Abstract
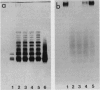
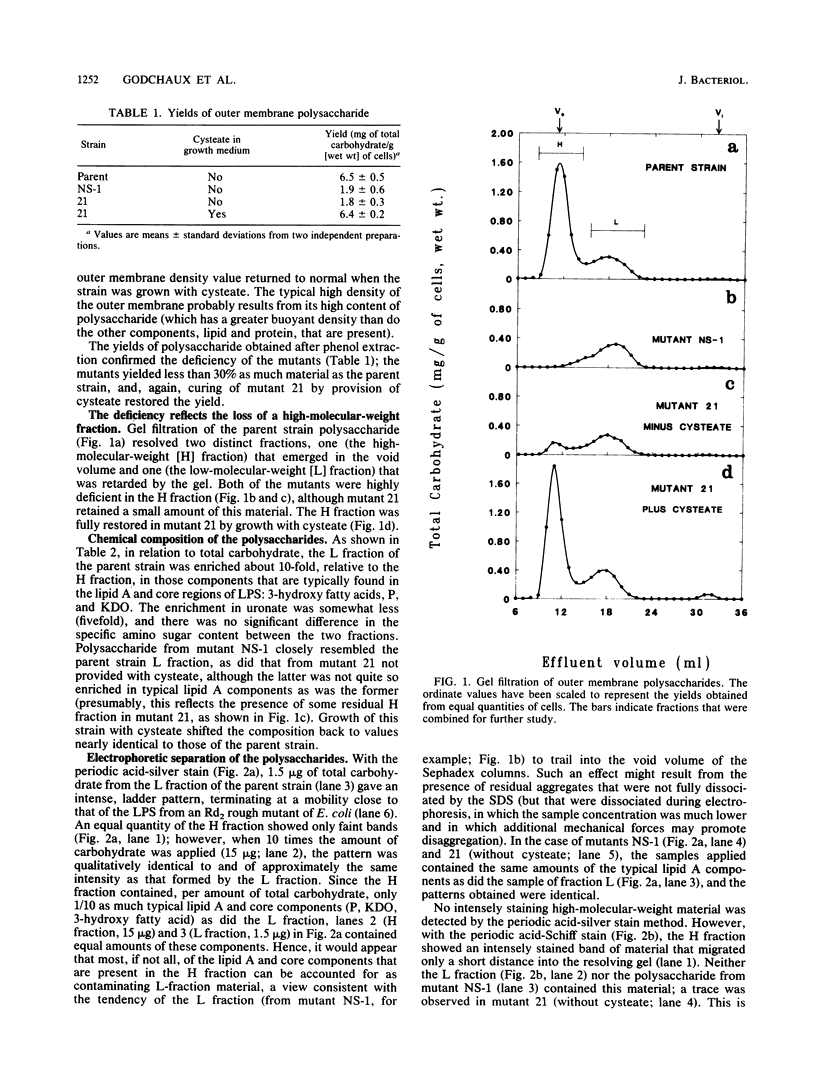
Phenol-extractable polysaccharides firmly associated with the outer membrane of the gliding bacterium Cytophaga johnsonae could be resolved by gel filtration in sodium dodecyl sulfate (SDS) or by SDS-polyacrylamide gel electrophoresis into a high-molecular-weight (H) fraction (excluded by Sephadex G-200) and a low-molecular-weight (L) fraction. Fraction L was rich in components typical of lipid A and the core region of lipopolysaccharide (P, 3-hydroxy fatty acids, and 2-keto-3-deoxyoctonate) and evidently was a lipopolysaccharide with a limited number of distal, repeating polysaccharide units, as judged by SDS-polyacrylamide gel electrophoresis. In relation to total carbohydrate, the H fraction was rich in amino sugar but poor in (possibly devoid of) the lipid A and core components. Two nongliding mutants were highly deficient in the H fraction; one of these was deficient in sulfonolipid but could be cured by provision of a specific sulfonolipid precursor, a process that also resulted in the return of both the H fraction and gliding, as well as the ability to move polystyrene latex spheres over the cell surface. Hence, the polysaccharide may be the component that is directly involved in motility, and the presence of sulfonolipids in the outer membrane is necessary for the synthesis or accumulation of the polysaccharide. This conclusion was reinforced by the fact that the second nongliding, polysaccharide-deficient mutant had a normal sulfonolipid content.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbanat D. R., Godchaux W., 3rd, Polychroniou G., Leadbetter E. R. Biosynthesis of a sulfonolipid in gliding bacteria. Biochem Biophys Res Commun. 1985 Jul 31;130(2):873–878. doi: 10.1016/0006-291x(85)90497-8. [DOI] [PubMed] [Google Scholar]
- Burchard R. P. Gliding motility of prokaryotes: ultrastructure, physiology, and genetics. Annu Rev Microbiol. 1981;35:497–529. doi: 10.1146/annurev.mi.35.100181.002433. [DOI] [PubMed] [Google Scholar]
- Caroff M., Lebbar S., Szabó L. Do endotoxins devoid of 3-deoxy-D-manno-2-octulosonic acid exist? Biochem Biophys Res Commun. 1987 Mar 30;143(3):845–847. doi: 10.1016/0006-291x(87)90326-3. [DOI] [PubMed] [Google Scholar]
- Chang L. E., Pate J. L., Betzig R. J. Isolation and characterization of nonspreading mutants of the gliding bacterium Cytophaga johnsonae. J Bacteriol. 1984 Jul;159(1):26–35. doi: 10.1128/jb.159.1.26-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bezard G. A highly sensitive periodic acid-silver stain for 1,2-diol groups of glycoproteins and polysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 15;119(2):325–329. doi: 10.1016/0003-2697(82)90593-0. [DOI] [PubMed] [Google Scholar]
- Gatt R., Berman E. R. A rapid procedure for the estimation of amino sugars on a micro scale. Anal Biochem. 1966 Apr;15(1):167–171. doi: 10.1016/0003-2697(66)90262-4. [DOI] [PubMed] [Google Scholar]
- Godchaux W., 3rd, Leadbetter E. R. Sulfonolipids of gliding bacteria. Structure of the N-acylaminosulfonates. J Biol Chem. 1984 Mar 10;259(5):2982–2990. [PubMed] [Google Scholar]
- Godchaux W., 3rd, Leadbetter E. R. Unusual sulfonolipids are characteristic of the Cytophaga-Flexibacter group. J Bacteriol. 1983 Mar;153(3):1238–1246. doi: 10.1128/jb.153.3.1238-1246.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman R. C., White D., Orskov F., Orskov I., Rick P. D., Lewis M. S., Bhattacharjee A. K., Leive L. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol. 1982 Sep;151(3):1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lapidus I. R., Berg H. C. Gliding motility of Cytophaga sp. strain U67. J Bacteriol. 1982 Jul;151(1):384–398. doi: 10.1128/jb.151.1.384-398.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitta T. P., Leadbetter E. R., Godchaux W., 3rd Increase of ornithine amino lipid content in a sulfonolipid-deficient mutant of Cytophaga johnsonae. J Bacteriol. 1989 Feb;171(2):952–957. doi: 10.1128/jb.171.2.952-957.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M., Bryan L. E., Hancock R. E., McGroarty E. J. Heterogeneity of lipopolysaccharides from Pseudomonas aeruginosa: analysis of lipopolysaccharide chain length. J Bacteriol. 1988 Feb;170(2):512–521. doi: 10.1128/jb.170.2.512-521.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfelder G., Lüderitz O., Westphal O. Composition of lipopolysaccharides from Myxococcus fulvus and other fruiting and non-fruiting myxobacteria. Eur J Biochem. 1974 May 15;44(2):411–420. doi: 10.1111/j.1432-1033.1974.tb03499.x. [DOI] [PubMed] [Google Scholar]
- Rothfield L., Romeo D. Role of lipids in the biosynthesis of the bacterial cell envelope. Bacteriol Rev. 1971 Mar;35(1):14–38. doi: 10.1128/br.35.1.14-38.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland I. W. Biosynthesis and composition of gram-negative bacterial extracellular and wall polysaccharides. Annu Rev Microbiol. 1985;39:243–270. doi: 10.1146/annurev.mi.39.100185.001331. [DOI] [PubMed] [Google Scholar]
- Verma J. P., Martin H. H. Uber die Oberflächenstruktur von Myxobakterien. I. Chemie und Morphologie der Zellwände von Cytophaga hutchinsonii und Sporocytophaga myxococcoides. Arch Mikrobiol. 1967;59(4):355–380. [PubMed] [Google Scholar]
- Whitfield C. Bacterial extracellular polysaccharides. Can J Microbiol. 1988 Apr;34(4):415–420. doi: 10.1139/m88-073. [DOI] [PubMed] [Google Scholar]