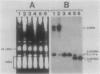
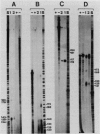
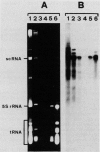
Abstract
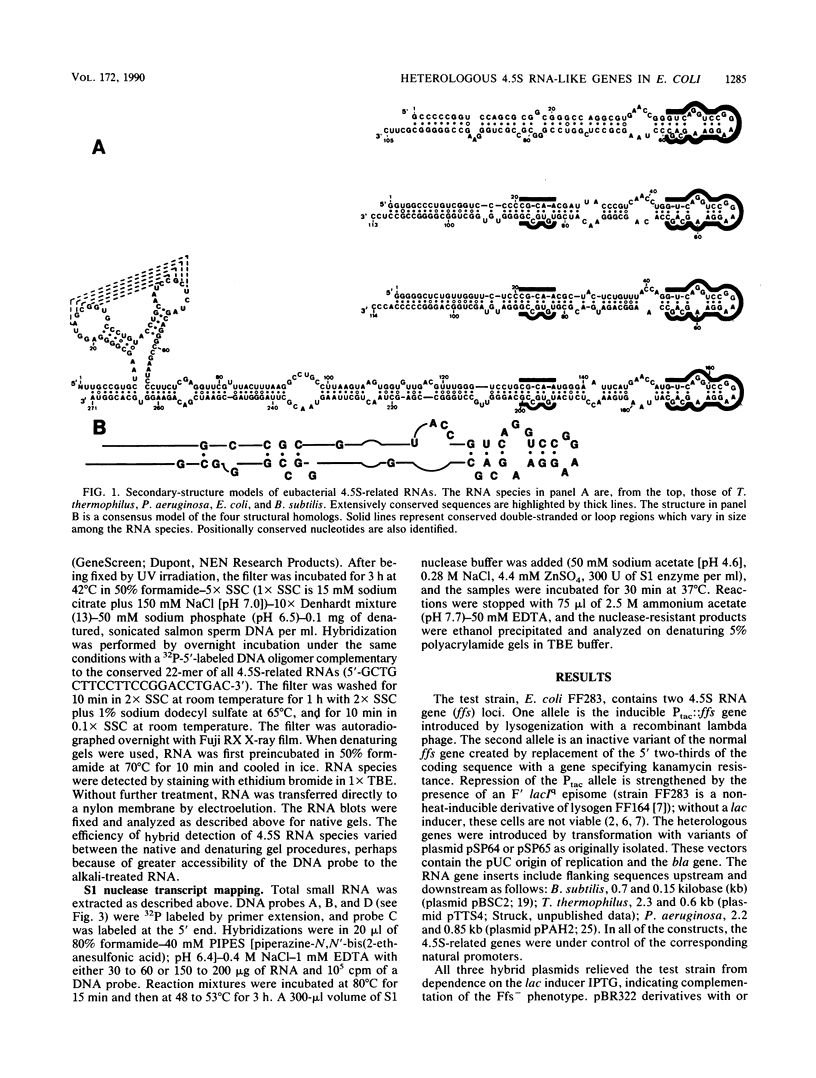
The essential 4.5S RNA gene of Escherichia coli can be complemented by 4.5S RNA-like genes from three other eubacteria, including both gram-positive and gram-negative organisms. Two of the genes encode RNAs similar in size to the E. coli species; the third, from Bacillus subtilis, specifies an RNA more than twice as large. The heterologous genes are expressed efficiently in E. coli, and the product RNAs resemble those produced by cognate cells. We conclude that the heterologous RNAs can replace E. coli 4.5S RNA and that the essential function of 4.5S RNA is evolutionarily conserved. A consensus structure is presented for the functionally related 4.5S RNA homologs.
Full text
PDF
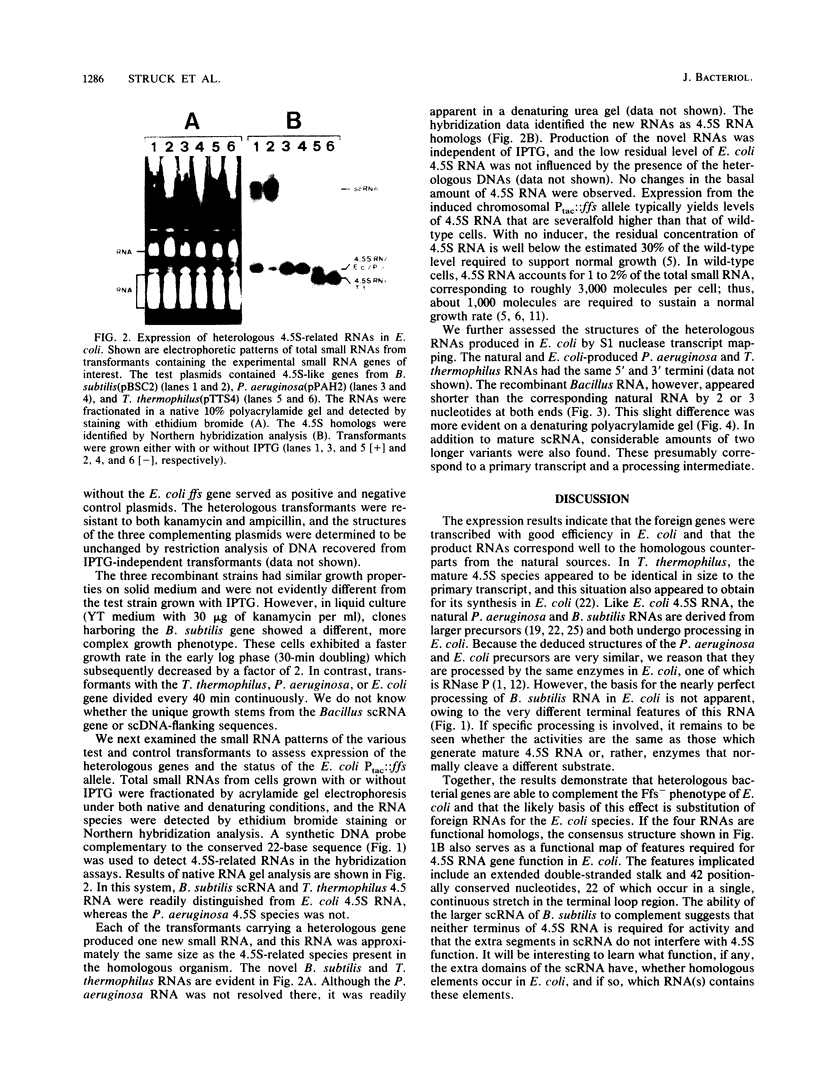


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bothwell A. L., Garber R. L., Altman S. Nucleotide sequence and in vitro processing of a precursor molecule to Escherichia coli 4.5 S RNA. J Biol Chem. 1976 Dec 10;251(23):7709–7716. [PubMed] [Google Scholar]
- Bourgaize D. B., Fournier M. J. Initiation of translation is impaired in E. coli cells deficient in 4.5S RNA. Nature. 1987 Jan 15;325(6101):281–284. doi: 10.1038/325281a0. [DOI] [PubMed] [Google Scholar]
- Bourgaize D. B., Phillips T. A., VanBogelen R. A., Jones P. G., Neidhardt F. C., Fournier M. J. Loss of 4.5S RNA induces the heat shock response and lambda prophage in Escherichia coli. J Bacteriol. 1990 Feb;172(2):1151–1154. doi: 10.1128/jb.172.2.1151-1154.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimacombe R., Atmadja J., Stiege W., Schüler D. A detailed model of the three-dimensional structure of Escherichia coli 16 S ribosomal RNA in situ in the 30 S subunit. J Mol Biol. 1988 Jan 5;199(1):115–136. doi: 10.1016/0022-2836(88)90383-x. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Brown S., Fournier M. J. The 4.5 S RNA gene of Escherichia coli is essential for cell growth. J Mol Biol. 1984 Sep 25;178(3):533–550. doi: 10.1016/0022-2836(84)90237-7. [DOI] [PubMed] [Google Scholar]
- Brown S. Mutations in the gene for EF-G reduce the requirement for 4.5S RNA in the growth of E. coli. Cell. 1987 Jun 19;49(6):825–833. doi: 10.1016/0092-8674(87)90620-9. [DOI] [PubMed] [Google Scholar]
- Brown S. Time of action of 4.5 S RNA in Escherichia coli translation. J Mol Biol. 1989 Sep 5;209(1):79–90. doi: 10.1016/0022-2836(89)90171-x. [DOI] [PubMed] [Google Scholar]
- Green C. J., Stewart G. C., Hollis M. A., Vold B. S., Bott K. F. Nucleotide sequence of the Bacillus subtilis ribosomal RNA operon, rrnB. Gene. 1985;37(1-3):261–266. doi: 10.1016/0378-1119(85)90281-1. [DOI] [PubMed] [Google Scholar]
- Hsu L. M., Zagorski J., Fournier M. J. Cloning and sequence analysis of the Escherichia coli 4.5 S RNA gene. J Mol Biol. 1984 Sep 25;178(3):509–531. doi: 10.1016/0022-2836(84)90236-5. [DOI] [PubMed] [Google Scholar]
- Höpfl P., Ulrich N., Hartmann R. K., Ludwig W., Schleifer K. H. Complete nucleotide sequence of a 23S ribosomal RNA gene from Thermus thermophilus HB8. Nucleic Acids Res. 1988 Sep 26;16(18):9043–9043. doi: 10.1093/nar/16.18.9043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T., Shimura Y., Sakano H., Ozeki H. Precursor molecules of Escherichia coli transfer RNAs accumulated in a temperature-sensitive mutant. J Mol Biol. 1975 Jul 25;96(1):69–86. doi: 10.1016/0022-2836(75)90182-5. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D., Robertson J. M., Noller H. F. Interaction of elongation factors EF-G and EF-Tu with a conserved loop in 23S RNA. Nature. 1988 Jul 28;334(6180):362–364. doi: 10.1038/334362a0. [DOI] [PubMed] [Google Scholar]
- Poritz M. A., Strub K., Walter P. Human SRP RNA and E. coli 4.5S RNA contain a highly homologous structural domain. Cell. 1988 Oct 7;55(1):4–6. doi: 10.1016/0092-8674(88)90003-7. [DOI] [PubMed] [Google Scholar]
- Powers T., Changchien L. M., Craven G. R., Noller H. F. Probing the assembly of the 3' major domain of 16 S ribosomal RNA. Quaternary interactions involving ribosomal proteins S7, S9 and S19. J Mol Biol. 1988 Mar 20;200(2):309–319. doi: 10.1016/0022-2836(88)90243-4. [DOI] [PubMed] [Google Scholar]
- Sköld S. E. Chemical crosslinking of elongation factor G to the 23S RNA in 70S ribosomes from Escherichia coli. Nucleic Acids Res. 1983 Jul 25;11(14):4923–4932. doi: 10.1093/nar/11.14.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck J. C., Hartmann R. K., Toschka H. Y., Erdmann V. A. Transcription and processing of Bacillus subtilis small cytoplasmic RNA. Mol Gen Genet. 1989 Feb;215(3):478–482. doi: 10.1007/BF00427046. [DOI] [PubMed] [Google Scholar]
- Struck J. C., Toschka H. Y., Erdmann V. A. Nucleotide sequence of the 4.5S RNA gene from Thermus thermophilus HB8. Nucleic Acids Res. 1988 Sep 26;16(18):9042–9042. doi: 10.1093/nar/16.18.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck J. C., Toschka H. Y., Specht T., Erdmann V. A. Common structural features between eukaryotic 7SL RNAs, eubacterial 4.5S RNA and scRNA and archaebacterial 7S RNA. Nucleic Acids Res. 1988 Aug 11;16(15):7740–7740. doi: 10.1093/nar/16.15.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck J. C., Vogel D. W., Ulbrich N., Erdmann V. A. The Bacillus subtilis scRNA is related to the 4.5S RNA from Escherichia coli. Nucleic Acids Res. 1988 Mar 25;16(6):2719–2719. doi: 10.1093/nar/16.6.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschka H. Y., Höpfl P., Ludwig W., Schleifer K. H., Ulbrich N., Erdmann V. A. Complete nucleotide sequence of a 16S ribosomal RNA gene from Pseudomonas aeruginosa. Nucleic Acids Res. 1988 Mar 25;16(5):2348–2348. doi: 10.1093/nar/16.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschka H. Y., Höpfl P., Ludwig W., Schleifer K. H., Ulbrich N., Erdmann V. A. Complete nucleotide sequence of a 23S ribosomal RNA gene from Pseudomonas aeruginosa. Nucleic Acids Res. 1987 Sep 11;15(17):7182–7182. doi: 10.1093/nar/15.17.7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschka H. Y., Struck J. C., Erdmann V. A. The 4.5S RNA gene from Pseudomonas aeruginosa. Nucleic Acids Res. 1989 Jan 11;17(1):31–36. doi: 10.1093/nar/17.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wower J., Hixson S. S., Zimmermann R. A. Labeling the peptidyltransferase center of the Escherichia coli ribosome with photoreactive tRNA(Phe) derivatives containing azidoadenosine at the 3' end of the acceptor arm: a model of the tRNA-ribosome complex. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5232–5236. doi: 10.1073/pnas.86.14.5232. [DOI] [PMC free article] [PubMed] [Google Scholar]