Abstract
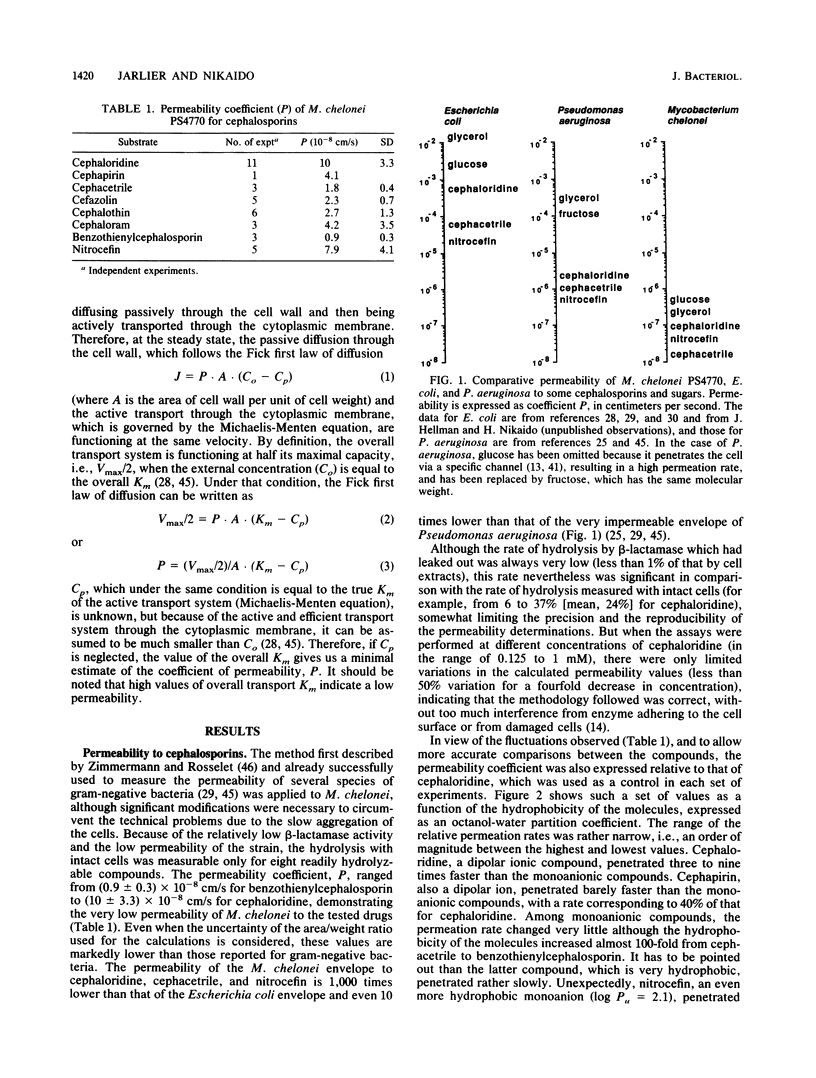
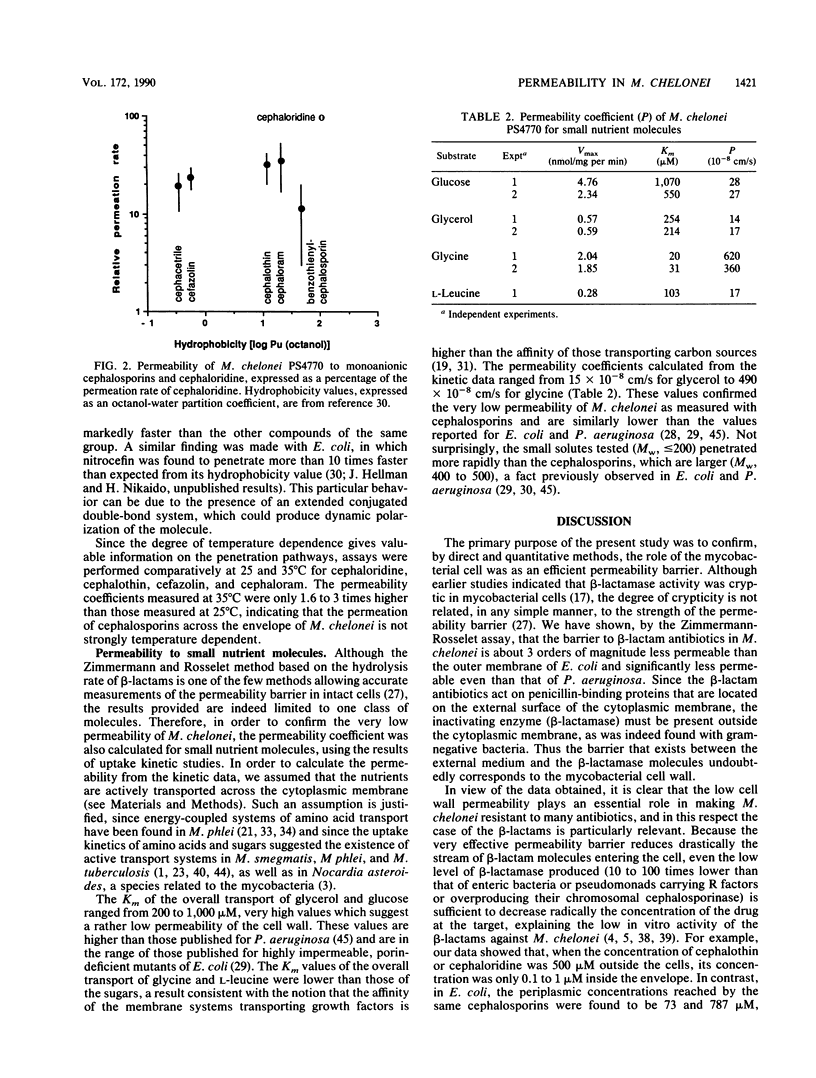
In order to define the permeability barrier to hydrophilic molecules in mycobacteria, we used as a model a smooth, beta-lactamase-producing strain of Mycobacterium chelonei. The rates of hydrolysis of eight cephalosporins by intact and sonicated cells were measured, and the permeability coefficient (P) was calculated from these rates by the method of Zimmermann and Rosselet (W. Zimmermann and A. Rosselet, Antimicrob. Agents Chemother. 12:368-372, 1977). P ranged from (0.9 +/- 0.3) x 10(-8) (benzothienylcephalosporin) to (10 +/- 3.3) x 10(-8) cm/s (cephaloridine); i.e., the P values were lower than those reported for Pseudomonas aeruginosa and Escherichia coli by 1 and 3 orders of magnitude, respectively. The permeability barrier was shown to reduce drastically the stream of drug molecules entering the cell, allowing the rather low level of beta-lactamase (0.1 U/mg of protein with penicillin G) to decrease radically the concentration of the drug at the target; this explains the poor in vitro activities of the beta-lactams against M. chelonei. We also estimated P for small, hydrophilic molecules (glucose, glycerol, glycine, leucine), by studying their uptake kinetics. The values found, ranging from 15 x 10(-8) to 490 x 10(-8) cm/s, were consistent again with a very low permeability of M. chelonei cell wall. The permeation of cephalosporins was not very dependent on the hydrophobicity of the molecules or on the temperature, suggesting a hydrophilic pathway of penetration for these molecules.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bai N. J., Pai M. R., Murthy P. S., Venkitasubramanian T. A. Uptake & transport of hexoses in Mycobacterium smegmatis. Indian J Biochem Biophys. 1978 Oct;15(5):369–372. [PubMed] [Google Scholar]
- Brennan P. J. The phthiocerol-containing surface lipids of Mycobacterium leprae--a perspective of past and present work. Int J Lepr Other Mycobact Dis. 1983 Sep;51(3):387–396. [PubMed] [Google Scholar]
- Calmes R., Deal S. J. Glycerol transport by Nocardia asteroides. Can J Microbiol. 1972 Nov;18(11):1703–1708. doi: 10.1139/m72-264. [DOI] [PubMed] [Google Scholar]
- Casal M. J., Rodriguez F. C., Benavente M. C. In vitro susceptibility of Mycobacterium fortuitum and Mycobacterium chelonei to cefmetazole. Antimicrob Agents Chemother. 1985 Feb;27(2):282–283. doi: 10.1128/aac.27.2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casal M., Rodríguez F. In vitro susceptibility of mycobacterium fortuitum and mycobacterium chelonei to cefoxitin. Tubercle. 1982 Jun;63(2):125–127. doi: 10.1016/s0041-3879(82)80049-4. [DOI] [PubMed] [Google Scholar]
- Cohen B. E., Bangham A. D. Diffusion of small non-electrolytes across liposome membranes. Nature. 1972 Mar 24;236(5343):173–174. doi: 10.1038/236173a0. [DOI] [PubMed] [Google Scholar]
- David H. L., Clavel-Seres S., Clement F., Goh K. S. Uptake of selected antibacterial agents in Mycobacterium avium. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Jul;265(3-4):385–392. [PubMed] [Google Scholar]
- David H. L., Lévy-Frébault V., Thorel M. F. Characterization of distinct layers of the Mycobacterium avium envelope in respect of their composition by fatty acids, proteins, oligosaccharides and antigens. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Apr;268(2):193–208. doi: 10.1016/s0176-6724(88)80003-8. [DOI] [PubMed] [Google Scholar]
- David H. L., Rastogi N., Clavel-Sérès S., Clément F., Thorel M. F. Structure of the cell envelope of Mycobacterium avium. Zentralbl Bakteriol Mikrobiol Hyg A. 1987 Apr;264(1-2):49–66. doi: 10.1016/s0176-6724(87)80124-4. [DOI] [PubMed] [Google Scholar]
- Galey W. R., Owen J. D., Solomon A. K. Temperature dependence of nonelectrolyte permeation across red cell membranes. J Gen Physiol. 1973 Jun;61(6):727–746. doi: 10.1085/jgp.61.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewinson R. G., Lane D. C., Slack M. P., Nichols W. W. The permeability parameter of the outer membrane of Pseudomonas aeruginosa varies with the concentration of a test substrate, cephalosporin C. J Gen Microbiol. 1986 Jan;132(1):27–33. doi: 10.1099/00221287-132-1-27. [DOI] [PubMed] [Google Scholar]
- Hou J. P., Poole J. W. Measurement of beta-lactamase activity and rate of inactivation of penicillins by a pH-stat alkalimetric titration method. J Pharm Sci. 1972 Oct;61(10):1594–1598. doi: 10.1002/jps.2600611010. [DOI] [PubMed] [Google Scholar]
- Hui J., Gordon N., Kajioka R. Permeability barrier to rifampin in mycobacteria. Antimicrob Agents Chemother. 1977 May;11(5):773–779. doi: 10.1128/aac.11.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak A., Philippon A., Chardon H., Labia R., Le Goffic F. Constantes enzymatiques (Km et Vmax) des beta-lactamases mesurées par une méthode micro-acidimétrique couplée a l'ordinateur. Ann Microbiol (Paris) 1973 Oct;124(3):259–268. [PubMed] [Google Scholar]
- Koch A. L. The adaptive responses of Escherichia coli to a feast and famine existence. Adv Microb Physiol. 1971;6:147–217. doi: 10.1016/s0065-2911(08)60069-7. [DOI] [PubMed] [Google Scholar]
- Labia R., Barthélémy M. L'enzymogramme des beta-lactamases: adaptation en cel de la méthode iodométrique. Ann Microbiol (Paris) 1979 Oct;130B(3):295–304. [PubMed] [Google Scholar]
- Lee S. H., Cohen N. S., Jacobs A. J., Brodie A. F. Isolation, purification, and reconstitution of a proline carrier protein from Mycobacterium phlei. Biochemistry. 1979 May 29;18(11):2232–2239. doi: 10.1021/bi00578a015. [DOI] [PubMed] [Google Scholar]
- Lyon R. H., Rogers P., Hall W. H., Lichtein H. C. Inducible glutamate transport in Mycobacteria and its relation to glutamate oxidation. J Bacteriol. 1967 Jul;94(1):92–100. doi: 10.1128/jb.94.1.92-100.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol Ther. 1985;27(2):197–231. doi: 10.1016/0163-7258(85)90069-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981 Feb;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Transport through the outer membrane of bacteria. Methods Enzymol. 1986;125:265–278. doi: 10.1016/s0076-6879(86)25023-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R., Kalra V. K., Brodie A. F. Active transport of glutamine and glutamic acid in membrane vesicles from Mycobacterium phlei. Biochem Biophys Res Commun. 1975 Mar 3;63(1):50–56. doi: 10.1016/s0006-291x(75)80009-x. [DOI] [PubMed] [Google Scholar]
- Prasad R., Kalra V. K., Brodie A. F. Different mechanisms of energy coupling for transport of various amino acids in cells of Mycobacterium phlei. J Biol Chem. 1976 Apr 25;251(8):2493–2498. [PubMed] [Google Scholar]
- Swenson J. M., Thornsberry C., Silcox V. A. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob Agents Chemother. 1982 Aug;22(2):186–192. doi: 10.1128/aac.22.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Wallace R. J., Jr, Silcox V. A., Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985 Dec;28(6):807–811. doi: 10.1128/aac.28.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper B. S. Differences in the utilization of glycerol and glucose by Mycobacterium phlei. J Bacteriol. 1968 May;95(5):1713–1717. doi: 10.1128/jb.95.5.1713-1717.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trias J., Rosenberg E. Y., Nikaido H. Specificity of the glucose channel formed by protein D1 of Pseudomonas aeruginosa. Biochim Biophys Acta. 1988 Mar 3;938(3):493–496. doi: 10.1016/0005-2736(88)90148-4. [DOI] [PubMed] [Google Scholar]
- Udou T., Mizuguchi Y., Yamada T. Biochemical mechanisms of antibiotic resistance in a clinical isolate of Mycobacterium fortuitum. Presence of beta-lactamase and aminoglycoside-acetyltransferase and possible participation of altered drug transport on the resistance mechanism. Am Rev Respir Dis. 1986 Apr;133(4):653–657. doi: 10.1164/arrd.1986.133.4.653. [DOI] [PubMed] [Google Scholar]
- Yabu K. Amino acid transport in Mycobacterium smegmatis. J Bacteriol. 1970 Apr;102(1):6–13. doi: 10.1128/jb.102.1.6-13.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Permeability of Pseudomonas aeruginosa outer membrane to hydrophilic solutes. J Bacteriol. 1982 Nov;152(2):636–642. doi: 10.1128/jb.152.2.636-642.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]



