Abstract
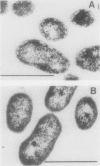
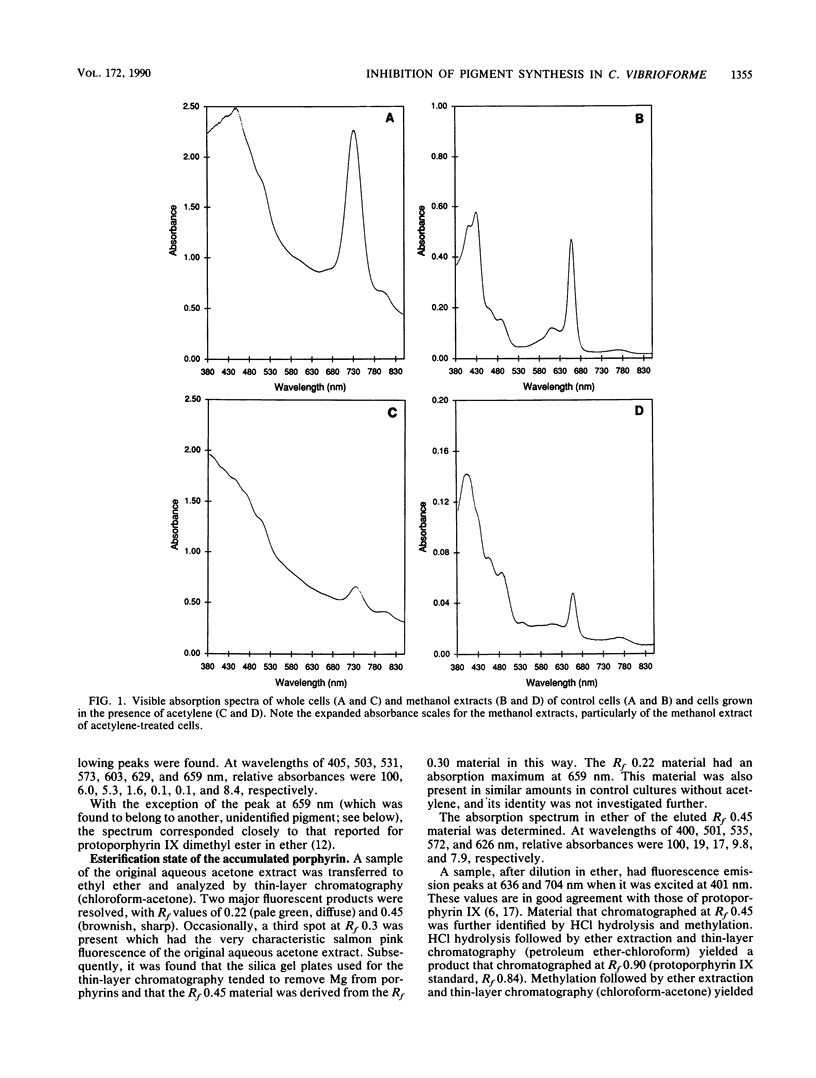
The green sulfur bacterium Chlorobium vibrioforme contains two types of bacteriochlorophyll (Bchl). The minor pigment, Bchl a, is associated primarily with the cell membrane and its reaction centers; and the major light-harvesting antenna pigment, Bchl d, is found primarily in the chlorosomes, which are attached to the inner surface of the cell membrane. Anesthetic gases, such as N2O, ethylene, and acetylene, were found to inhibit the synthesis of Bchl d, but not of Bchl a, thus allowing the cells to grow at high light intensities with a greatly diminished content of antenna pigment. Chlorosomes were absent or sparse in inhibited cells. Porphyrins accumulated in the inhibited cells. The major one was identified as the Bchl precursor magnesium-protoporphyrin IX monomethyl ester (Mg-PPME) by comparative absorption and fluorescence spectroscopy and thin-layer chromatography of the porphyrin and its derivatives with those of authentic protoporphyrin IX. Small amounts of Mg-PPME were present in control cells, but the addition of inhibitor caused a rapid increase in the Mg-PPME concentration, accompanying the inhibition of Bchl d synthesis. Cells grown in the presence of ethephon (as a source of ethylene) and allowed to stand in dim light for long periods accumulated large amounts of PPME and other porphyrins and excreted or released porphyrins, which accumulated as a brown precipitate in the culture. Inhibition of Bchl d synthesis was relieved upon removal of the inhibitor. These results suggest that the gases act at a step in pigment biosynthesis that affects the utilization of Mg-PPME for isocyclic ring formation. Synthesis of Bchl d and Bchl a may be differentially affected by the gases because of compartmentation of their biosynthetic apparatus or because competition for precursors favors Bchl a synthesis. An ethephon-resistant mutant strain was isolated by selection for growth in dim, long-wavelength light. The mutant cells were also resistant to acetylene, but not to N2O. The ability to reversibly generate viable Chlorobium cells that lack antenna pigments may be useful in photosynthesis research. The ethephon- and acetylene-resistant strain may be useful in the study of the enzymes and genes that are involved in the biosynthetic step that the gases affect.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARRETT J. Detection of hydroxyl groups in porphyrins and chlorins. Nature. 1959 Apr 25;183(4669):1185–1186. doi: 10.1038/1831185a0. [DOI] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: I. Accumulation of delta-Aminolevulinic Acid in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):291–296. doi: 10.1104/pp.53.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I. The biosynthesis of delta-aminolevulinic acid in Chlorella. Plant Physiol. 1970 Apr;45(4):504–506. doi: 10.1104/pp.45.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger F. C., Rebeiz C. A. Chloroplast biogenesis. Detection of monovinyl magnesium-protoporphyrin monoester and other monovinyl magnesium-porphyrins in higher plants. J Biol Chem. 1982 Feb 10;257(3):1360–1371. [PubMed] [Google Scholar]
- Broch-Due M., Ormerod J. G., Fjerdingen B. S. Effect of light intensity of vesicle formation in chlorobium. Arch Microbiol. 1978 Mar;116(3):269–274. doi: 10.1007/BF00417850. [DOI] [PubMed] [Google Scholar]
- Cardinal R. A., Bossenmaier I., Petryka Z. J., Johnson L., Watson C. J. Isolation of porphyrins from porphyria urine by preparative thin-layer chromatography. J Chromatogr. 1968 Nov 5;38(1):100–105. doi: 10.1016/0021-9673(68)85012-5. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Harel E., Klein S., Meller E. Control of delta-Aminolevulinic Acid and Chlorophyll Accumulation in Greening Maize Leaves upon Light-Dark Transitions. Plant Physiol. 1975 Oct;56(4):497–501. doi: 10.1104/pp.56.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. Molecular mechanisms of general anaesthesia. Nature. 1982 Dec 9;300(5892):487–493. doi: 10.1038/300487a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mayer S. M., Beale S. I., Weinstein J. D. Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem. 1987 Sep 15;262(26):12541–12549. [PubMed] [Google Scholar]
- Richards W. R., Lascelles J. The biosynthesis of bacteriochlorophyll. The characterization of latter stage intermediates from mutants of Rhodopseudomonas spheroides. Biochemistry. 1969 Aug;8(8):3473–3482. doi: 10.1021/bi00836a051. [DOI] [PubMed] [Google Scholar]
- Richards W. R., Rapoport H. The biosynthesis of chlorobium chlorophylls-660. The production of magnesium protoporphyrin monomethyl ester, bacteriochlorophyll, and chlorobium pheoporphyrins by Chlorobium thiosulfatophilum-660. Biochemistry. 1967 Dec;6(12):3830–3839. doi: 10.1021/bi00864a029. [DOI] [PubMed] [Google Scholar]
- Rieble S., Ormerod J. G., Beale S. I. Transformation of glutamate to delta-aminolevulinic acid by soluble extracts of Chlorobium vibrioforme. J Bacteriol. 1989 Jul;171(7):3782–3787. doi: 10.1128/jb.171.7.3782-3787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STANIER R. Y., SMITH J. H. The chlorophylis of green bacteria. Biochim Biophys Acta. 1960 Jul 15;41:478–484. doi: 10.1016/0006-3002(60)90045-7. [DOI] [PubMed] [Google Scholar]
- Schwartz D. P., Bright R. S. A column procedure for the esterification of organic acids with diazomethane at the microgram level. Anal Biochem. 1974 Sep;61(1):271–274. doi: 10.1016/0003-2697(74)90354-6. [DOI] [PubMed] [Google Scholar]
- Sirevåg R., Ormerod J. G. Carbon dioxide fixation in green sulphur bacteria. Biochem J. 1970 Nov;120(2):399–408. doi: 10.1042/bj1200399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]
- Walker C. J., Mansfield K. E., Rezzano I. N., Hanamoto C. M., Smith K. M., Castelfranco P. A. The magnesium-protoporphyrin IX (oxidative) cyclase system. Studies on the mechanism and specificity of the reaction sequence. Biochem J. 1988 Oct 15;255(2):685–692. [PMC free article] [PubMed] [Google Scholar]
- Walker C. J., Mansfield K. E., Smith K. M., Castelfranco P. A. Incorporation of atmospheric oxygen into the carbonyl functionality of the protochlorophyllide isocyclic ring. Biochem J. 1989 Jan 15;257(2):599–602. doi: 10.1042/bj2570599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Y., Huang D. D., Stachon D., Gough S. P., Kannangara C. G. Purification, Characterization, and Fractionation of the delta-Aminolevulinic Acid Synthesizing Enzymes from Light-Grown Chlamydomonas reinhardtii Cells. Plant Physiol. 1984 Mar;74(3):569–575. doi: 10.1104/pp.74.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J. D., Beale S. I. Enzymatic conversion of glutamate to delta-aminolevulinate in soluble extracts of the unicellular green alga, Chlorella vulgaris. Arch Biochem Biophys. 1985 Mar;237(2):454–464. doi: 10.1016/0003-9861(85)90299-1. [DOI] [PubMed] [Google Scholar]
- With T. K. Thin-layer chromatography of free and esterified porphyrins on talc. J Chromatogr. 1969 Jul;42(3):389–395. doi: 10.1016/s0021-9673(01)80639-7. [DOI] [PubMed] [Google Scholar]
- Wong Y. S., Castelfranco P. A. Resolution and Reconstitution of Mg-Protoporphyrin IX Monomethyl Ester (Oxidative) Cyclase, the Enzyme System Responsible for the Formation of the Chlorophyll Isocyclic Ring. Plant Physiol. 1984 Jul;75(3):658–661. doi: 10.1104/pp.75.3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]