Abstract
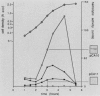
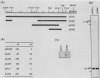
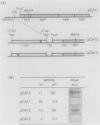
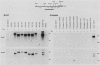
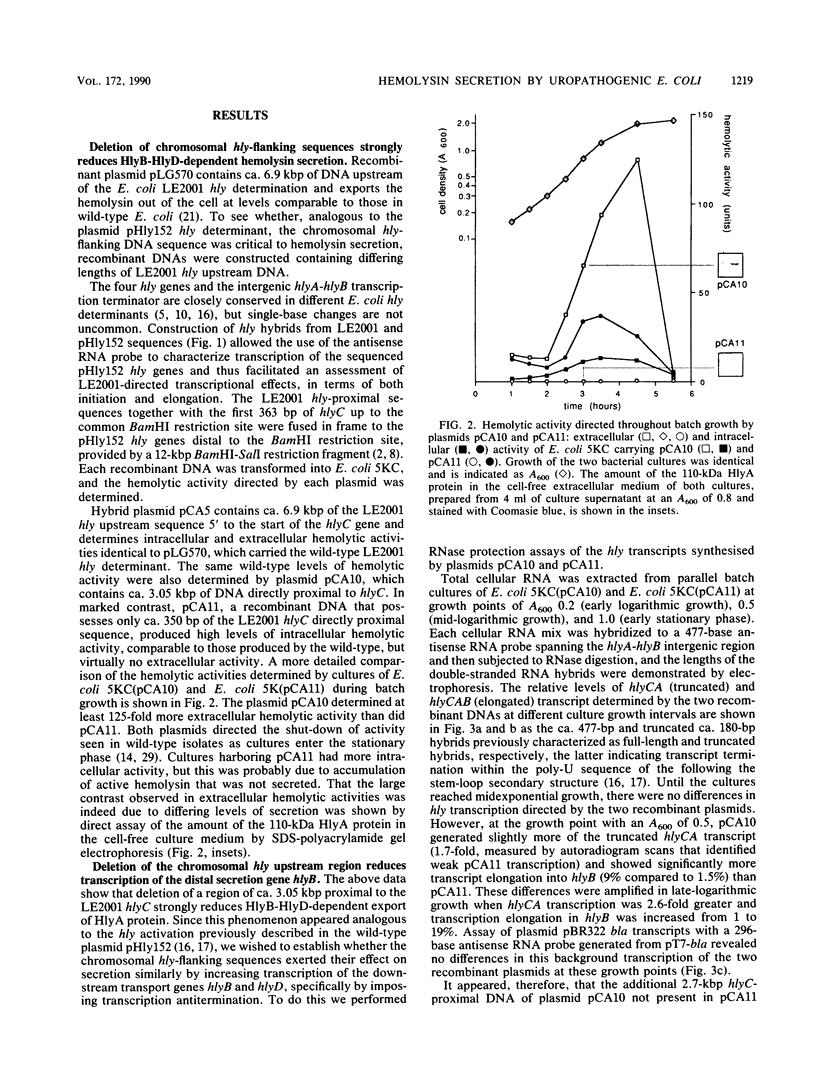
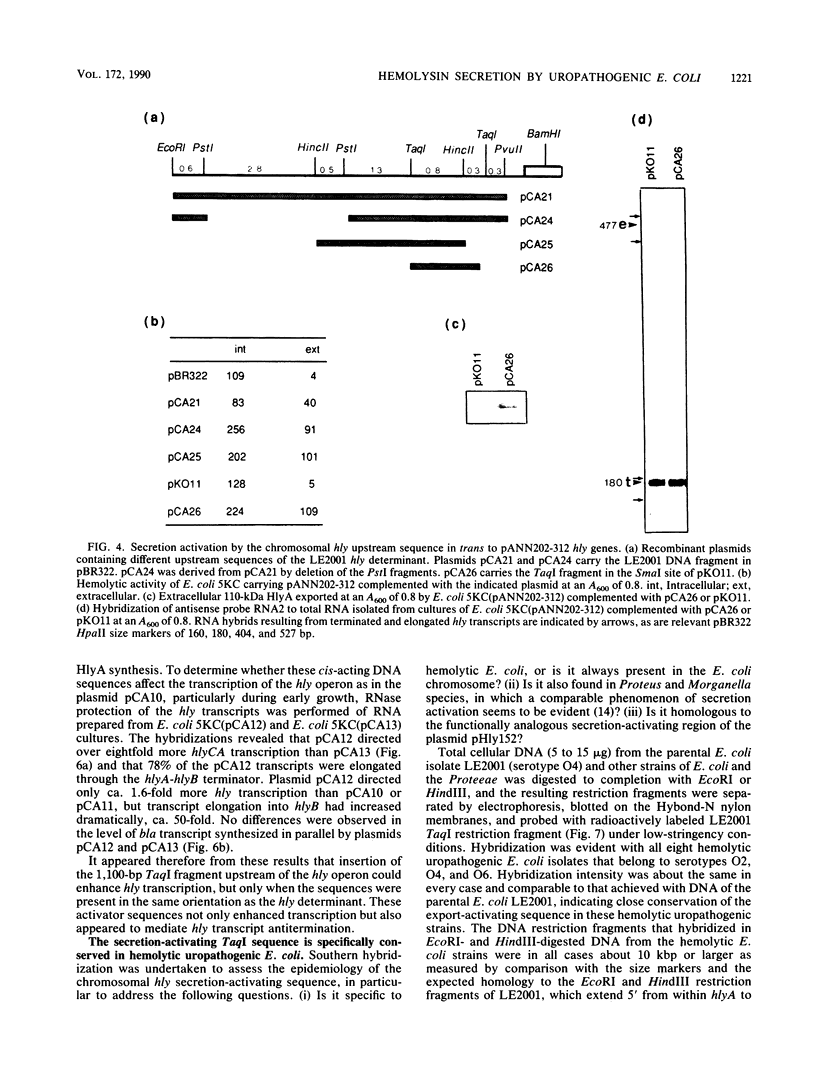
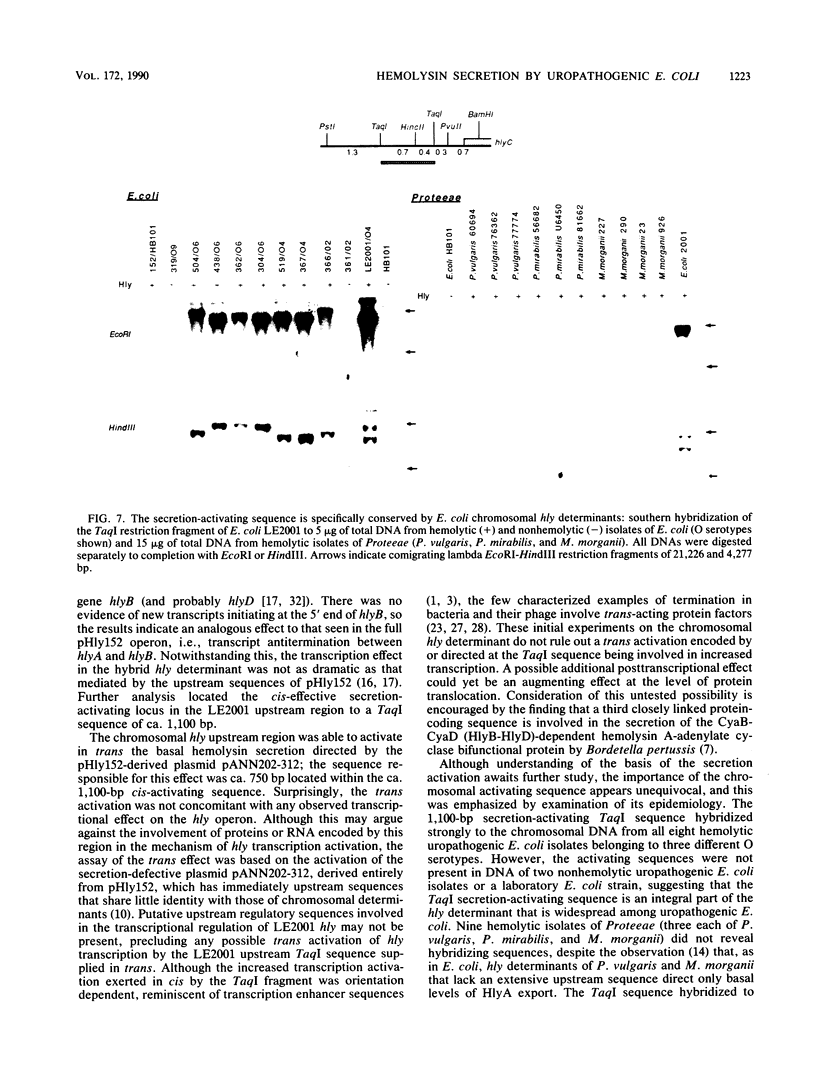
The synthesis and secretion of hemolysin (HlyA) by Escherichia coli are governed by four contiguous genes (hlyCABD) that are closely conserved on plasmids and, among human pathogenic strains, on the chromosome. We have previously shown that in plasmid pHly152 the coexpressed synthesis and export functions are uncoupled by intraoperon transcription termination, which is in turn alleviated by antitermination dictated in cis by a region upstream of the hly operon. In this study we describe an analogous region of ca. 1,100 base pairs flanking the chromosomal hly determinant of the uropathogenic strain E. coli 2001. This region had no significant effect on intracellular levels of hemolysin but activated strongly, both in cis and in trans, the specific hlyB-hlyD-dependent hemolysin secretion function. The secretion-activating region increased the transcription of the secretion gene hlyB, but the transcription effect was not as pronounced as that seen in the pHly152 determinant and was not evident when the region was present in trans to the hemolysin genes, suggesting that, in addition to transcriptional activation, the region may possibly exert a secondary posttranscriptional influence. Southern hybridizations with the 1,100-base pairs secretion-activating sequence showed low identity to plasmid pHly152 and no identity with total DNA from nonhemolytic uropathogenic E. coli or hemolytic isolates of Proteus vulgaris, Proteus mirabilis, and Morganella morganii. In contrast, hybridization to total DNA from hemolytic E. coli isolates belonging to different serotypes showed strong conservation of the activating sequence, indicating that it is an integral component of the chromosomal hly determinant that is widespread among uropathogenic E. coli.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Berger H., Hacker J., Juarez A., Hughes C., Goebel W. Cloning of the chromosomal determinants encoding hemolysin production and mannose-resistant hemagglutination in Escherichia coli. J Bacteriol. 1982 Dec;152(3):1241–1247. doi: 10.1128/jb.152.3.1241-1247.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandanell G., Valentin-Hansen P., Larsen J. E., Hammer K. Long-range cooperativity between gene regulatory sequences in a prokaryote. 1987 Feb 26-Mar 4Nature. 325(6107):823–826. doi: 10.1038/325823a0. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Lee E. Y., Welch R. A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985 Jul;163(1):88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J. H., Endicott J. A., Juranka P. F., Henderson G., Sarangi F., Deuchars K. L., Ling V. Homology between P-glycoprotein and a bacterial haemolysin transport protein suggests a model for multidrug resistance. Nature. 1986 Dec 4;324(6096):485–489. doi: 10.1038/324485a0. [DOI] [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebel W., Hedgpeth J. Cloning and functional characterization of the plasmid-encoded hemolysin determinant of Escherichia coli. J Bacteriol. 1982 Sep;151(3):1290–1298. doi: 10.1128/jb.151.3.1290-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Hiles I. D., Salmond G. P., Gill D. R., Downie J. A., Evans I. J., Holland I. B., Gray L., Buckel S. D., Bell A. W. A family of related ATP-binding subunits coupled to many distinct biological processes in bacteria. Nature. 1986 Oct 2;323(6087):448–450. doi: 10.1038/323448a0. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Juarez A., Hughes C., Vogel M., Goebel W. Expression and regulation of the plasmid-encoded hemolysin determinant of Escherichia coli. Mol Gen Genet. 1984;197(2):196–203. doi: 10.1007/BF00330963. [DOI] [PubMed] [Google Scholar]
- Knapp S., Then I., Wels W., Michel G., Tschäpe H., Hacker J., Goebel W. Analysis of the flanking regions from different haemolysin determinants of Escherichia coli. Mol Gen Genet. 1985;200(3):385–392. doi: 10.1007/BF00425721. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Hughes C. Expression of the E.coli hemolysin secretion gene hlyB involves transcript anti-termination within the hly operon. Nucleic Acids Res. 1988 Jun 10;16(11):4789–4800. doi: 10.1093/nar/16.11.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Hughes C. Transcription antitermination in an Escherichia coli haemolysin operon is directed progressively by cis-acting DNA sequences upstream of the promoter region. Mol Microbiol. 1989 Oct;3(10):1397–1404. doi: 10.1111/j.1365-2958.1989.tb00122.x. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Cross M., Senior B., Koronakis E., Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987 Apr;169(4):1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koronakis V., Hughes C. Identification of the promoters directing in vivo expression of hemolysin genes in Proteus vulgaris and Escherichia coli. Mol Gen Genet. 1988 Jul;213(1):99–104. doi: 10.1007/BF00333404. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Comparison of the haemolysin secretion protein HlyB from Proteus vulgaris and Escherichia coli; site-directed mutagenesis causing impairment of export function. Mol Gen Genet. 1988 Aug;213(2-3):551–555. doi: 10.1007/BF00339631. [DOI] [PubMed] [Google Scholar]
- Koronakis V., Koronakis E., Hughes C. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 1989 Feb;8(2):595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Baker K., Gray L., Haigh R., Nicaud J. M., Holland I. B. Release of a chimeric protein into the medium from Escherichia coli using the C-terminal secretion signal of haemolysin. EMBO J. 1987 Sep;6(9):2835–2841. doi: 10.1002/j.1460-2075.1987.tb02580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Genetical and functional organisation of the Escherichia coli haemolysin determinant 2001. Mol Gen Genet. 1985;201(2):282–288. doi: 10.1007/BF00425672. [DOI] [PubMed] [Google Scholar]
- Mackman N., Nicaud J. M., Gray L., Holland I. B. Secretion of haemolysin by Escherichia coli. Curr Top Microbiol Immunol. 1986;125:159–181. doi: 10.1007/978-3-642-71251-7_10. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Wright A. A bacterial gene involved in transcription antitermination: regulation at a rho-independent terminator in the bgl operon of E. coli. Cell. 1987 Jul 31;50(3):485–494. doi: 10.1016/0092-8674(87)90502-2. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D., Hughes C., Goebel W. Relationship between plasmid and chromosomal hemolysin determinants of Escherichia coli. J Bacteriol. 1983 Feb;153(2):846–851. doi: 10.1128/jb.153.2.846-851.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Phage lambda and the regulation of transcription termination. Cell. 1988 Jan 15;52(1):5–6. doi: 10.1016/0092-8674(88)90523-5. [DOI] [PubMed] [Google Scholar]
- Schnetz K., Rak B. Regulation of the bgl operon of Escherichia coli by transcriptional antitermination. EMBO J. 1988 Oct;7(10):3271–3277. doi: 10.1002/j.1460-2075.1988.tb03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer W., Goebel W. Synthesis and secretion of hemolysin by Escherichia coli. J Bacteriol. 1980 Oct;144(1):53–59. doi: 10.1128/jb.144.1.53-59.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel M., Hess J., Then I., Juarez A., Goebel W. Characterization of a sequence (hlyR) which enhances synthesis and secretion of hemolysin in Escherichia coli. Mol Gen Genet. 1988 Apr;212(1):76–84. doi: 10.1007/BF00322447. [DOI] [PubMed] [Google Scholar]
- Wagner W., Vogel M., Goebel W. Transport of hemolysin across the outer membrane of Escherichia coli requires two functions. J Bacteriol. 1983 Apr;154(1):200–210. doi: 10.1128/jb.154.1.200-210.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch R. A., Pellett S. Transcriptional organization of the Escherichia coli hemolysin genes. J Bacteriol. 1988 Apr;170(4):1622–1630. doi: 10.1128/jb.170.4.1622-1630.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabala J. C., García-Lobo J. M., Diaz-Aroca E., de la Cruz F., Ortiz J. M. Escherichia coli alpha-haemolysin synthesis and export genes are flanked by a direct repetition of IS91-like elements. Mol Gen Genet. 1984;197(1):90–97. doi: 10.1007/BF00327927. [DOI] [PubMed] [Google Scholar]