Abstract
Numerous studies have demonstrated that low birthweight (LBW) is associated with the development of medical conditions, such as hypertension and diabetes, and psychiatric disorders, such as depression. One possible mechanism through which LBW might increase risk for both medical and psychiatric disorders is by altering the biological systems, such as the HPA-axis function, that govern emotion regulation and physical reactivity. In this study, we conducted secondary data analyses in a longitudinal study originally designed to understand the intergenerational transmission of MDD. We examined risk for both medical and psychiatric illnesses known to be influenced by HPA-axis dysregulation in the context of parental depression. The study had two primary objectives: 1) to examine whether LBW increases the risk of selected adult illness that may be influenced by the HPA-axis; and 2) to examine whether the increased risk of illness varies by parental depression status.
We conducted longitudinal assessments of 244 offspring of depressed and non-depressed parents for over 20 years. Psychopathology and medical illness were assessed by direct interview conducted by clinicians blind to risk status and previous diagnosis. We examined the effect of birthweight in three categories: birthweight less than 2.5kg (LBW); 2.5kg-3.5kg; and greater than 3.5kg (reference group).
Offspring with LBW had a significantly increased risk of MDD, anxiety disorders, phobia, suicidal ideation, impaired functioning, allergies, and hypertension compared to those with BW greater than 3.5kg. The association between LBW and depression was stronger among children of depressed parents than among children of non-depressed parents, with an interaction term (birthweight and parental depression status) significant for MDD (p=.05), suggesting that parental depression may augment the impact of LBW on offspring depression:
1. Introduction
Low birthweight (LBW), defined as birthweight less than 2.5kg, may increase the risk of medical and psychiatric problems across the life cycle. School-aged children born with LBW are vulnerable to behavioral1, emotional2, and medical problems3, poor cognitive functioning4 and learning difficulties4,5. Barker's fetal origins hypothesis6 also suggests a link between LBW and adult hypertension, stroke7, and diabetes mellitus8. Furthermore, recent studies showed that LBW increased the risk of psychopathology, such as emotional distress and depression in adolescents and adults9-12.
Psychosocial stress in pregnancy13 may contribute to decreased BW through increases in hypothalamic CRH release14. Exposure to stress in utero may also underlie vulnerability to subsequent normative stress and biophysiological changes in the hypothalamic-pituitary-adrenal (HPA) axis. Taken together, having LBW may compromise the integrity of the HPA-axis functionality15 which may be associated with emotional disorders and behavioral inhibition as well as medical illnesses related to cardiovascular reactivity and respiratory, and immune functionality16. The present study examined whether the effect of LBW was found in other illnesses associated with HPA-axis functionality such as depression, anxiety disorders, hypertension, allergies and respiratory illness17 as well as global functioning of the offspring. It further examined whether the risk was greater if concomitant with parental depression, which might place offspring with LBW at increased risk for disorders from familial and environmental factors.18
2. METHODS
2.1. Subjects
The study sample consisted of offspring of depressed and non-depressed probands participating in an ongoing longitudinal study of the impact of parental depression on the offspring. Depressed probands were recruited from a treatment center and non-depressed probands were recruited from the same community where the treatment center was located. The depressed and non-depressed probands were group-matched by age and sex (see Weissman et al., 1987, 1992, 1997, 2005 for description of design and sample assessment).19-22 After the initial assessment, 244 offspring with information on BW were interviewed four times over a 20-year period and followed into adulthood (mean age, sd=33, 8.8). Response rates were 80% or higher at each wave of data collection. Non-response rates did not vary by proband depression status or birthweight. Of these 244 offspring, 162 were offspring of depressed parents (defined as either parent depressed); 82 were offspring of non-depressed parents; 8.2% of offspring had LBW (<2.5kg), 54.5% had BW between 2.5kg and 3.5kg and 37.3% had BW greater than 3.5kg. All interview waves were approved by the Institutional Review Board at New York State Psychiatric Institute/Columbia University. After providing a complete description of the study to the subjects, written informed consent was obtained from adults and assent was obtained from minors who also had written consent from their parents.
2.2. Assessments
Psychiatric diagnosis was obtained through the Schedule for Affective Disorders and Schizophrenia-Lifetime Version [SADS-L] for adults23 and a slightly modified version of the Schedule for Affective Disorders and Schizophrenia for School-Aged Children, Epidemiologic Version [K-SADS-E]24 for ages 6-17. The diagnostic assessments were administered by trained doctoral and masters level mental health professionals, who were blind to the clinical status of the parents and to previous history. Multiple sources of information were used, including direct and informant interviews and medical records wherever available. The Global Assessment Scale [GAS] was completed at each wave25. This instrument is rated on a 100 point scale and provides an overall estimate of the person's current functional adjustment. Another version for children, the CGAS26 was used when the offspring were between ages 6 and 17. Lower scores on the GAS or CGAS indicated more overall impairment. Diagnoses, GAS, and episodes of suicidal ideation were based on the best estimate (BE) procedure, which is described elsewhere.22,27 All diagnoses for the offspring were cumulative across all waves.
Information on medical illness in offspring was collected at each wave. Using a checklist format, either the parent or the offspring (depending on age) indicated “yes” or “no” to both a lifetime and current history of medical problems. The age at first onset of each medical problem was ascertained as well. All ambiguous reports of medical problems, including any discrepancy between medical charts obtained from their physicians and the interview, were recoded by a physician blind to the depression status of the offspring and parents. Information on each medical illness was pooled to create cumulative variables indicating a lifetime history of medical conditions.
BW was extracted from parents' report of the child's developmental history at the baseline assessment, except for 17 cases (9%) whose BW was collected again at a later assessment. Although a very small proportion, the correlation between the two reports of BW was high (r=.87). BW was split into three categories (< 2.5kg, 2.5-3.5kg, > 3.5kg). We will refer to BW less than 2.5kg as “LBW”, BW between 2.5-3.5kg as “mid BW” and BW greater than 3.5kg as “high BW” throughout the paper. Normal BW will refer to BW greater than 2.5kg (mid and high BW). Information on maternal smoking, drinking, and substance use during pregnancy was also gathered from parents' report (mostly mothers). Information on SES was determined with the Hollingshead Four-Factor Index, which incorporates the education and occupation of mothers and fathers.28
2.3. Data analysis
In order to examine differences in rates of disorders in offspring among the three BW groups (<2.5kg, 2.5-3.5kg, >3.5kg), univariate analysis was conducted using X2 tests. The same analysis was repeated after stratification by parental depression status.
The univariate analyses were followed by multivariate analyses to adjust for potential confounders: the Cox proportional hazard regression model was used to adjust for the differences in follow-up time for each offspring29. Sex and preterm birth status of offspring, parental depression, SES, mother's smoking, alcohol, and drug use during pregnancy, parity, maternal age at the birth of the offspring were considered a priori as potential confounders and were included in the model for statistical adjustment. Age of the offspring was not included in the model as a covariate because Cox proportional hazard analyses implicitly adjusts for differences in age at follow-up (i.e., age at the last interview). We used an average of CGAS or GAS scores across waves as a measure of global functioning, since it reflects functioning from childhood to early adulthood. We used a cut-off score of 65 or less as impaired functioning, the suggested cut-off for children and adolescents.30 Logistic regression analyses were used to examine the effect of BW on the offspring's global functioning, since the functioning measure was averaged across waves. The product term of birthweight (<2.5kg, 2.5-3.5kg, >3.5kg) and parental depression measures was added for testing possible interaction effect. The same set of potential confounders was included in this model as covariates, and age of offspring was added as a covariate. Lastly, lifetable analyses were conducted to explore differences in age-specific rates of MDD, anxiety disorders, phobia and any medical illness by BW status. We used dichotomous BW (LBW vs. normal BW), instead of the three categories, as patterns of age-specific incidence for the two normal BW groups (mid BW and high BW) were similar. Since no formal tests of differences in patterns were performed, these analyses should be viewed as descriptive.
The study allowed for the inclusion of more than one offspring from the same family. Consequently, the assumption of independence of the outcome variable implicit in the use of the standard Cox proportional hazard model may be violated. To overcome this problem, we used the methods of Binder31 who extended the methods of Lin and Wei.32 They proposed a method for estimating the covariance matrix of the estimated parameters when the model is misspecified in those situations where there is correlation among sample units. We used SUDAAN to obtain the adjusted variance for the relevant parameters.33
3. Results
3.1. Characteristics of offspring and their mothers
There were no significant differences among the three BW groups (<2.5kg, 2.5-3.5kg, >3.5kg) on any major demographic variable, except for age of offspring. Mean (sd) age of offspring with LBW, with mid BW (2.5-3.5kg), and with high BW (>3.5kg) was 36.3 (5.7), 33.1 (7.8), and 30.5 (10.2) respectively (F2,241=4.8, p=.009). Fifty-five percent of the offspring were female, 51% were married, 73% Catholic, 16% Protestant, and 11% other religions. Mean (sd) family, and individual incomes were $64,574 (28,053) and $39,608 (22,646) respectively.
With regards to mothers' characteristics that may be associated with birthweight, a little over half (58.6%) of the mothers did not smoke at all, 82% did not have any alcoholic beverages, and almost all (99%) did not use any illicit drug during pregnancy. While the rate of maternal drinking and substance use during pregnancy did not differ among the three BW groups, the rate of maternal smoking during pregnancy (ever-smoked) was significantly different among the three groups: 60% of mothers of LBW, 36% of mothers of mid BW, and 20% of mothers of high BW offspring smoked during pregnancy respectively (p=.001). Half of the LBW offspring were born preterm, and approximately 8% of mid and high BW offspring were born preterm (p<.0001). There was no difference in maternal age at birth (mean=27.2, sd=5.6) and parity (mean=2.4, sd=1.4) among the three BW groups.
3.2. Cumulative rates of offspring disorders by birthweight
We examined the rate of each offspring disorder according to BW. We began by evaluating the overall differences in the rates of each disorder (both psychiatric and medical) among the three BW groups. As can be seen in table 1, the rate of disorder for LBW was substantially higher than that for high BW offspring, eg., MDD (75% vs. 32%), any anxiety disorder (65% vs. 36%), phobia (50% vs. 19%), suicidal ideation (40% vs. 11%), impaired functioning (45% vs. 24%), respiratory illness (44% vs. 19%), hypertension (15% vs. 4%), and allergies (67% vs. 36%). The cumulative rate of disorders for offspring with mid BW fell in between that of LBW and high BW. Overall group differences for the rate of offspring disorders among the three birthweight groups were significant, for all outcome variables except impaired functioning and hypertension. For impaired functioning and hypertension, although offspring with LBW had the highest rates, the two other groups (mid BW and high BW) had similar rates, contributing to the non-significant findings.
Table 1.
Rates per 100 (standard error) of psychiatric and medical problems in offspring according to birthweight
| Offspring birth weight | statistics | |||||
|---|---|---|---|---|---|---|
| Offspring disorders | < 2.5 kg (n=20) % (SE) |
2.5 – 3.5 kg (n=133) % (SE) |
> 3.5 kg (n=91) % (SE) |
Overall difference | ||
| X2 | df | p | ||||
| MDD | 75.0 (9.9) | 47.4 (4.3) | 31.9 (4.9) | 13.9 | 2 | .001 |
| Any anxiety disorder | 65.0 (10.9) | 44.4 (4.3) | 36.3 (5.1) | 5.8 | 2 | .05 |
| Any phobia | 50.0 (11.5) | 24.1 (3.7) | 18.7 (4.1) | 8.8 | 2 | .01 |
| Suicidal ideation | 40.0 (11.2) | 18.0 (3.3) | 11.0 (3.3) | 9.8 | 2 | .007 |
| Impaired functioning | 45.0 (11.4) | 27.1 (3.9) | 24.1 (4.6) | 3.6 | 2 | .17 |
| Respiratory illness | 44.4 (12.1) | 31.0 (4.0) | 17.6 (4.3) | 6.1 | 2 | .04 |
| Hypertension | 15.0 (8.2) | 7.8 (2.2) | 3.6 (2.0) | 3.6 | 2 | .17 |
| Allergies | 66.7 (11.4) | 52.7 (4.5) | 36.3 (5.5) | 8.7 | 2 | .01 |
NB: N may vary due to missing values. Any anxiety disorder includes separation anxiety disorder, overanxious disorder, generalized anxiety disorder, obsessive compulsive disorder, panic disorder, PTSD and phobia; (c) any phobia includes simple phobia, social phobia and agoraphobia. Impaired functioning was based on overall functioning scores across.
Df= degree of freedom; SE=standard error.
3.3. Cumulative rates of offspring disorders by birthweight stratified by parental depression status
We examined the rate of each offspring disorder according to BW (table 2) stratified by parental depression status. Among offspring of depressed parents, the rate of MDD, any anxiety disorder, any phobia, suicidal ideation, impaired functioning, and allergy was significantly different among the three BW groups. As compared to high BW (>3.5kg) offspring, LBW offspring had substantially higher rates of MDD (81.3% vs. 32.8%), any anxiety disorder (75% vs. 41.8%), phobia (56.3% vs. 22.4%), suicidal ideation (43.8% vs. 13.4%), impaired functioning (50% vs. 23.8%), and allergies (66.7% vs. 36.3%). In contrast, among offspring of non-depressed parents, there was no overall difference in the rate of any disorder.
Table 2.
Rates per 100 (standard error) of psychiatric and medical problems in offspring according to birthweight by parental depression status
| Neither parent depressed | ||||||
|---|---|---|---|---|---|---|
| Offspring birth weight | statistics | |||||
| Offspring disorders | < 2.5 kg (n=4) % (SE) |
2.5 – 3.5 kg (n=54) % (SE) |
> 3.5 kg (n=24) % (SE) |
Overall difference | ||
| X2 | df | p | ||||
| MDD | 50.0 (28.9) | 24.1 (5.9) | 29.2 (9.5) | 1.4 | 2 | .50 |
| Any anxiety disorder | 25.0 (25.0) | 22.2 (5.7) | 20.8 (8.5) | 0.4 | 2 | .98 |
| Any phobia | 25.0 (25.0) | 9.3 (3.6) | 8.3 (4.2) | 1.1 | 2 | .57 |
| Suicidal ideation | 25.0 (25.0) | 7.4 (3.6) | 4.2 (4.2) | 2.2 | 2 | .38 |
| Impaired functioning | 25.0 (25.0) | 9.3 (4.0) | 25.0 (9.0) | 3.7 | 2 | .16 |
| Respiratory illness | 75.0 (25.0) | 30.8 (6.5) | 21.7 (8.8) | 4.6 | 2 | .10 |
| Hypertension | 0 (--) | 4.3 (3.0) | 0 (--) | 1.0 | 2 | .60 |
| Allergies | 50.0 (28.9) | 53.8 (7.0) | 37.5 (10.4) | 1.8 | 2 | .42 |
| ≥ 1 parent depressed | ||||||
| Offspring birth weight | statistics | |||||
| Offspring disorders | < 2.5 kg (n=16) % (SE) |
2.5 – 3.5 kg (n=79) % (SE) |
> 3.5 kg (n=69) % (SE) |
Overall difference | ||
| X2 | df | p | ||||
| MDD | 81.3 (10.1) | 63.3 (5.5) | 32.8 (5.8) | 19.4 | 2 | .0001 |
| Any anxiety disorder | 75.0 (11.2) | 59.5 (5.6) | 41.8 (6.1) | 7.8 | 2 | .02 |
| Any phobia | 56.3 (12.8) | 34.2 (5.4) | 22.4 (5.1) | 7.4 | 2 | .03 |
| Suicidal ideation | 43.8 (12.8) | 25.3 (4.9) | 13.4 (4.2) | 7.7 | 2 | .02 |
| Impaired functioning | 50.0 (12.9) | 39.2 (5.5) | 23.8 (5.4) | 5.7 | 2 | .058 |
| Respiratory illness | 35.7 (13.3) | 31.2 (5.3) | 17.7 (4.9) | 3.9 | 2 | .14 |
| Hypertension | 18.8 (10.1) | 7.9 (3.5) | 4.9 (3.3) | 3.4 | 2 | .19 |
| Allergies | 66.7 (12.5) | 52.7 (5.7) | 36.3 (6.3) | 7.5 | 2 | .02 |
NB: N may vary due to missing values. Any anxiety disorder includes separation anxiety disorder, overanxious disorder, generalized anxiety disorder, obsessive compulsive disorder, panic disorder, PTSD and phobia; (c) any phobia includes simple phobia, social phobia and agoraphobia. Impaired functioning was based on overall functioning scores across.
Df= degree of freedom; SE=standard error.
3.4. Risk of offspring disorders by birthweight
Results from Cox proportional hazards analyses are presented in Table 3. Relative risk (RR) was used as a risk indicator for all variables except for impaired functioning, where odds ratio (OR) was used (see data analysis section for detail). Offspring who had LBW, relative to those with high BW, had an over 4-fold increased risk of hypertension (RR=4.5 [95% CI 0.8,25.2]) and impaired global functioning (OR=6.1 [95% CI 1.5, 22.8]), an approximately 3-fold increased risk of MDD (RR=2.9 [95% CI 1.4, 6.0]), any anxiety disorder (RR=3.0 95% CI 1.4, 6.7), phobias (RR=3.1 95% CI 1.2, 8.0) and suicidal ideation (RR=2.7 [95% CI 1.0, 7.2]), as well as a 2-fold increased risk of allergies (RR=2.0 [95% CI 1.0, 4.2]). Offspring who had mid BW (2.5-3.5kg), relative to those with high BW, had an almost two-fold increased risk of MDD (RR=1.7 [95% CI 1.0, 2.7]) and allergies (RR=1.65 [95% CI 1.0, 2.6]), after adjusting for potential confounder variables. There was no significant increased risk of respiratory illness for offspring with LBW relative to those with high BW, after controlling for potential confounders.
Table 3.
Risk and 95% confidence interval (CI) for lifetime psychiatric and medical illness according to birthweight (kg)
| Illness in offspring Offspring birthweight |
N | Unadjusted Risk (95% CI), p-value |
Adjusted Risk (95% CI), p-value |
Interactiona Wald X2, p- value |
|---|---|---|---|---|
| Major depressive disorder |
3.7, p=.050 | |||
| <2.50 | 20 | 2.7 (1.5, 5.1), p=.0017 | 2.9 (1.4, 6.1), p=.004 | |
| 2.50-3.50 | 133 | 1.7 (1.1, 2.7), p=.02 | 1.7 (1.0, 2.7), p=.04 | |
| >3.50 | 91 | 1.0 | 1.0 | |
| Any anxiety disorder | 1.2, p=.28 | |||
| <2.50 | 20 | 2.2 (1.2, 4.2), p=.02 | 3.0 (1.4, 6.7), p=.006 | |
| 2.50-3.50 | 133 | 1.4 (.9, 2.1), p=.14 | 1.3 (.8, 2.1), p=.24 | |
| >3.50 | 91 | 1.0 | 1.0 | |
| Any phobia | .09, p=.77 | |||
| <2.50 | 20 | 2.9 (1.3, 6.3), p=.0085 | 3.1 (1.2, 8.0), p=.02 | |
| 2.50-3.50 | 133 | 1.4 (.8, 2.6), p=.24 | 1.3 (.7, 2.4), p=.50 | |
| >3.50 | 91 | 1.0 | 1.0 | |
| Suicidal ideation | ||||
| <2.50 | 20 | 3.2 (1.3, 8.2), p=.01 | 2.7 (1.0, 7.2), p=.05 | .07, p=.78 |
| 2.50-3.50 | 133 | 1.5 (.7, 3.5), p=.32 | 1.5 (.7, 3.3), p=.30 | |
| >3.50 | 91 | 1.0 | 1.0 | |
| Impaired functioning | ||||
| <2.50 | 20 | 2.6 (.9, 7.3), p=.06 | 6.1 (1.5, 22.8), p=.008 | 3.2, p=.098 |
| 2.50-3.50 | 133 | 2.4 (1.0, 5.6), p=.05 | 1.8 (.8, 3.8), p=.13 | |
| >3.50 | 87 | 1.0 | 1.0 | |
| Respiratory illness | ||||
| <2.50 | 18 | 2.3 (1.0, 5.4), p=.05 | 1.8 (.9, 3.6), p=.11 | .24, p=.74 |
| 2.50-3.50 | 129 | 1.5 (.8, 2.6), p=.19 | 1.3 (.7, 4.4), p=.74 | |
| >3.50 | 85 | 1.0 | 1.0 | |
| Hypertension | ||||
| <2.50 | 20 | 3.4 (.7, 16.4), p=.12 | 4.5 (.80, 25.2), p=.09 | .65, p=.42 |
| 2.50-3.50 | 128 | 1.5 (.2, 16.6), p=.74 | 2.0 (.15, 25.6), p=.59 | |
| >3.50 | 83 | 1.0 | 1.0 | |
| Allergies | .88, p=1.7 | |||
| <2.50 | 18 | 2.0 (1.1, 3.9), p=.03 | 2.0 (1.0, 4.2), p=.05 | |
| 2.50-3.50 | 129 | 1.6 (1.0, 2.4), p=.04 | 1.6 (1.0, 2.6), p=.04 | |
| >3.50 | 84 | 1.0 | 1.0 |
NB: Impaired functioning was based on overall functioning scores across waves and there is no age of onset. Therefore logistic regression analysis was used and the risk reported on the table is based on odds ratio. For other diagnoses, proportional hazards model was used and the risk reported is based on hazards ratio. Adjusted risk=adjusted for preterm birth, sex, family SES, maternal risk behavior during pregnancy (drinking, smoking, and druguse), parity, maternal age at the birth of the offspring, and parental lifetime depression status.
Any anxiety disorder includes separation anxiety disorder, overanxious disorder, generalized anxiety disorder, obsessive compulsive disorder, panic disorder, post traumatic stress disorders, and phobia; and any phobia includes simple phobia, social phobia and agoraphobia.
95% CI=95% confidence interval
The interaction between birthweight (<2.50, 2.50-3.50, >3.50) and parental depression status.
The possible moderating effect of parental depression was explored and the results are presented in the last column of Table 3. There was a statistically significant interaction between parental depression and birthweight status on MDD (p=.05) and a marginally significant effect on impaired functioning (p=.098), indicating that the adverse impact of birthweight (low, mid, and high) on offspring's MDD and impaired functioning may be stronger in the offspring of depressed parents, compared to the offspring of non-depressed parents. No other interactions between parental depression and BW status on offspring disorders were statistically significant.
3.5. Age-specific rates of MDD, anxiety disorders, phobia, and any medical illness
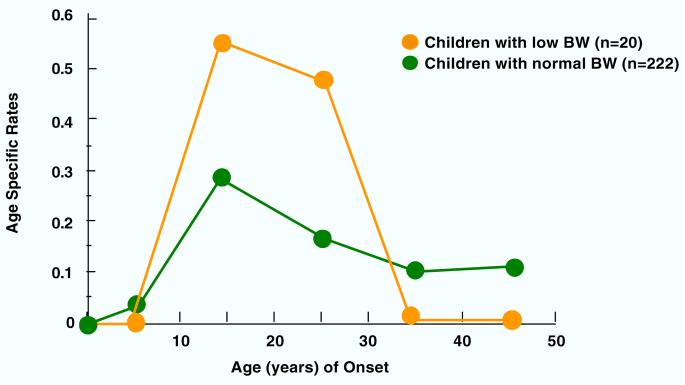
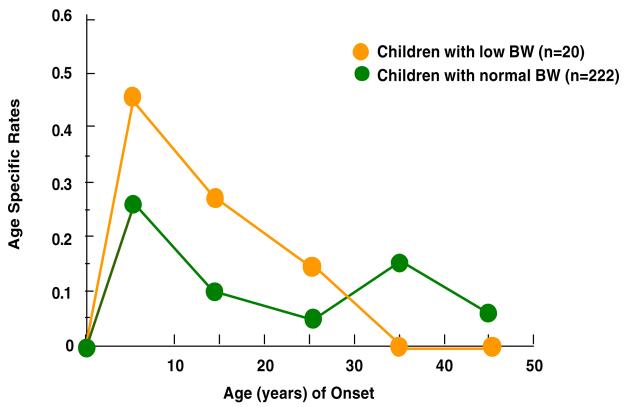
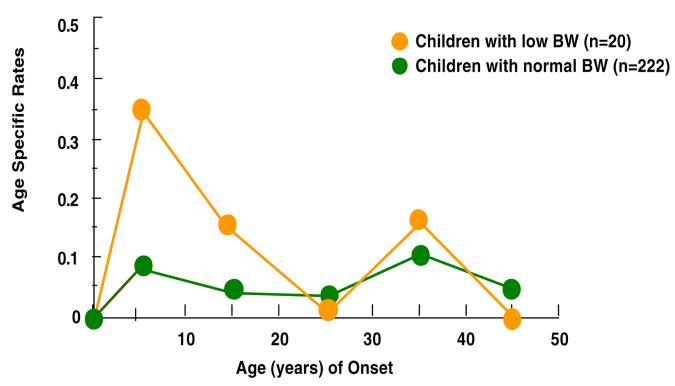
Figures 1-4 show the results of estimating age-specific rates using lifetable analyses for MDD, anxiety disorders, phobia, and any medical illness under examination by dichotomous BW status (LBW vs. normal BW). Visual inspection suggests that there was a difference in rates between the two groups. Figure 1 shows that the peak incidence of MDD was between ages 15 and 25, and it was higher in the offspring with LBW compared to offspring with normal BW. Figures 2 and 3 show that the peak incidence of anxiety disorders and phobia was between ages 5 and 15, and it was higher in the offspring with LBW compared to offspring with normal BW. Figure 4 shows that the peak incidence of any medical illness occurs later in adulthood, especially after age 25, where a greater difference in rates between offspring with LBW and normal birthweight was observed.
Figure 1.
Age Specific Rates of Major Depression in Children (2nd Gen.) by their Low Birthweight (BW) Status
Figure 4.
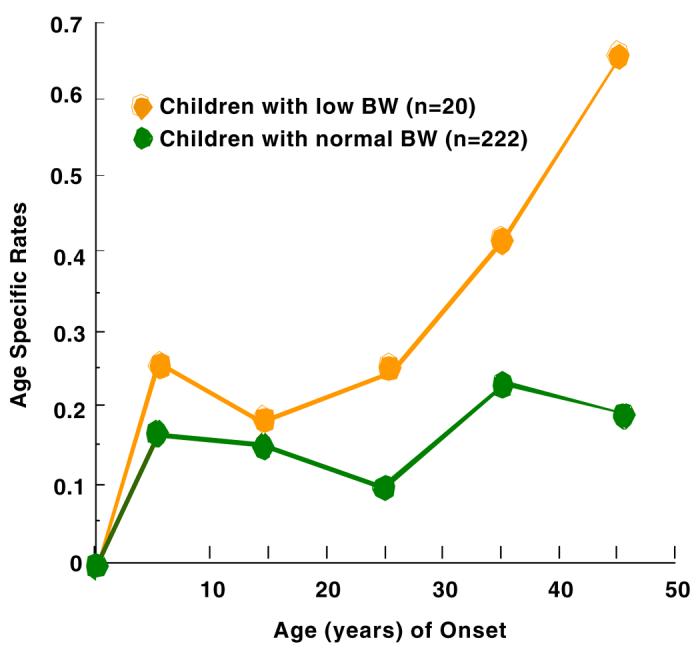
Age Specific Rates of any Medical Illness in Children (2nd Gen.) by their Low Birth Weight (BW) Status
Figure 2.
Age Specific Rates of any Anxiety Disorder in Children (2nd Gen.) by their Low Birthweight (BW) Status
Figure 3.
Age Specific Rates of any Phobia in Children (2nd Gen.) by their Low Birthweight (BW) Status
4. Discussion
Using a longitudinal design, we examined whether birthweight was associated with psychopathology, selected medical illness and global functioning in young adulthood, and whether the severity of sequelae increased with decreasing birthweight. We further examined whether parental depression amplified the adverse effect of LBW on offspring disorders and functioning. The findings suggest a 2- to 4-fold increased risk of MDD (p=.004), anxiety disorders (p=.006), phobia (p=.02), suicidal ideation (p=.05), hypertension (p=.09) and allergies (p=.05), and a 6-fold increased risk of impaired global functioning (p=.008) in LBW offspring compared to those with high BW. Furthermore, the interaction between parental depression and offspring birthweight status on offspring MDD was significant (p=.05), suggesting that parental MDD amplified the effect of birthweight on offspring MDD. Notably, among offspring of depressed parents, 81% of LBW offspring had MDD.
Our findings on the increased risk of medical illness in relation to birthweight status are consistent with previously demonstrated links between LBW and medical illness such as cardiovascular illness7 and diabetes8. The current study, however, examined a wider range of medical illnesses, including several related to HPA-axis functionality (hypertension, respiratory illness, and allergies). We found that in offspring with LBW relative to those with high BW, not only was the risk of hypertension elevated but so was the risk of allergies. However, the risk of hypertension became only marginally significant after adjusting for potential confounders. Post-hoc analysis revealed that although the overall prevalence of hypertension in female and male offspring was similar (4.7% vs. 4.3%), the risk of developing hypertension was much higher in male offspring with LBW (RR=16.7, p=.08) than in female offspring with LBW (RR=3.3, p=.38). Thus, when the gender effect was removed from the analysis, there was only a marginally increased risk of hypertension among offspring with LBW relative to those with high BW (>3.5kg). Since the prevalence of medical illness in young adulthood is relatively low, the examination of a possible differential gender effect might be more reliable as offspring grow older. In sum, the risk of medical illnesses that were potentially related to HPA-axis functionality appeared to be elevated in offspring with LBW relative to those with birthweight greater than 3.5kg, even in young adulthood. However, the magnitude of this association may not be fully appreciated until we follow the sample into the ages of greater risk for these conditions.
As the magnitude of increased risk among offspring with LBW has public health implications, we had chosen to analyze the effect of birthweight using a discrete category [< 2.5kg (LBW); 2.5kg-3.5kg, and >3.5kg] while recognizing that the risk of adult illness could increase in a dose-response manner as birthweight decreases. However, we also examined whether the data better fit a model in which the effects of BW were exerted dimensionally. The results showed that the risk was increased as BW decreased for MDD (p=.03), suicidal ideation (p=.04), allergies (p=.04) and functional impairment (p=.01), with trends for asthma (p=.09) and hypertension (p=.10).
Results from related twin studies are consistent with our findings : twins with lower BW had higher psychological distress2, more behavioral problems34, and a higher prevalence of psychopathology35. However, the hypothesized association between LBW and increased risk of depression or emotional distress in adolescence and adulthood in community-based studies4,10.12 is still inconclusive. While Tompson et al. (2001)12 reported the association between LBW and depression only in men, another study did so only in women10. We found that both men (RR=2.7 [95% CI 0.95,7.2]) and women (RR=2.7 [95% CI 1.2,5.8]) who were born with LBW were at increased risk of MDD. The interaction between BW status and gender was not significant (p=.96), indicating that LBW status increased the risk of MDD equally by gender in this sample.
One recent study36 followed a cohort of 10,753 male singletons from age 15 to 49 years for over 30 years and reported no evidence for increased risk of depression by LBW. Their findings were based on hospital discharge records. Indeed in our sample, among those who met the criteria for MDD, only 33% received treatment and only 13% were ever hospitalized for their psychiatric condition. Those who were hospitalized, relative to those who were not, may have suffered from more severe forms of depression with different causal pathways.
Taking advantage of the high-risk study design, we examined separately the impact of LBW on the risk of psychiatric and medical illnesses among offspring of depressed and non-depressed parents. This is not possible with other community-based studies of the prevalence of depression among adolescents and adults born with low or normal birthweight. We hypothesized that the adverse impact of LBW on psychopathology would be stronger among offspring of depressed, relative to non-depressed, parents. Analyses stratified by parental depression did appear to suggest that risks for all illnesses, except the respiratory illness, were greater among offspring of depressed relative to offspring of non-depressed. However, a formal test of interaction between parental depression and birthweight status was not significant for most of disorders except depression and impaired functioning (only marginally significant). Unfortunately, the number of subjects, especially in the group with LBW and non-depressed parents, may have been too small to detect an interaction for many of the disorders. In some twin studies, the interaction of familial history of psychopathology and BW status has been tested. One study found no evidence for a synergistic interaction of BW and parent psychopathology on child psychopathology34, but others reported an antagonistic interaction, in which children with LBW (adjusted for gestational age), compared to those with normal BW, was less sensitive to the familial or genetic effects on increased risk of behavioral problems37 and cognitive development38. Twin studies have a methodological strength over community and/or epidemiological studies in controlling for extraneous effects. However, none of the twin studies above utilized DSM-IV diagnoses. Future studies including those with twins, could benefit from application of the DSM-IV diagnostic criteria.
In our study, offspring with LBW showed a higher incidence of psychiatric disorders in their early years (5-15) relative to those with normal BW (mid and high BW). The occurrence of medical illnesses was more prominent after childhood and adolescence. In our sample, because of the high risk design, the prevalence of affective disorders was higher than that found in community samples, which permitted documentation of these disorders throughout the life cycle. However, among the medical illnesses we studied, with the exception of allergies, prevalence rates were relatively low. Given the low prevalence rates of medical illnesses, the clear difference in age-specific rates in offspring in later life by BW is noteworthy.
Previous studies suggest that stressful prenatal events associated with maternal smoking, substance use, pre-term birth, and LBW, may contribute to abnormalities in brain morphology and function (cognition, emotionality and behavior), and may compromise the integrity of the endocrine and immune system.15 One possible mechanism which could explain susceptibility to physical and psychiatric illness throughout the lifespan in infants with LBW is alteration in HPA-axis activity and reactivity. In order to determine whether this is a reasonable hypothesis to pursue in more focused research investigations, we conducted secondary data analyses in a longitudinal study originally designed to understand the intergenerational transmission of MDD. We examined risk for both physical and psychiatric illnesses known to be influenced by HPA-axis dysregulation. Our findings provided preliminary evidence of increased risk for both physical and psychiatric illnesses that are associated with HPA-axis functioning. Incorporating direct measures of HPA-functionality into future studies could significantly enhance our ability to confirm the hypothesis that increased adult illness among offspring with LBW is mediated by HPA-axis dysregulation. This longitudinal study of intergenerational transmission of MDD provides the preliminary evidence to validate further studies into this link.
While we have considered maternal risk behavior, such as smoking, drinking and substance use during pregnancy as confounders, we also examined the extent to which the risk behavior during pregnancy was associated with psychiatric and medical illness outcomes in offspring, mediated through birthweight status. Although a previous study in this sample demonstrated that maternal smoking during pregnancy increased risk for conduct disorder and Attention Deficit Hyperactivity Disorder in their offspring39.The results show that smoking was associated with an increased risk for LBW but not associated with any of the offspring outcomes under examination. In sum, while LBW was increased risk for affective and anxiety disorders (psychiatric illness) and medical illnesses related to reactivity (respiratory, allergies and hypertension), maternal risk behavior during pregnancy, such as smoking, was not directly associated with the risk of these illnesses.
Strengths and limitations
Strengths of our study include, diagnoses based on standardized, in-depth psychiatric interviews with best estimate procedures; a very long follow-up time (20-year) affording examination of age-specific incidence rates by BW status; and analyses based on proportional hazards models rather than logistic regression, permitting variable follow-up times. In using a high-risk sample, we could maximize the potential case yield for affective disorders, minimize heterogeneity, and identify early patterns of illness, while evaluating whether parental depression status moderated the relationship between BW and offspring disorders.
However, the study also has some limitations. First, the number of LBW offspring is relatively small, so results should be interpreted cautiously. Due to this limitation, we were unable to divide the LBW further into LBW (2.5kg-1.5kg) and very LBW (<1.5kg) groups, although we divided normal BW into the two groups (2.5kg-3.5kg and >3.5g) and examined a gradient relationship across the BW range on offspring disorders. Second, we found partial evidence to support an interaction between parental depression and LBW on offspring illness outcomes (MDD [p=.05] and impaired functioning [p=.098]). The absence of statistically significant interactions between LBW and parental depression on other disorders, however, has to be interpreted cautiously as the small number of LBW cases in offspring of non-depressed parents may have limited the statistical power to examine the significance of interactions. Third, the measure of BW relied on parents' retrospective reports. Although several studies reported that BW reported by mothers was valid40, it may be subject to recall bias. We have multiple reports of BW with excellent reliability (r=.87). Additionally, 66 offspring now have their own children and reported their BW at both wave 3 and 4, which also showed high reliability (r=.94). Taken together, it is reasonable to assume that parental reports of BW are fairly accurate in our sample. Finally, results are based on offspring of depressed parents who sought treatment for their depression as well as non-depressed age and sex matched control, not a community sample. Thus, generalizability may be limited. However, unlike other community- based studies, diagnoses were based on rigorous best-estimate procedure deriving from the DSM-IV criteria; and medical illness status was updated through interviews and medical records. Any discrepancy was consolidated by independent assessors blind to parental depression and birthweight status. Despite the small sample size, our clinical data inform a future direction of the investigation in association between LBW and psychiatric and medical illnesses across life. Future community-based studies could benefit from application of the DSM-IV diagnostic criteria.
Acknowledgement
The study was supported by R01MH036197 (P.I., Myrna M. Weissman) from the NIMH, Young Investigator Award (P.I., Yoko Nomura) from the National Alliance for Research on Schizophrenia and Depression (NARSAD), R03 MH 067761 (P.I., Yoko Nomura) from the NIMH, the Sackler Institute for Developmental Psychobiology, and Child and Families Resilience Program at Mount Sinai School of Medicine (Director, Claude M. Chemtob). We appreciate the helpful comments of Virginia Warner on the earlier version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Gray RF, Indurkhya A, McCormick MC. Prevalence, stability, and predictors of clinically significant behavior problems in low birth weight children at 3, 5, and 8 years of age. Pediatrics. 2004;114:932–40. doi: 10.1542/peds.2003-1150-L. [DOI] [PubMed] [Google Scholar]
- 2.Cheung YB, Ma S, Machin D, et al. Birthweight and psychological distress in adult twins: a longitudinal study. Acta Paediatics. 2004;93:965–968. doi: 10.1111/j.1651-2227.2004.tb02697.x. [DOI] [PubMed] [Google Scholar]
- 3.Bhutta AT, Cleves MA, Casey PH, et al. Cognitive and behavioral outcome of school-aged children who were born pre-term: a meta-analysis. JAMA. 2002;288:728–737. doi: 10.1001/jama.288.6.728. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S, Pinelli J, Hoult L, et al. Psychopathology and social competencies of adolescents who were extremely low birth weight. Pediatrics. 2003;111:969–975. doi: 10.1542/peds.111.5.969. [DOI] [PubMed] [Google Scholar]
- 5.Rickards Al, Kelly EA, Doyle LW, et al. Cognition, academic progress, behavior and self-concept at 14 years of very low birth weight children. J Dev Behav Pediatrics. 2001;22:11–8. doi: 10.1097/00004703-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatrics Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 7.Leon DA, Lithell HO, Vagero D, et al. Reduced fetal growth rate and increased risk of death from ishcaemic heart disease: cohort study of 15000 Swedish men and women born 1915-1929. BMJ. 1998;317:241–245. doi: 10.1136/bmj.317.7153.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lithell HO, McKeigue PM, Berglund L, et al. Relation of size and birth to non-insulin dependent diabetes and insulin concentrations in men aged 50-60 years. BMJ. 1996;312:406–410. doi: 10.1136/bmj.312.7028.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Indredavik MS, Vik T, Heyerdahl S, et al. Psychiatric symptoms and disorders in adolescents with low birth weight. Arch Dis Child Fetal Neonatal Ed. 2004;89:F445–50. doi: 10.1136/adc.2003.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale CR, Martyn CN. Birth weight and later risk of depression in a national birth cohort. Bri J Psychiatry. 2004;184:28–33. doi: 10.1192/bjp.184.1.28. [DOI] [PubMed] [Google Scholar]
- 11.Wiles N, Peters TJ, Leon DA, et al. Birth weight and psychological distress at age 45-51 years. Bri J Psychiatry. 2005;187:21–28. doi: 10.1192/bjp.187.1.21. [DOI] [PubMed] [Google Scholar]
- 12.Thompson C, Syddall H, Rodin I, et al. Birth weight and the risk of depressive disorder in later life. Bri J Psychiatry. 2001;179:450–455. doi: 10.1192/bjp.179.5.450. [DOI] [PubMed] [Google Scholar]
- 13.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinology. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- 15.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 16.McEwen BS. Protective and damaging effects of stress mediators. New Eng J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 17.Nomura Y, Brooks-Gunn J, Davey C, et al. The role of perinatal problems for risk of comorbid psychiatric and medical disorders in adulthood. Psychol Med. doi: 10.1017/S0033291707000736. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nomura Y, Wickramaratne P, Warner V, et al. Family discord, parental depression and psychopathology in offspring: 10-Year follow-up. J Am Acad Child Adoles Psychiatry. 2002;41:402–409. doi: 10.1097/00004583-200204000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Weissman MM, Gammon GD, John K, et al. Children of depressed parents: increased psychopathology and early onset of major depression. Arch Gen Psychiatry. 1987;44:847–853. doi: 10.1001/archpsyc.1987.01800220009002. [DOI] [PubMed] [Google Scholar]
- 20.Weissman MM, Fendrich M, Warner V, et al. Incidence of psychiatric disorder in offspring at high and low risk for depression. J Am Acad Child Adoles Psychiatry. 1992;31:640–648. doi: 10.1097/00004583-199207000-00010. [DOI] [PubMed] [Google Scholar]
- 21.Weissman MM, Warner V, Wickramaratne P, et al. Offspring of depressed parents: 10 years later. Arch Gen Psychiatry. 1997;54:932–940. doi: 10.1001/archpsyc.1997.01830220054009. [DOI] [PubMed] [Google Scholar]
- 22.Weissman MM, Wickramaratne P, Nomura Y, et al. Families at High and Low Risk for Depression: A 3-Generation Study. Arch Gen Psychiatry. 2005;62:9–36. doi: 10.1001/archpsyc.62.1.29. [DOI] [PubMed] [Google Scholar]
- 23.Mannuzza S, Fyer AJ, Klein DF, et al. Schedule for affective disorders and schizophrenia-lifetime version modified for the study of anxiety disorders (SADS-LA): rationale and conceptual development. J Psychiatric Res. 1986;20:317–325. doi: 10.1016/0022-3956(86)90034-8. [DOI] [PubMed] [Google Scholar]
- 24.Orvaschel H, Puig-Antich, Chambers W, et al. Retrospective assessments of prepubertal major depression with Kiddie-SADS-E. J Am Acad Child Adoles Psychiatry. 1982;21:392–397. doi: 10.1016/s0002-7138(09)60944-4. [DOI] [PubMed] [Google Scholar]
- 25.Endicott J, Spitzer RL, Fleiss JL, et al. The global assessment scale: a procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 26.Shaffer D, Gould MS, Brasic J, et al. Children's global assessment scale (C-GAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 27.Leckman JF, Sholomskas D, Thompson D, et al. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry. 1982;39:879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- 28.Hollingshead AB, Redlich FC. Social Class and Mental Illness. John Wiley; New York, NY: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cox DR. Regression models and life tables. Applied Statistics. 1972;34:87–220. [Google Scholar]
- 30.Weissman MM, Warner V, Fendrich M. Applying impairment criteria to children's psychiatry diagnosis. J Am Academy Child Adoles Psychiatry1990. 1990;29:789–795. doi: 10.1097/00004583-199009000-00019. [DOI] [PubMed] [Google Scholar]
- 31.Binder DA. Fitting Cox's Proportional Hazards Models from Survey Data. Biometrika. 1992;79:139–147. [Google Scholar]
- 32.Lin DY, Wey LJ. The robust inference for the proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 33.Shah BV, Barnwell BG, Gayle GS. SUDAAN user's manual 7.5. Research Triangle Institute; Research Triangle Park, NC: 1997. [Google Scholar]
- 34.Van Os J, Wichers M, Danckaerts M, et al. A prospective twin study of birth weight discordance and child problem behaviour. Biol Psychiatry. 2001;50:593–599. doi: 10.1016/s0006-3223(01)01085-x. [DOI] [PubMed] [Google Scholar]
- 35.Wals M, Reichart CG, Hillegers MH, et al. Impact of birth weight and genetic liability on psychopathology in children of bipolar parents. J Am Acad Child Adoles Psychiatry. 2003;42:1116–21. doi: 10.1097/01.CHI.0000070242.24125.78. [DOI] [PubMed] [Google Scholar]
- 36.Osler M, Nordentoft M, Andersen AMN. Birth dimensions and risk of depression in adulthood: cohort study of Danish men born in 1953. Bri J Psychiatry. 2004;186:400–403. doi: 10.1192/bjp.186.5.400. [DOI] [PubMed] [Google Scholar]
- 37.Wichers MC, Purcell S, Danckaerts M, et al. Prenatal life and post-natal psychopathology: evidence for negative gene-birth weight interaction. Psychol Med. 2002;32:1165–1174. doi: 10.1017/s0033291702006372. [DOI] [PubMed] [Google Scholar]
- 38.Koeppen-Schomerus G, Eley TC, Wolke D, et al. The interaction of prematurity with genetic and environmental influences on cognitive development in twins. J Pediatrics. 2000;137:527–533. doi: 10.1067/mpd.2000.108445. [DOI] [PubMed] [Google Scholar]
- 39.Weissman MM, Warner V, Wickramaratne PJ, et al. Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J. Am. Acad.Child Adolesc. Psychiatry. 1999;38:892–899. doi: 10.1097/00004583-199907000-00020. [DOI] [PubMed] [Google Scholar]
- 40.Olson JE, Shu XO, Ross JA, et al. Medical record validation of maternally reported birth characteristics and pregnancy-related events: A report from the Children's Cancer Group. Am J Epidemiol. 1997;145:58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]