Abstract
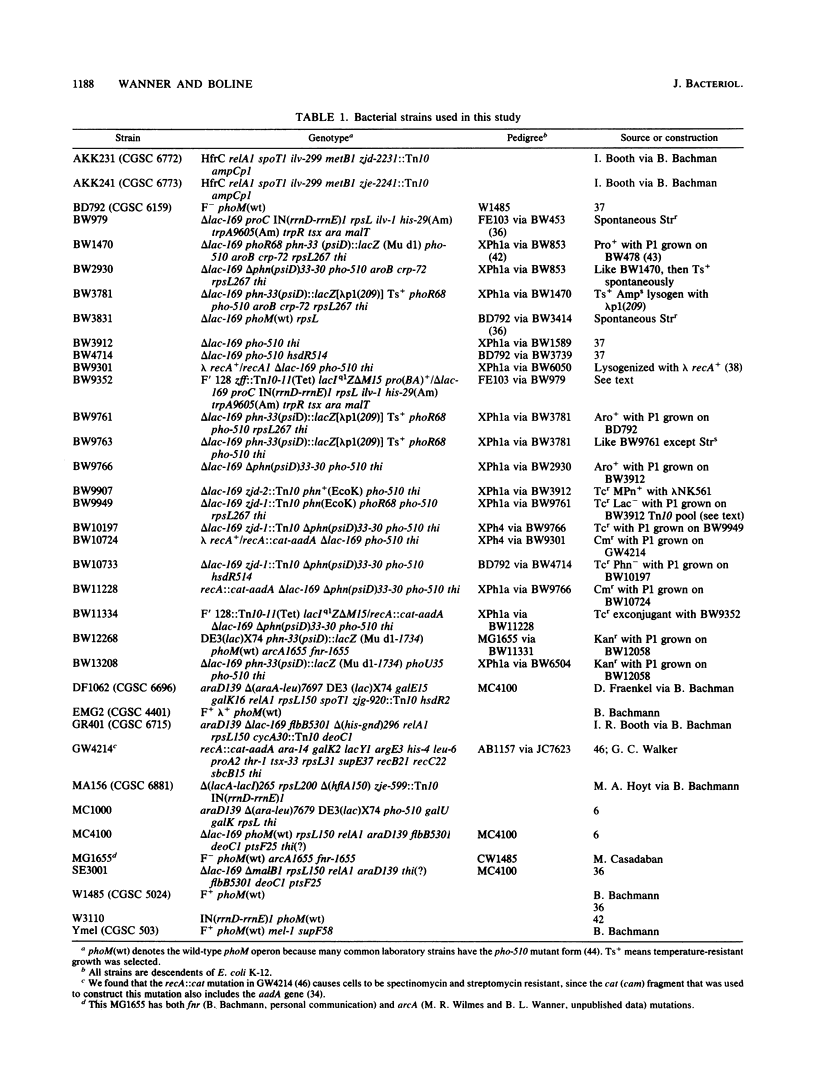
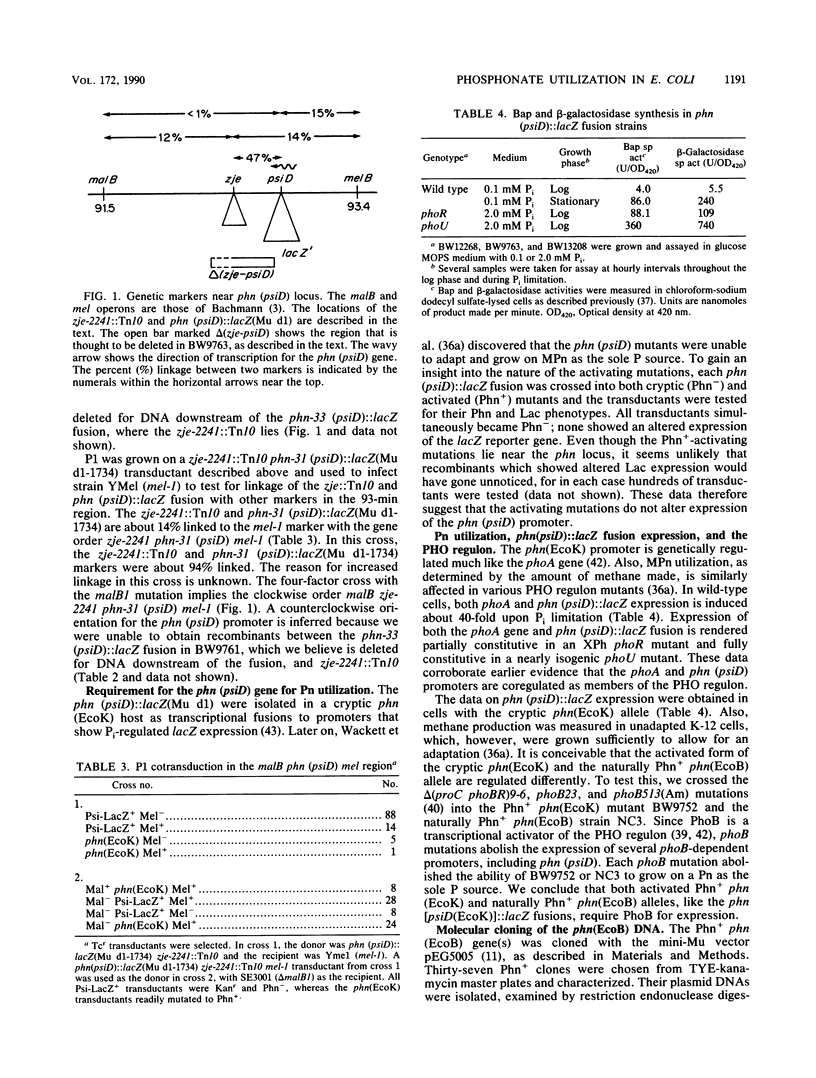
The Escherichia coli phn (psiD) locus encodes genes for phosphonate (Pn) utilization, for phn (psiD) mutations abolish the ability to use as a sole P source a Pn with a substituted C-2 or unsubstituted hydrocarbon group such as 2-aminoethylphosphonate (AEPn) or methylphosphonate (MPn), respectively. Even though the E. coli K-12 phosphate starvation-inducible (psi) phn (psiD) gene(s) shows normal phosphate (Pi) control, Pn utilization is cryptic in E. coli K-12, as well as in several members of the E. coli reference (ECOR) collection which are closely related to K-12. For these bacteria, an activating mutation near the phn (psiD) gene is necessary for growth on a Pn as the sole P source. Most E. coli strains, including E. coli B, are naturally Phn+; a few E. coli strains are Phn- and are deleted for phn DNA sequences. The Phn+ phn(EcoB) DNA was molecularly cloned by using the mini-Mu in vivo cloning procedure and complementation of an E. coli K-12 delta phn mutant. The phn(EcoB) DNA hybridized to overlapping lambda clones in the E. coli K-12 gene library (Y. Kohara, K. Akiyama, and K. Isono, Cell 50:495-508, 1987) which contain the 93-min region, thus showing that the phn (psiD) locus was itself cloned and verifying our genetic data on its map location. The cryptic phn(EcoK) DNA has an additional 100 base pairs that is absent in the naturally Phn+ phn(EcoB) sequence. However, no gross structural change was detected in independent Phn+ phn(EcoK) mutants that have activating mutations near the phn locus.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atherton F. R., Hassall C. H., Lambert R. W. Synthesis and structure-activity relationships of antibacterial phosphonopeptides incorporating (1-aminoethyl)phosphonic acid and (aminomethyl)phosphonic acid. J Med Chem. 1986 Jan;29(1):29–40. doi: 10.1021/jm00151a005. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilho B. A., Olfson P., Casadaban M. J. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J Bacteriol. 1984 May;158(2):488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan T. T., Newman E. B. Threonine as a carbon source for Escherichia coli. J Bacteriol. 1981 Mar;145(3):1150–1153. doi: 10.1128/jb.145.3.1150-1153.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Daughton C. G., Alexander M. Benzene from bacterial cleavage of the carbon-phosphorus bond of phenylphosphonates. Biochem J. 1979 Nov 15;184(2):453–455. doi: 10.1042/bj1840453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington G. K., Kumar A., Wedler F. C. Design and synthesis of phosphonate inhibitors of glutamine synthetase. J Med Chem. 1987 Nov;30(11):2062–2067. doi: 10.1021/jm00394a022. [DOI] [PubMed] [Google Scholar]
- Groisman E. A., Casadaban M. J. Cloning of genes from members of the family Enterobacteriaceae with mini-Mu bacteriophage containing plasmid replicons. J Bacteriol. 1987 Feb;169(2):687–693. doi: 10.1128/jb.169.2.687-693.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIGUCHI M., KANDATSU M. Isolation of 2-aminoethane phosphonic acid from rumen protozoa. Nature. 1959 Sep 19;184(Suppl 12):901–902. doi: 10.1038/184901b0. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Betts P. W. Cryptic genes for cellobiose utilization in natural isolates of Escherichia coli. Genetics. 1987 Mar;115(3):431–439. doi: 10.1093/genetics/115.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B. G., Betts P. W., Kricker M. Maintenance of the cellobiose utilization genes of Escherichia coli in a cryptic state. Mol Biol Evol. 1986 Sep;3(5):389–402. doi: 10.1093/oxfordjournals.molbev.a040406. [DOI] [PubMed] [Google Scholar]
- Harkness D. R. Bacterial growth on aminoalkylphosphonic acids. J Bacteriol. 1966 Sep;92(3):623–627. doi: 10.1128/jb.92.3.623-627.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittredge J. S., Roberts E. A carbon-phosphorus bond in nature. Science. 1969 Apr 4;164(3875):37–42. doi: 10.1126/science.164.3875.37. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- La Nauze J. M., Coggins J. R., Dixon H. B. Aldolase-like imine formation in the mechanism of action of phosphonoacetaldehyde hydrolase. Biochem J. 1977 Aug 1;165(2):409–411. doi: 10.1042/bj1650409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Nauze J. M., Rosenberg H., Shaw D. C. The enzymic cleavage of the carbon-phosphorus bond: purification and properties of phosphonatase. Biochim Biophys Acta. 1970 Aug 15;212(2):332–350. doi: 10.1016/0005-2744(70)90214-7. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Adams C. W., Hauser C. A., Gray J., Hatfield G. W. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981 Feb;78(2):922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawther R. P., Calhoun D. H., Gray J., Adams C. W., Hauser C. A., Hatfield G. W. DNA sequence fine-structure analysis of ilvG (IlvG+) mutations of Escherichia coli K-12. J Bacteriol. 1982 Jan;149(1):294–298. doi: 10.1128/jb.149.1.294-298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MASTALERZ P., WIECZOREK Z., KOCHMAN M. UTILIZATION OF CARBON-BOUND PHOSPHORUS BY MICROORGANISMS. Acta Biochim Pol. 1965;12:151–156. [PubMed] [Google Scholar]
- Moore J. K., Braymer H. D., Larson A. D. Isolation of a Pseudomonas sp. Which Utilizes the Phosphonate Herbicide Glyphosate. Appl Environ Microbiol. 1983 Aug;46(2):316–320. doi: 10.1128/aem.46.2.316-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Higaki N., Kimura A. A microbial carbon-phosphorus bond cleavage enzyme requires two protein components for activity. J Bacteriol. 1989 Aug;171(8):4504–4506. doi: 10.1128/jb.171.8.4504-4506.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata K., Higaki N., Kimura A. Detection of carbon-phosphorus lyase activity in cell free extracts of Enterobacter aerogenes. Biochem Biophys Res Commun. 1988 Nov 30;157(1):190–195. doi: 10.1016/s0006-291x(88)80031-7. [DOI] [PubMed] [Google Scholar]
- ROWLEY D. Interrelationships between amino-acids in the growth of coliform organisms. J Gen Microbiol. 1953 Aug;9(1):37–43. doi: 10.1099/00221287-9-1-37. [DOI] [PubMed] [Google Scholar]
- Reynolds A. E., Felton J., Wright A. Insertion of DNA activates the cryptic bgl operon in E. coli K12. Nature. 1981 Oct 22;293(5834):625–629. doi: 10.1038/293625a0. [DOI] [PubMed] [Google Scholar]
- Robinson C. L., Jackson J. H. New acetohydroxy acid synthase activity from mutational activation of a cryptic gene in Escherichia coli K-12. Mol Gen Genet. 1982;186(2):240–246. doi: 10.1007/BF00331856. [DOI] [PubMed] [Google Scholar]
- Schaefler S., Maas W. K. Inducible system for the utilization of beta-glucosides in Escherichia coli. II. Description of mutant types and genetic analysis. J Bacteriol. 1967 Jan;93(1):264–272. doi: 10.1128/jb.93.1.264-272.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N. Structural and functional analysis of cloned DNA segments containing the replication and incompatibility regions of a miniplasmid derived from a copy number mutant of NR1. J Bacteriol. 1979 Jan;137(1):92–104. doi: 10.1128/jb.137.1.92-104.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij A., Boter H. L., Degenhardt C. E. Chemical warfare agents: verification of compounds containing the phosphorus-methyl linkage in waste water. Science. 1979 May 11;204(4393):616–618. doi: 10.1126/science.204.4393.616. [DOI] [PubMed] [Google Scholar]
- Wackett L. P., Shames S. L., Venditti C. P., Walsh C. T. Bacterial carbon-phosphorus lyase: products, rates, and regulation of phosphonic and phosphinic acid metabolism. J Bacteriol. 1987 Feb;169(2):710–717. doi: 10.1128/jb.169.2.710-717.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Wanner B. L., Venditti C. P., Walsh C. T. Involvement of the phosphate regulon and the psiD locus in carbon-phosphorus lyase activity of Escherichia coli K-12. J Bacteriol. 1987 Apr;169(4):1753–1756. doi: 10.1128/jb.169.4.1753-1756.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Chang B. D. The phoBR operon in Escherichia coli K-12. J Bacteriol. 1987 Dec;169(12):5569–5574. doi: 10.1128/jb.169.12.5569-5574.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Physiological regulation of a decontrolled lac operon. J Bacteriol. 1977 Apr;130(1):212–222. doi: 10.1128/jb.130.1.212-222.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., McSharry R. Phosphate-controlled gene expression in Escherichia coli K12 using Mudl-directed lacZ fusions. J Mol Biol. 1982 Jul 5;158(3):347–363. doi: 10.1016/0022-2836(82)90202-9. [DOI] [PubMed] [Google Scholar]
- Wanner B. L. Molecular cloning of Mu d(bla lacZ) transcriptional and translational fusions. J Bacteriol. 1987 May;169(5):2026–2030. doi: 10.1128/jb.169.5.2026-2030.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L. Novel regulatory mutants of the phosphate regulon in Escherichia coli K-12. J Mol Biol. 1986 Sep 5;191(1):39–58. doi: 10.1016/0022-2836(86)90421-3. [DOI] [PubMed] [Google Scholar]
- Wanner B. L., Wieder S., McSharry R. Use of bacteriophage transposon Mu d1 to determine the orientation for three proC-linked phosphate-starvation-inducible (psi) genes in Escherichia coli K-12. J Bacteriol. 1981 Apr;146(1):93–101. doi: 10.1128/jb.146.1.93-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Wilmes M. R., Young D. C. Control of bacterial alkaline phosphatase synthesis and variation in an Escherichia coli K-12 phoR mutant by adenyl cyclase, the cyclic AMP receptor protein, and the phoM operon. J Bacteriol. 1988 Mar;170(3):1092–1102. doi: 10.1128/jb.170.3.1092-1102.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way J. C., Davis M. A., Morisato D., Roberts D. E., Kleckner N. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene. 1984 Dec;32(3):369–379. doi: 10.1016/0378-1119(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- ZELEZNICK L. D., MYERS T. C., TITCHENER E. B. GROWTH OF ESCHERICHIA COLI ON METHYL- AND ETHYLPHOSPHONIC ACIDS. Biochim Biophys Acta. 1963 Nov 15;78:546–547. doi: 10.1016/0006-3002(63)90921-1. [DOI] [PubMed] [Google Scholar]