suggests that the ability of oocytes to fuse with sperm is hindered. Trichloroethylene metabolism in the ovary may decrease the functionality of oocyte plasma membrane proteins indirectly by adversely affecting cumulus-oocyte interactions which take place during oocyte growth and maturation. The disruption of cumulus-oocyte interactions could alter the ability of cumulus cells to support oocyte cytoplasmic maturation, which includes synthesis of plasma membrane receptors for sperm [43]. Thus, the effect of TCE and its metabolites on the oocyte could be indirect.
The presence of increased ovarian protein carbonyls following TCE-exposure as demonstrated by immunohistochemistry and Western blotting suggests the ovary may participate in the metabolism of TCE. Increased protein carbonyls, previously reported presence of cytochrome P4502E1 [13], presence of TCE metabolites [42] and the p-nitrophenol hydroxylase activity are consistent with metabolism of some TCE by the cytochrome P450 pathway in the ovary. Evidence from this study suggests the rat ovary, in addition to the liver, is capable of metabolizing TCE via the cytochrome P450 dependent oxidative pathway. The results demonstrate that oocytes are less fertilizable as a result of short-term TCE-exposure, and there is an increased incidence of protein carbonyls in granulosa cells of the ovaries from TCE-treated rats. Given these results, oocyte fertilizability in vivo may be temporarily hindered after a female is exposed to high levels of TCE. Although litter size might not be reduced in rats following this in vivo exposure and natural mating, female gamete quality is compromised. This is an important finding when the rat is used as a model for other species where excess sperm numbers may not be as available to compensate for less fertile females [25, 26]. Bioactivation of the environmental toxicant, TCE, within the rat ovary by metabolism may play an important role in reducing rat oocyte fertilizability after in vivo TCE-exposure and be a model for effects of toxicants on female gametes.
Acknowledgments
Portions of this paper were presented at the 38th Annual Meeting of the Society for the Study of Reproduction, Quebec City, Quebec, Canada, July 24–27, 2005. Funding for KLW was provided in part by the National Institutes of Health, National Institute of Child Health and Human Development (NICHD) training grant in Fertilization and Early Development (T32 HD071131), and the University of California Toxic Substances Research and Teaching Program (UC TSR&TP) student fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.EPA, Sources, Emission and Exposure for Trichloroethylene (TCE) and Related Chemicals., National Center for Environmental Assessment–Washington Office, Office of Research and Development, U.S. Environmental Protection Agency, 2001. pp. 1–125.
- 2.Howard PH, Boethling RS, Jarvis WF, Michalenko MWMEM. Handbook of Environmental Degradation Rates. Lewis Publishers; Chelsea, MI: 1991. [Google Scholar]
- 3.ATSDR, ToxFAQs for Trichloroethylene, U.S. Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, 2003.
- 4.Wu C, Schaum J. Exposure assessment of trichloroethylene. Environ Health Perspect. 2000;108:359–363. doi: 10.1289/ehp.00108s2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DuTeaux SB, Berger T, Hess RA, Sartini BL, Miller MG. Male reproductive toxicity of trichloroethylene: sperm protein oxidation and decreased fertilizing ability. Biol Reprod. 2004;70:1518–1526. doi: 10.1095/biolreprod.103.022210. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Tanphaichitr N, Forkert PG, Anupriwan A, Weerachatyanukul W, Vincent R, Leader A, Wade MG. Exposure to trichloroethylene and its metabolites causes impairment of sperm fertilizing ability in mice. Toxicol Sci. 2004;82:590–597. doi: 10.1093/toxsci/kfh277. [DOI] [PubMed] [Google Scholar]
- 7.Lamb JC, Hentz KL. Toxicological review of male reproductive effects and trichloroethylene exposure: Assessing the relevance to human male reproductive health. Reproductive Toxicology. 2006;22:557–563. doi: 10.1016/j.reprotox.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.van Zutphen LFM, Baumans V, Beynen AC. Principles of Laboratory Animal Science: a Contribution to the Humane Use and Care of Animals and to the Quality of Experimental Results. Elsevier; New York: 2001. [Google Scholar]
- 9.Berger T, Horner CM. In vivo exposure of female rats to toxicants may affect oocyte quality. Reprod Toxicol. 2003;17:273–281. doi: 10.1016/s0890-6238(03)00009-1. [DOI] [PubMed] [Google Scholar]
- 10.Cosby NC, Dukelow WR. Toxicology of maternally ingested trichloroethylene (TCE) on embryonal and fetal development in mice and of TCE metabolites on in vitro fertilization. Fundam Appl Toxicol. 1992;19:268–274. doi: 10.1016/0272-0590(92)90160-j. [DOI] [PubMed] [Google Scholar]
- 11.Lash LH, Fisher JW, Lipscomb JC, Parker JC. Metabolism of trichloroethylene. Environ Health Perspect. 2000;108:177–200. doi: 10.1289/ehp.00108s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson IW, Beliles RP. Consideration of the target organ toxicity of trichloroethylene in terms of metabolite toxicity and pharmacokinetics. Drug Metab Rev. 1991;23:493–599. doi: 10.3109/03602539109029772. [DOI] [PubMed] [Google Scholar]
- 13.Cannady E, Dyer CA, Christian PJ, Sipes IG, Hoyer PB. Expression and activity of cytochromes P450 2E1, 2A, and 2B in the mouse ovary: the effect of 4-vinylcyclohexene and its diepoxide metabolite. Toxicol Sci. 2003;73:423–430. doi: 10.1093/toxsci/kfg077. [DOI] [PubMed] [Google Scholar]
- 14.Singh D, Pandey RS. Glutathione-S-transferase in rat ovary: its changes during estrous cycle and increase in its activity by estradiol-17 beta. Indian J Exp Biol. 1996;34:1158–1160. [PubMed] [Google Scholar]
- 15.Cummings BS, Parker JC, Lash LH. Role of cytochrome P450 and glutathione S-transferase alpha in the metabolism and cytotoxicity of trichloroethylene in rat kidney. Biochem Pharmacol. 2000;59:531–543. doi: 10.1016/s0006-2952(99)00374-3. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council (NRC) Drinking Water and Health: Selected Issues in Risk Assessment. Washington D.C: National Academies Press Washington D.C; 1989. [PubMed] [Google Scholar]
- 17.International Agency for Research on Cancer (IARC), Monographs on the evaluation of carcinogenic risks to humans: dry cleaning, some chlorinated solvents and other industrial chemicals, World Health Organization, 1995.
- 18.Berger T, Miller MG, Horner CM. In vitro fertilization after in vivo treatment of rats with three reproductive toxicants. Reprod Toxicol. 2000;14:45–53. doi: 10.1016/s0890-6238(99)00062-3. [DOI] [PubMed] [Google Scholar]
- 19.Evans G, Armstrong DT. Reduction in fertilization rate in vitro of oocytes from immature rats induced to superovulate. J Reprod Fertil. 1984;70:131–135. doi: 10.1530/jrf.0.0700131. [DOI] [PubMed] [Google Scholar]
- 20.Nicolson GL, Yanagimachi R, Yanagimachi H. Ultrastructural localization of lectin-binding sites on the zonae pellucidae and plasma membranes of mammalian eggs. J Cell Biol. 1975;66:263–274. doi: 10.1083/jcb.66.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schacter E, Williams JA, Lim M, Levine RL. Differential Susceptibility of Plasma Proteins to Oxidative Modification: Examination by Western Blot Immunoassay. Free Radical Biology & Medicine. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 22.Forkert PG, Millen B, Lash LH, Putt DA, Ghanayem BI. Pulmonary bronchiolar cytotoxicity and formation of dichloroacetyl lysine protein adducts in mice treated with trichloroethylene. The Journal of pharmacology and experimental therapeutics. 2006;316:520–529. doi: 10.1124/jpet.105.093062. [DOI] [PubMed] [Google Scholar]
- 23.Koop DR. Inhibition of ethanol-inducible cytochrome P450IIE1 by 3-amino-1,2,4-triazole. Chem Res Toxicol. 1990;3:377–383. doi: 10.1021/tx00016a017. [DOI] [PubMed] [Google Scholar]
- 24.Lilly PD, Thornton-Manning JR, Gargas ML, Clewell HJ, Andersen ME. Kinetic characterization of CYP2E1 inhibition in vivo and in vitro by the chloroethylenes. Archives of toxicology. 1998;72:609–621. doi: 10.1007/s002040050551. [DOI] [PubMed] [Google Scholar]
- 25.Aafjes JH, Vels JM, Schenck E. Fertility of rats with artificial oligozoospermia. J Reprod Fertil. 1980;58:345–351. doi: 10.1530/jrf.0.0580345. [DOI] [PubMed] [Google Scholar]
- 26.Working PK. Male reproductive toxicology: comparison of the human to animal models. Environ Health Perspect. 1988;77:37–44. doi: 10.1289/ehp.887737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith K, Rodriguez-Rigau LJ, Steinberger E. Relation between indices of semen analysis and pregnancy rate in infertile couples. Fertility and Sterility. 1977;28:1314–1319. doi: 10.1016/s0015-0282(16)42976-6. [DOI] [PubMed] [Google Scholar]
- 28.Steinberger E, Smith KD, Tcholakian RK, Rodriguez-Rigau LJ. Testosterone levels in female partners of infertile couples. Relationship between androgen levels in the woman, the male factor, and the incidence of pregnancy. American journal of obstetrics and gynecology. 1979;133:133–138. doi: 10.1016/0002-9378(79)90463-0. [DOI] [PubMed] [Google Scholar]
- 29.Bachvarova R. Gene expression during oogenesis and oocyte development in mammals. Dev Biol (N Y 1985) 1985;1:453–524. doi: 10.1007/978-1-4615-6814-8_11. [DOI] [PubMed] [Google Scholar]
- 30.Bachvarova R, Paynton BV. Gene expression during growth and meiotic maturation of mouse oocytes. Prog Clin Biol Res. 1988;267:67–85. [PubMed] [Google Scholar]
- 31.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocrine Reviews. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 32.Chevion M, Berenshtein E, Stadtman ER. Human studies related to protein oxidation: protein carbonyl content as a marker of damage. Free Radical Research. 2000;33:S99–S108. [PubMed] [Google Scholar]
- 33.Levine RL. Carbonyl modified proteins in cellular regulation, aging, and disease. Free Radic Biol Med. 2002;32:790–796. doi: 10.1016/s0891-5849(02)00765-7. [DOI] [PubMed] [Google Scholar]
- 34.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 35.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metab Rev. 2000;32:307–326. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 36.Beal MF. Oxidatively modified proteins in aging and disease. Free Radic Biol Med. 2002;32:797–803. doi: 10.1016/s0891-5849(02)00780-3. [DOI] [PubMed] [Google Scholar]
- 37.Levine RL, Garland D, Oliver CN, Amic iA, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 38.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 39.Stadtman ER, Levine RL. Protein oxidation. Ann N Y Acad Sci. 2000;899:191–208. doi: 10.1111/j.1749-6632.2000.tb06187.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee KM, Muralidhara S, White CA, Bruckner JV. Mechanisms of the dose-dependent kinetics of trichloroethylene: oral bolus dosing of rats. Toxicology and applied pharmacology. 2000;164:55–64. doi: 10.1006/taap.2000.8892. [DOI] [PubMed] [Google Scholar]
- 41.Coberly S, Oudiz DJ, Overstreet JW, Wiley LM. Effects of maternal exposure to trichloroethylene (TCE) on cell proliferation in the mouse preimplantation embryo. Reprod Toxicol. 1992;6:241–245. doi: 10.1016/0890-6238(92)90179-w. [DOI] [PubMed] [Google Scholar]
- 42.Manson JM, Murphy M, Richdale N, Smith MK. Effects of Oral Exposure to Trichloroethylene on Female Reproductive Function. Toxicology. 1984;32:229–242. doi: 10.1016/0300-483x(84)90076-3. [DOI] [PubMed] [Google Scholar]
- 43.Zuccotti M, Yanagimachi R, Yanagimachi H. The ability of hamster oolemma to fuse with spermatozoa: its acquisition during oogenesis and loss after fertilization. Development (Cambridge, England) 1991;112:143–152. doi: 10.1242/dev.112.1.143. [DOI] [PubMed] [Google Scholar]