Abstract
Polyglycylation is a polymeric post-translational modification of tubulin that is ubiquitous and widely present in cilia and flagella. It consists of the addition of highly variable numbers of glycyl residues as side chains onto the γ carboxyl group of specific glutamyl residues at the C-termini of α- and β-tubulin. The function of polyglycylation is poorly understood, however, studies in Tetrahymena have shown that the mutation of polyglycylation sites in β-tubulin resulted in axonemal abnormality or lethality. This suggests that polyglycylation is functionally essential in protists. We hypothesize that polyglycylation is also essential in mammalian cilia and that the extent of polyglycylation has functional significance. In this study, we examined polyglycylation states in ciliated tissues and in mouse tracheal epithelial cell cultures. We utilized two antibodies, TAP 952 and AXO 49, which recognize glutamyl sites possessing monomeric glycylation sites and glutamyl sites possessing polymeric glycylation sites, respectively. Monomeric glycylation sites were observed in cilia of all the ciliated tissues examined but were invariably excluded from the distal tips. In contrast, polymeric glycylation sites were rare, but when observed, they were localized at the bases of cilia. During ciliogenesis, in epithelial cell cultures, monomeric glycylation sites were observed, but the extent of polymeric glycylation sites were variable and were only observed during the early stages of the cultures. Our observations suggest that while monomeric glycylation sites are universal and likely essential in mammalian cilia, polymeric glycylation sites are not required for ciliary beating. Rather, our observations suggest that the number of added glycyl residues increases progressively from the tips of cilia toward their bases.
Keywords: polyglycylation, monoglycylation, post-translational modification, tubuli, cilia
INTRODUCTION
Microtubules are composed of α- and β-tubulin dimers and play fundamental roles in many cell processes. These roles include supporting the cell’s shape, providing means of intracellular transport, assisting sister chromatid separation during cell division, and supporting motility in cilia and flagella. The α- and β-tubulin subunits of microtubules undergo many post-translational modifications (PTMs); these include detyrosination, acetylation, phosphorylation, and polyglutamylation. [MacRae, 1997; Luduena, 1998; Westermann and Weber, 2003]. Another polymeric PTM called polyglycylation is particularly abundant in axonemal tubulin [Redeker et al., 1994; Rudiger et al., 1995; Plessmann and Weber, 1997]. This PTM involves the addition of variable numbers of glycyl residues as side chains onto the γ carboxyl group of specific C-terminal glutamyl residues; for α-tubulin glycylation occurs at glutamates (445, 446, and 447) and for β-tubulin it occurs at glutamates (437, 438, 439, and 441) [Redeker et al., 1994; Vinh et al., 1999; Xia et al., 2000]. Up to 34 glycyl residues have been observed on the α- and β-tubulin subunits of Paramecium [Redeker et al., 1994]. Polyglycylation has been extensively studied in ciliated protist models such as Paramecium and Tetrahymena. Tetrahymena mutants possessing three or more mutated glutamyl residues at the C-terminus of β-tubulin displayed either abnormal axonemes or lethal phenotypes [Xia et al., 2000]. This strongly suggests that polyglycylation is essential for ciliary function. However, the functional role of polyglycylation in both protists and mammals is still unclear.
We hypothesize that polyglycylation is functionally essential in mammalian cilia. If this hypothesis is correct, the extent of polyglycylation should be similar in cilia of all ciliated tissues examined. To determine the extent of polyglycylation in mammals, we examined a variety of gerbil ciliated tissues and mouse tracheal epithelial cell (MTEC) cultures that undergo ciliogenesis, using two well-characterized antibodies, TAP 952 and AXO 49, which respectively identify monomeric glycylation sites (one glycyl residue) and polymeric glycylation sites (at least three glycyl residues) [Bre et al., 1996, 1998]. Glycylation sites possessing two glycyl residues are not identified by the antibodies. Thus it is very possible that glycyl chains with two glycyl residues are present but not detected.
In this study, we will refer to monomeric glycylation as (MG) and polymeric glycylation as (PG). Contrary to expectations, our results show different levels of tubulin polyglycylation between different tissue types, and between cilia of neighboring cells in a tissue. In particular, MG was ubiquitous in cilia of all tissues while PG was absent from cilia of most, but not all, ciliated tissues. Additionally, a transient occurrence of MG was exhibited in nonaxonemal microtubules during ciliogenesis in MTEC primary cultures. Thus, while the PTM, polyglycylation, is likely involved in ciliary function, PG does not appear to be necessary for ciliary beating in mammals.
MATERIALS AND METHODS
Animal care and handling was performed according to approved protocols of Creighton University School of Medicine Institutional Animal Care and Use Committee.
Gerbil Frozen Sections
Ciliated tissue types, including trachea, oviduct, testis efferent duct, brain ependyma, nasal respiratory epithelium, and olfactory epithelium, were examined using frozen section of tissues obtained from mature adult gerbils (21 days or older). Gerbils were selected as the test species to facilitate comparison with previous studies of ciliated tissue from this laboratory [Woo et al., 2002; Jensen-Smith et al., 2003; Perry et al., 2003]. The tissues were dissected and processed as described previously [Jensen-Smith et al., 2003].
Vestibular Organ Whole Mount Preparations
Vestibular organ whole mounts were prepared from mature adult gerbils. The animals were anesthetized with Nembutal and cardiac-perfused with 4% paraformaldehyde dissolved in phosphate buffered saline (PBS). Temporal bones were than removed and fixed in 4% paraformaldehyde dissolved in PBS for 1 h. The temporal bones were then decalcified in ethylenediamine tetraacetic acid for 24–48 h and vestibular organ whole mounts were dissected out. Whole mounts were processed for immunohistochemistry as described below.
Mouse Tracheal Epithelial Cell Cultures
MTEC cultures were established using the method of [Hastie, 1995]. Adult C57BL6 mice were euthanized with carbon dioxide (CO2), decapitated, and the tracheas were dissected and placed in chilled F-12 Nutrient Mixture (Ham) (Invitrogen, Carlsbad, CA). The tracheas were then placed in a pronase (Hoffmann-La Roche, Nutley, NJ) and F-12 Nutrient Mixture solution (1.5 mg/ml) and allowed to dissociate for 18–24 h at 4°C. The dissociated cells were harvested in a basic medium containing Dulbecco’s modified minimal essential medium (Invitrogen), F-12 Nutrient Mixture, glutamine (Cambrex Bio Science, Walkersville, MD), Penicillin-Streptomycin (Sigma-Aldrich, St. Louis, MO), Gentamycin (Sigma-Aldrich) and Amphotericin B (Pharmacia Corporation, Kalamazoo, MI). They were then allowed to incubate on 35 × 10 mm² Falcon culture dishes (Fisher Scientific, Hampton, NH) for 3 h at 37°C in air supplemented with 5% CO2. The cells were resuspended in supplementary medium composed of basic medium, Fetal Bovine Serum (Fisher Scientific), Insulin (Cambrex), transferrin (Cambrex), recombinant Human Epidermal Growth Factor (Cambrex), Bovine Pituitary Extract (Cambrex) and retinoic acid (Sigma-Aldrich). They were seeded at a density of 106 cells/mm³ on 6.5 mm diameter Costar membrane inserts (Corning Corporation, Corning, NY) coated with rat tail collagen, type I (Becton Dickinson, Bedford, MA) and allowed to incubate at 37°C in air supplemented with 5% CO2. The supplementary medium was replaced after 3 days of incubation. When the cultures were confluent (6 days in vitro), an air liquid interface (ALI) was established. In establishing the ALI, medium was removed from the apical portion of the membrane and resulted in the exposure of the cultures to air on the apical surface and medium on the base. During the ALI, the supplementary medium was replaced with a maintenance medium containing the basic medium plus NuSerum (Becton Dickinson) and retinoic acid. The medium was replaced every 3–4 days. To preserve the cultures for immunohistochemistry, the membranes containing the cultures were fixed with 4% paraformaldehyde in PBS.
Immunohistochemistry
To detect the presence of MG and PG in tubulin, the preserved cultures, whole mounts and sectioned tissues were blocked and permeabilized in PBS with 1% bovine serum albumin (BSA) (Fisher Scientific), normal goat serum (NGS) (Jackson ImmunoResearch) and 0.25% Triton-X. MTEC membranes were processed for 1 h, frozen sections for 2 h and whole mounted tissues for 24 h. Cells were labeled with the mouse monoclonal antibodies, TAP 952 (1:50,000) or AXO 49 (1:10,000), which identify tubulin MG and PG, respectively. These antibodies were visualized using a goat anti-mouse secondary antibody conjugated to Alexa 488 (Molecular Probes, Eugene, OR). The cells or tissues were double-labeled with a rabbit anti-α-tubulin polyclonal antibody (1:1000) (Abcam Incorporated, Cambridge, MA) which was visualized with goat anti-rabbit CY3 antibody (Jackson ImmunoResearch). Primary and secondary antibodies were incubated for 1 h each with MTEC membranes, for 3 and 2 h, respectively, with frozen sections and 24 h each with whole mounted tissues. After removal from the well inserts, the MTEC membranes were sealed under cover slips on glass slides with mounting medium containing 50% PBS, 50% glycerol and 0.1% n-propylgallate (Sigma-Aldrich), an anti-fade agent. Whole mounts and sectioned tissues were also mounted in this manner.
Fluorescence Microscopy
Cells and tissues were analyzed using an upright Zeiss Axioskop II microscope (Carl Zeiss, Germany) and photographed with a SPOT RT camera (Diagnostic Instruments, Sterling Heights, MI). Confocal images were taken using a Zeiss LSM 510 confocal microscope (Carl Zeiss).
Ciliary Beat Frequency Measurement
MTEC membranes containing living epithelial cells were removed from the well inserts, placed on glass slides, and covered with maintenance media incubated at 37°C in air supplemented with 5% CO2. Ciliary beat frequency (CBF) of cilia in the cultures was measured with a Sisson Ammons Video Analysis (SAVA) system (Ammons Engineering, Mt. Morris, MI) [Sisson et al., 2003] via an Axioscope upright microscope (Carl Zeiss) and an A60f monochrome digital camera (Basler Vision Technologies, Ahrensburg, Germany). CBF measurements were taken every 20–30 s for a total of 20 min. Random areas on each membrane were analyzed at room temperature.
RESULTS
Tubulin Monomeric Glycylation in Gerbil Tissues
Tubulin MG was observed in cilia of all the adult ciliated tissues examined. In Fig. 1a, cilia in a section of gerbil trachea were labeled with an antibody against α-tubulin (red), while in Fig. 1b the same cilia were labeled with TAP 952 (green). In Fig. 1c, we show merged images of Fig. 1a and 1b superimposed on a transmitted light image of the same section. Co-localization of label is shown as yellow. Figure 1 shows that label for MG extends from the bases of cilia and almost to the tips of cilia. The tips of cilia display no evidence of MG. Additionally, the extent of MG is consistent within the cilia of a cell but varies from cell to cell. We regularly observe such variations in the extent of polyglycylation between cells in all our samples. Thus, MG always extended from the bases of cilia toward their tips and generally encompassed at least 70% of the ciliary length. However the distal tips are excluded.
Fig. 1.

Confocal immunofluorescence images of gerbil trachea were labeled with anti α-tubulin antibody (a) to reveal the lengths of cilia and TAP 952 for tubulin MG (b). (c) Merged image (yellow) of (a) and (b) superimposed on transmitted light. Tubulin MG was observed in cilia (b) and was excluded from the tips of cilia (c). Scale bars = 5 µm.
Identical patterns of label for MG were observed in other ciliated tissues. These include oviduct (Fig. 2a), testis efferent duct (Fig. 2b), brain ependyma (Fig. 2c), nasal respiratory epithelium (Fig. 2d), olfactory epithelium (Fig. 2e), and vestibular utricle hair cells (Fig. 2f). MG was observed in all cilia and was present along most of the ciliary length. In Fig. 2f, cilia of olfactory neurons are truncated by tissue processing, however MG was still present. As described for the trachea, the extent of MG was the same in the cilia of the same cell but varied between cells. MG was consistently absent from the distal tips of cilia, which were labeled with only the anti-α-tubulin antibody.
Fig. 2.

Confocal immunofluorescence images of gerbil tissues labeled with TAP 952 for tubulin MG (green) and anti α-tubulin antibody (red) to reveal the lengths of cilia (yellow = merged). Frozen sections [oviduct (a), efferent duct (b), brain ependyma (c), nasal respiratory epithelium (d), olfactory epithelium (e)] and vestibular tissue whole mount (f). Tubulin MG was observed in all ciliated tissues and varied lengthwise between cilia of neighboring cells. (a–e) MG is excluded from the tips of most cilia. (f) Tubulin MG is present (cilia fractured). Inserts show magnified regions of the ciliated tissues. Scale bars = 5 µm.
Tubulin Polymeric Glycylation in Gerbil Tissues
In the same tissues, tubulin PG was less frequently observed than MG (Fig. 3a–3g). PG was only detected in cilia of the trachea and oviduct (Fig. 3a and 3b). Even in these tissues, PG was present in only about one quarter of cilia present. Additionally, it was restricted to the bases of cilia and generally covered at most 20% of the ciliary length (Fig. 3).
Fig. 3.

Confocal immunofluorescence images of gerbil tissues labeled with AXO 49 for PG (green) and anti α-tubulin antibody (red) to distinguish the lengths of cilia (yellow = merged). Frozen sections [trachea (a), oviduct (b), efferent duct (c), brain ependyma (d), nasal respiratory epithelium (e), olfactory epithelium (f)] and vestibular tissue whole mount (g). (a–b) PG was only observed in the trachea and the oviduct, and was restricted to the bases of some cilia. Scale bars = 5 µm.
Fig. 4 is a schematic diagram providing an overview of the polyglycylation patterns observed in mature mammalian ciliated tissues.
Fig. 4.

Schematic diagram of the general MG and PG trends observed in gerbil tissue types. Unglycylated regions are represented by red, MG regions are represented by green and regions presumably containing both MG and PG are represented yellow. Breaks in cilia of olfactory neuron represent cilia fractured during tissue preparation. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Tubulin Polyglycylation During Ciliogenesis
Ciliogenesis in MTEC cultures occurred principally after the ALI was established (6 days in vitro), but, in a limited number of cells, ciliogenesis was observed before the establishment of the ALI. By the 4th day post-ALI (10 days in vitro) over 25% of cells began to generate or already generated cilia. CBF analysis of cilia generated from 4 days post-ALI to 35 days post-ALI showed that the cultured cilia were functional (Fig. 5). As the cultures matured, average CBF measurements increased from 4 Hz and plateaued at 7 Hz, a frequency similar to this was observed in in vivo trachea epithelium cilia at room temperature. This similarity demonstrates that the MTEC cultures successfully mimic the in vivo conditions of the tracheal epithelium. For these reasons, MTEC cultures were used to examine the temporal course of MG and PG in tubulin during ciliogenesis. Several patterns of MG and PG were observed at different stages of development.
Fig. 5.

CBF analysis of MTEC cultures with respect to days post-ALI using SAVA. Mature cultures maintain CBF when PG is absent. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Early Stages (3–6 days in vitro and 1 day post-ALI)
The presence of tubulin MG and PG were observed in cells that generated cilia during the early stages of the culture, 3 days in vitro (Fig. 6a and 6b). MG was observed in most cilia present (Fig. 6a). It was present along most of the lengths of cilia and was excluded from the distal tips (Fig. 6a). Interestingly, MG was observed in cytoplasmic microtubules of cultured cells that had begun to generate cilia (Fig. 6a, arrow). In contrast, PG was limited to axonemal microtubules and thus was not detected in cytoplasmic microtubules (Fig. 6b). Unlike MG, PG was also not observed in all cilia present, it was only detected in some cilia (Fig. 6b). Furthermore, PG varied between cilia of cultured cells and thus, no obvious pattern could be correlated (Fig. 6b).
Fig. 6.
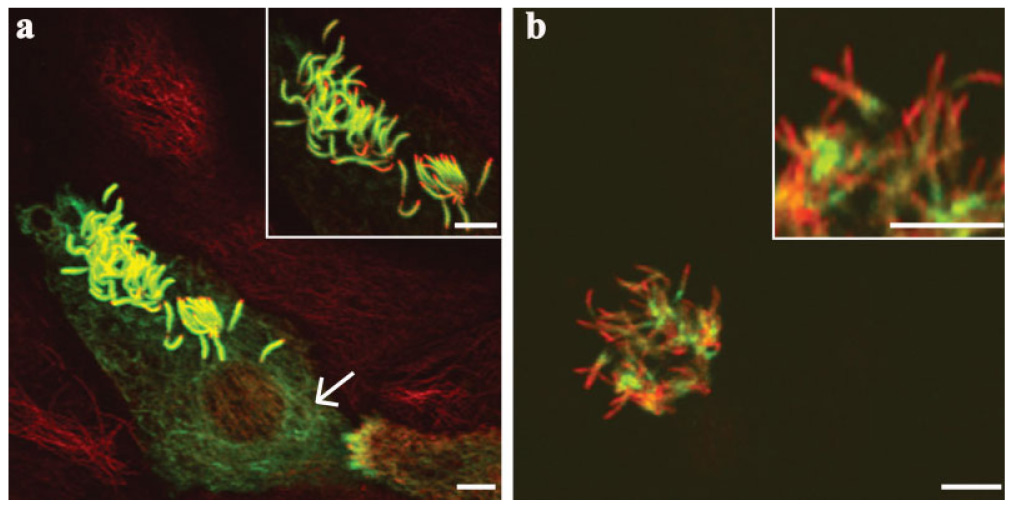
Confocal immunofluorescence images of MG (a) and PG (b) visualized with TAP 952 and AXO 49, respectively, in early stages of MTEC cultures (3 days in vitro). (a) Cell generating cilia during early stages of cultures lack MG at the distal tips of cilia. (a) MG was present in cytoplasmic microtubules of cells beginning ciliogenesis (arrow). (b) PG was present and variable in the early stages of the culture. Green = MG or PG. Red = α-tubulin (labels the entire lengths of cilia). Yellow (merged). Scale bars = 5 µm.
Mature stage (4–35 days post ALI)
Different patterns of MG and PG distribution became apparent as the cultures matured during the ALI (4–35 days post-ALI). In this stage, the patterns of labeling in the cultures became especially complex. The cultured cells were confluent and largely ciliated. After 4 days post-ALI, tubulin MG was again observed in most cilia present (Fig. 7a). MG was generally uniform within the cilia of an individual cell but varied considerably in extent between cilia of neighboring cells. Labeling along the lengths of cilia varied and once again the distal tips were excluded (Fig. 7a). Secondly, MG was not detected in cells with short cilia that appeared in a lower plane and were generally 0.33–2 µm shorter than those comprising MG. Contrary to MG, PG was not observed in cilia of the mature stage cultured cells (Fig. 7b).
Fig. 7.

Confocal immunofluorescence images of tubulin MG and PG in mature stages of MTEC cultures. (a) MG was maintained in mature cultures (32 days post-ALI). MG along the lengths of cilia varies between cilia of neighboring cells. (b) PG was absent in mature cultures (33 days post-ALI). Green = MG or PG. Red = α-tubulin (labels the entire lengths of cilia). Yellow (merged). Scale bars = 5 µm.
DISCUSSION
Polymeric Glycyl Side Chains are Not Required for Mammalian Cilia Function
Our observations show varying levels of polyglycylation in different gerbil ciliated tissues as well as between cilia of cells in the same tissue. MG was found in cilia of all the ciliated tissues examined and was consistently observed along the lengths of cilia but was not present at the distal tips. In contrast, PG was only observed in cilia of the trachea and oviduct and was localized at the bases of cilia. Thus, both MG and PG are present at the bases of cilia and likely coexist on different glutamyl residues on a tubulin monomer. It is difficult to ascribe a reason to this finding as there is nothing to indicate that tracheal and oviduct cilia are functionally different from cilia in other tissues. Furthermore, cilia in the MTEC cultures were able to beat at normal frequencies in the absence of detectable PG. Infrequent observations of PG in early stages of the MTEC cultures likely resulted from the seeding of a small quantity of ciliated epithelial cells during the establishment of the cultures. Although epithelial cells should eventually contain PG as their cilia mature, the production of mucus by the cultures limit the analysis of cilia in later stages. Hence we conclude that the presence of polymeric glycyl side chains of three of more glycyl residues is not required for rodent ciliary beating.
In contrast, the abundance of MG in mammalian tissues and the MTEC cultures suggests that MG may contribute to ciliary function. Similarly, MG has been shown in different axonemal models, including ciliated rodent lung cells [Péchart et al., 1999], human respiratory epithelial cells [Million et al., 1999], and flagella of human spermatozoa [Bré et al., 1996]. Xia et al. [2000] showed that Tetrahymena cells expressing β-tubulin (but not α-tubulin) with three or more mutated C-terminal polyglycylatable glutamyl residues were either abnormal or lethal. They also showed that, while polyglycylation deficient mutated β-tubulin could be incorporated into Tetrahymena cilia, the resulting cilia were defective. These defects included a decreased rate of assembly, a decrease in the lengths of cilia, and the absence of central pair microtubules.
The presence of tubulin PG in gerbil oviduct cilia is reminiscent of the result obtained in quail oviduct epithelial cells where the ciliary axonemes were found to be strongly labeled with a polyclonal antibody [Adoutte et al., 1991] having a specificity similar to AXO 49 [Levilliers et al., 1995].
Monomeric Glycylation in Cytoplasmic Microtubules
The discovery of polyglycylation in hyperstable microtubules of Paramecium cilia [Redeker et al., 1994] led to the suggestion that polyglycylation was associated with stable microtubular networks [Redeker et al., 1994] and not with dynamic, labile microtubules. However, tubulin polyglycylation of maximum side chain length of two glycyl residues has also been found in the cytoplasmic microtubules of Paramecium [Bré et al., 1998]. Given the difference in side chain length between axonemal and cytoplasmic microtubules, a correlation was established between the side chain length and microtubule stability in Paramecium. In human respiratory epithelial cell cultures, Million et al. [1999] presented evidence that TAP 952 was reactive with axonemal microtubules and not with cytoplasmic microtubules. Strikingly, in the MTEC cultures we observed the presence of MG in dynamic cytoplasmic microtubules. Thus, polyglycylation in cytoplasmic microtubules is not exclusive to ciliated protists. Additionally, since polyglycylation of cytoplasmic microtubules was only visible in some ciliated cells and at early stages of ciliogenesis, we hypothesize that the presence of MG in cytoplasmic microtubules may be a feature that contributes to the initiation of ciliogenesis in trachea epithelial cells.
Polyglycylation as a Marker for Tubulin Age (Postincorporation)
In the trachea and oviduct, we observed that the extent of polyglycylation along the lengths of cilia is progressive and varies from a lack of polyglycylation at the tips of cilia, to MG, and then (in some cases) to PG at the bases of cilia. If tubulin turnover in the axoneme is at a low rate, as has been suggested, then the extent of polyglycylation would in effect be a marker for the length of time that a tubulin monomer has been in the axoneme. This hypothesis is consistent with our observation that, while the extent of polyglycylation varies from cell to cell in a tissue, it is uniform in cilia of a cell. Considering that ciliated epithelial cells are removed and regularly replaced at intervals of days, the extent of polyglycylation would be expected to vary from cell to cell and should reflect the age of tubulin in cilia of a cell. This proposal is in accordance with Iftode et al. [2000], who showed in Paramecium the presence of MG in newly assembled axonemal structures of cilia, cortical and oral groove basal bodies, whereas PG was observed in older cilia and basal bodies. They also suggested that the length of glycyl residues on tubulin could serve as a discriminator between young and mature microtubules.
A question that should be addressed is what use the cell might make of an age marker. If turnover is occurring in cilia, then the cell would need a marker to indicate that tubulin monomers or dimers are ready for removal. The extent of polyglycylation may therefore function as a signal to initiate the removal of axonemal tubulin at the bases of cilia.
Polyglycylation Enzymes
At least two enzymatic reactions are necessary for tubulin polyglycylation. One enzyme (an initiase) would be required to attach the first glycyl residue onto the γ carboxyl group of glutamate, thus forming an α-γ isopeptide bond, and the second (an elongase) would be required to elongate the glycyl chain by the addition of increasing numbers of glycyl residues to the initial glycyl via α-α bonds. The initiase could be near the distal ends of cilia, but likely not at their tips since there is no detectable polyglycylation at the tips. The elongase could be located anywhere or everywhere in the cilium, since it cannot act until the initiase has added the first glycyl residue. Likewise, two enzymes would be required for deglycylation at the bases of cilia. Assuming that deglycylation occurs prior to the removal of tubulin dimers from the axoneme, the first enzyme would be a peptidase that is specific for the glycyl chain and quite likely one that cleaves at the first peptide bond between the first and second glycyl residues. The second enzyme would be an isopeptidase that cleaves the α-γ isopeptide bond between the glutamyl residue and the first glycyl residue. Both enzymes would be located at the bases of cilia, and possibly associated with the basal body. Alternatively, both polyglycylating and deglycylating enzymes could be employed to regulate side chain length, according to findings in Paramecium [Bré et al., 1998].
ACKNOWLEDGMENTS
We acknowledge the use of the confocal microscope facility of the Nebraska Center for Cell Biology. We thank Phillip Karp and Michael Welsh, University of Iowa School of Medicine, for their help with the primary MTEC culture and Heather Jensen-Smith, Creighton University School of Medicine, for her help with the gerbil tissue dissections.
Contract grant sponsors: National Center for Research Resources, National Institutes of Health; Contract grant number: 1 C06 RR17417-01; Contract grant sponsor: N.S.F.-E.P.S.Co.R; Contract grant number: EPS-0346476 (CFD 47.076).
Abbreviations used
- ALI
air liquid interphase
- BSA
bovine serum albumin
- CBF
ciliary beat frequency
- MG
monomeric glycylation
- MTEC
mouse tracheal epithelial cell
- NGS
normal goat serum
- PBS
phosphate buffered saline
- PG
polymeric glycylation
- PTMs
post-translational modifications
- SAVA
Sisson-Ammons video analysis
REFERENCES
- Adoutte A, Delgado P, Fleury A, Levilliers N, Laine MC, Marty MC, Boisvieux-Ulrich E, Sandoz D. Microtubule diversity in ciliated cells: Evidence for its generation by post-translational modification in the axonemes of Paramecium and quail oviduct cells. Biol Cell. 1991;71(12):227–245. doi: 10.1016/0248-4900(91)90069-y. [DOI] [PubMed] [Google Scholar]
- Bré MH, Redeker V, Quibell M, Darmanaden-Delorme J, Bressac C, Cosson J, Huitorel P, Schmitter JM, Rossler J, Johnson T, Adoutte A, Levilliers N. Axonemal tubulin polyglycylation probed with two monoclonal antibodies: Widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J Cell Sci. 1996;109(Part 4):727–738. doi: 10.1242/jcs.109.4.727. [DOI] [PubMed] [Google Scholar]
- Bré MH, Redeker V, Vinh J, Rossier J, Levilliers N. Tubulin polyglycylation: Differential posttranslational modification of dynamic cytoplasmic and stable axonemal microtubules in paramecium. Mol Biol Cell. 1998;9(9):2655–2665. doi: 10.1091/mbc.9.9.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie AT. Isolation of respiratory cilia. Methods Cell Biol. 1995;47:93–98. doi: 10.1016/s0091-679x(08)60795-5. [DOI] [PubMed] [Google Scholar]
- Iftode F, Clerot JC, Levilliers N, Bre MH. Tubulin polyglycylation: A morphogenetic marker in ciliates. Biol Cell. 2000;92(89):615–628. doi: 10.1016/s0248-4900(00)01107-2. [DOI] [PubMed] [Google Scholar]
- Jensen-Smith HC, Luduena RF, Hallworth R. Requirement for the betaI and betaIV tubulin isotypes in mammalian cilia. Cell Motil Cytoskeleton. 2003;55(3):213–220. doi: 10.1002/cm.10122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levilliers N, Fleury A, Hill AM. Monoclonal and polyclonal antibodies detect a new type of post-translational modification of axonemal tubulin. J Cell Sci. 1995;108(Part 9):3013–3028. doi: 10.1242/jcs.108.9.3013. [DOI] [PubMed] [Google Scholar]
- Luduena RF. Multiple forms of tubulin: Different gene products and covalent modifications. Int Rev Cytol. 1998;178:207–275. doi: 10.1016/s0074-7696(08)62138-5. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Tubulin post-translational modifications—Enzymes and their mechanisms of action. Eur J Biochem. 1997;244(2):265–278. doi: 10.1111/j.1432-1033.1997.00265.x. [DOI] [PubMed] [Google Scholar]
- Million K, Larcher J, Laoukili J, Bourguignon D, Marano F, Tournier F. Polyglutamylation and polyglycylation of alpha- and beta-tubulins during in vitro ciliated cell differentiation of human respiratory epithelial cells. J Cell Sci. 1999;112(Part 23):4357–4366. doi: 10.1242/jcs.112.23.4357. [DOI] [PubMed] [Google Scholar]
- Péchart I, Kann ML, Levilliers N, Bré MH, Fouquet JP. Composition and organization of tubulin isoforms reveals a variety of axonemal models. Biol Cell. 1999;91(9):685–697. doi: 10.1111/j.1768-322x.1999.tb01113.x. [DOI] [PubMed] [Google Scholar]
- Perry B, Jensen-Smith HC, Luduena RF, Hallworth R. Selective expression of beta tubulin isotypes in gerbil vestibular sensory epithelia and neurons. J Assoc Res Otolaryngol. 2003;4(3):329–338. doi: 10.1007/s10162-002-2048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessmann U, Weber K. Mammalian sperm tubulin: An exceptionally large number of variants based on several posttranslational modifications. J Protein Chem. 1997;16(5):385–390. doi: 10.1023/a:1026332621215. [DOI] [PubMed] [Google Scholar]
- Redeker V, Levilliers N, Schmitter JM, Le Caer JP, Rossier J, Adoutte A, Bre MH. Polyglycylation of tubulin: A posttranslational modification in axonemal microtubules. Science. 1994;266(5191):1688–1691. doi: 10.1126/science.7992051. [DOI] [PubMed] [Google Scholar]
- Rudiger M, Plessmann U, Rudiger AH, Weber K. Beta tubulin of bull sperm is polyglycylated. FEBS Lett. 1995;364(2):147–151. doi: 10.1016/0014-5793(95)00373-h. [DOI] [PubMed] [Google Scholar]
- Sisson JH, Stoner JA, Ammons BA, Wyatt TA. All-digital image capture and whole-field analysis of ciliary beat frequency. J Microsc. 2003;211(Part 2):103–111. doi: 10.1046/j.1365-2818.2003.01209.x. [DOI] [PubMed] [Google Scholar]
- Vinh J, Langridge JI, Bre MH, Levilliers N, Redeker V, Loyaux D, Rossier J. Structural characterization by tandem mass spectrometry of the posttranslational polyglycylation of tubulin. Biochemistry. 1999;38(10):3133–3139. doi: 10.1021/bi982304s. [DOI] [PubMed] [Google Scholar]
- Westermann S, Weber K. Post-translational modifications regulate microtubule function. Nat Rev Mol Cell Biol. 2003;4(12):938–947. doi: 10.1038/nrm1260. [DOI] [PubMed] [Google Scholar]
- Woo K, Jensen-Smith HC, Luduena RF, Hallworth R. Differential synthesis of beta-tubulin isotypes in gerbil nasal epithelia. Cell Tissue Res. 2002;309(2):331–335. doi: 10.1007/s00441-002-0591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L, Hai B, Gao Y, Burnette D, Thazhath R, Duan J, Bre MH, Levilliers N, Gorovsky MA, Gaertig J. Polyglycylation of tubulin is essential and affects cell motility and division in Tetrahymena thermophila. J Cell Biol. 2000;149(5):1097–1106. doi: 10.1083/jcb.149.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]


