Abstract
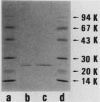
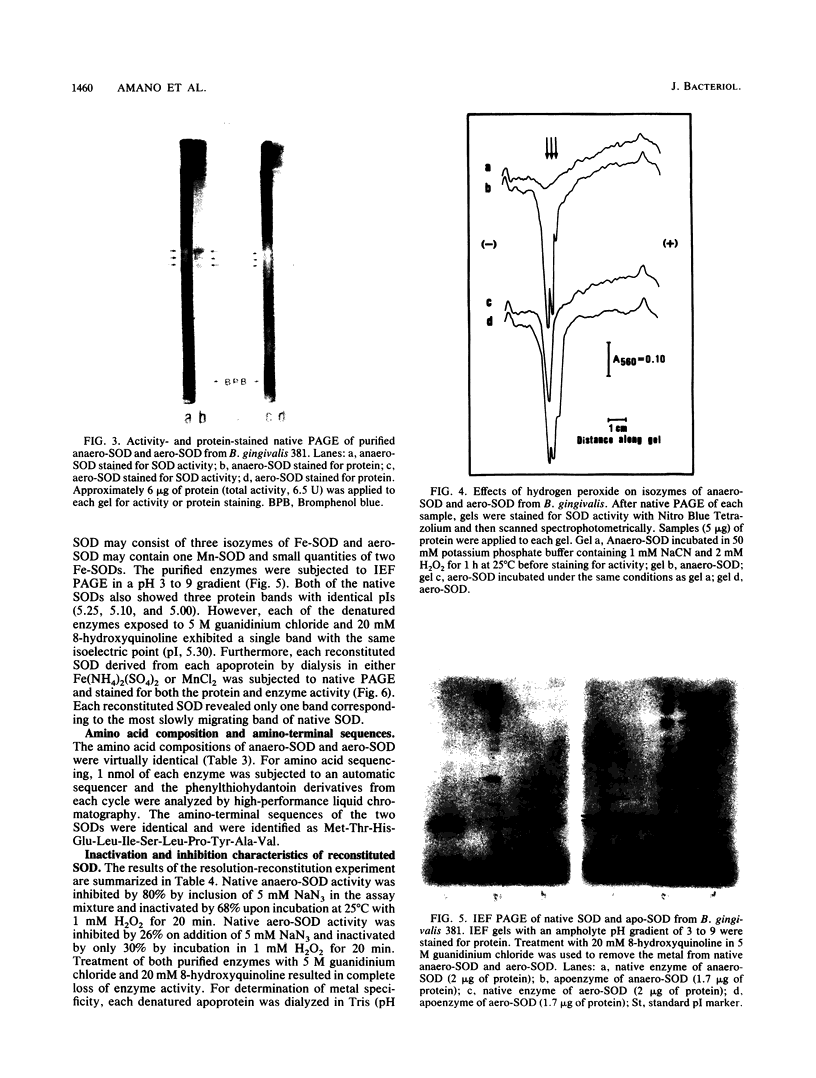
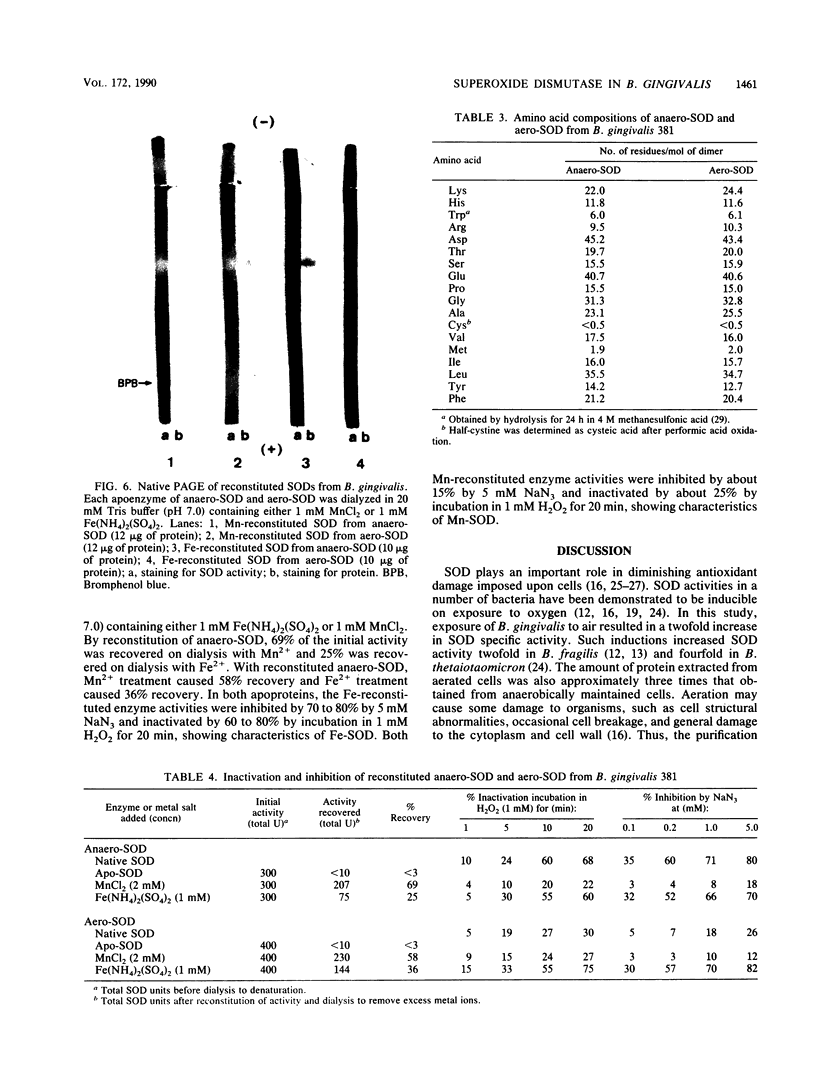
Superoxide dismutases (SODs) were purified from extracts of either anaerobically maintained or aerated Bacteroides gingivalis. Each purified enzyme (molecular weight, 46,000) was a dimer composed of two subunits of equal sizes. SOD from anaerobically maintained cells (anaero-SOD) contained 1.79 g-atom of Fe and 0.28 g-atom of Mn, and SOD from aerated cells (aero-SOD) contained 1.08 g-atom of Mn and 0.36 g-atom of Fe. Spectral analysis showed that anaero-SOD had the characteristic of Fe-SOD and that aero-SOD had that of Mn-SOD. Both enzyme preparations contained three isozymes with identical isoelectric points. On the basis of inactivation of SOD by H2O2, it was found that aero-SOD consisted of one Mn-SOD and a small quantity of two Fe-SODs, whereas anaero-SOD contained only Fe-SOD. However, each apoprotein from anaero-SOD and aero-SOD, prepared by dialysis in guanidinium chloride plus 8-hydroxyquinoline, showed only one protein band each with the same isoelectric point on an isoelectric focusing gel. Subsequent dialysis of both apoenzymes with either MnCl2 or Fe(NH4)2(SO4)2 restored the activity. These reconstituted SODs showed only one protein band with SOD activity on native polyacrylamide gel electrophoresis. Furthermore, the two enzymes had similar amino acid compositions, and their amino-terminal sequences were identical through the first 12 amino acids. These results suggest that the three isozymes of anaero-SOD and aero-SOD in B. gingivalis are formed from a single apoprotein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano A., Tamagawa H., Takagaki M., Murakami Y., Shizukuishi S., Tsunemitsu A. Relationship between enzyme activities involved in oxygen metabolism and oxygen tolerance in black-pigmented Bacteroides. J Dent Res. 1988 Sep;67(9):1196–1199. doi: 10.1177/00220345880670090901. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Maguchi S., Fujii S., Ishibashi H., Oikawa K., Taniguchi N. Glycation and inactivation of human Cu-Zn-superoxide dismutase. Identification of the in vitro glycated sites. J Biol Chem. 1987 Dec 15;262(35):16969–16972. [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Benovic J., Tillman T., Cudd A., Fridovich I. Electrostatic facilitation of the reaction catalyzed by the manganese-containing and the iron-containing superoxide dismutases. Arch Biochem Biophys. 1983 Mar;221(2):329–332. doi: 10.1016/0003-9861(83)90151-0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dougherty H. W., Sadowski S. J., Baker E. E. A new iron-containing superoxide dismutase from Escherichia coli. J Biol Chem. 1978 Jul 25;253(14):5220–5223. [PubMed] [Google Scholar]
- Fee J. A., Shapiro E. R., Moss T. H. Direct evidence for manganese (III) binding to the manganosuperoxide dismutase of Escherichia coli B. J Biol Chem. 1976 Oct 10;251(19):6157–6159. [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Fujita M., Taniguchi N., Makita A., Ono M., Oikawa K. Protein phosphorylation of beta-glucuronidase in human lung cancer--identification of serine- and threonine-phosphates. Biochem Biophys Res Commun. 1985 Jan 31;126(2):818–824. doi: 10.1016/0006-291x(85)90258-x. [DOI] [PubMed] [Google Scholar]
- Gregory E. M. Characterization of the O2-induced manganese-containing superoxide dismutase from Bacteroides fragilis. Arch Biochem Biophys. 1985 Apr;238(1):83–89. doi: 10.1016/0003-9861(85)90143-2. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Dapper C. H. Isolation of iron-containing superoxide dismutase from Bacteroides fragilis: reconstitution as a Mn-containing enzyme. Arch Biochem Biophys. 1983 Jan;220(1):293–300. doi: 10.1016/0003-9861(83)90413-7. [DOI] [PubMed] [Google Scholar]
- Harris J. I., Auffret A. D., Northrop F. D., Walker J. E. Structural comparisons of superoxide dismutases. Eur J Biochem. 1980 May;106(1):297–303. doi: 10.1111/j.1432-1033.1980.tb06023.x. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Enzymatic defenses against the toxicity of oxygen and of streptonigrin in Escherichia coli. J Bacteriol. 1977 Mar;129(3):1574–1583. doi: 10.1128/jb.129.3.1574-1583.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T., Blum J., Kahane I., Fridovich I. Distinguishing between Mn-containing and Fe-containing superoxide dismutases in crude extracts of cells. Arch Biochem Biophys. 1980 May;201(2):551–555. doi: 10.1016/0003-9861(80)90544-5. [DOI] [PubMed] [Google Scholar]
- Martin M. E., Byers B. R., Olson M. O., Salin M. L., Arceneaux J. E., Tolbert C. A Streptococcus mutans superoxide dismutase that is active with either manganese or iron as a cofactor. J Biol Chem. 1986 Jul 15;261(20):9361–9367. [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Meier B., Barra D., Bossa F., Calabrese L., Rotilio G. Synthesis of either Fe- or Mn-superoxide dismutase with an apparently identical protein moiety by an anaerobic bacterium dependent on the metal supplied. J Biol Chem. 1982 Dec 10;257(23):13977–13980. [PubMed] [Google Scholar]
- Ose D. E., Fridovich I. Manganese-containing superoxide dismutase from Escherichia coli: reversible resolution and metal replacements. Arch Biochem Biophys. 1979 May;194(2):360–364. doi: 10.1016/0003-9861(79)90628-3. [DOI] [PubMed] [Google Scholar]
- Pennington C. D., Gregory E. M. Isolation and reconstitution of iron- and manganese-containing superoxide dismutases from Bacteroides thetaiotaomicron. J Bacteriol. 1986 May;166(2):528–532. doi: 10.1128/jb.166.2.528-532.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Gregory E. M. Superoxide dismutase and O2 lethality in Bacteroides fragilis. J Bacteriol. 1979 Apr;138(1):139–145. doi: 10.1128/jb.138.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellhorn H. E., Hassan H. M. Response of hydroperoxidase and superoxide dismutase deficient mutants of Escherichia coli K-12 to oxidative stress. Can J Microbiol. 1988 Oct;34(10):1171–1176. doi: 10.1139/m88-206. [DOI] [PubMed] [Google Scholar]
- Schwartz C. E., Krall J., Norton L., McKay K., Kay D., Lynch R. E. Catalase and superoxide dismutase in Escherichia coli. J Biol Chem. 1983 May 25;258(10):6277–6281. [PubMed] [Google Scholar]
- Simpson R. J., Neuberger M. R., Liu T. Y. Complete amino acid analysis of proteins from a single hydrolysate. J Biol Chem. 1976 Apr 10;251(7):1936–1940. [PubMed] [Google Scholar]
- Slots J., Genco R. J. Black-pigmented Bacteroides species, Capnocytophaga species, and Actinobacillus actinomycetemcomitans in human periodontal disease: virulence factors in colonization, survival, and tissue destruction. J Dent Res. 1984 Mar;63(3):412–421. doi: 10.1177/00220345840630031101. [DOI] [PubMed] [Google Scholar]
- Slykhouse T. O., Fee J. A. Physical and chemical studies on bacterial superoxide dismutases. Purification and some anion binding properties of the iron-containing protein of Escherichia coli B. J Biol Chem. 1976 Sep 25;251(18):5472–5477. [PubMed] [Google Scholar]
- Steinman H. M. The amino acid sequence of mangano superoxide dismutase from Escherichia coli B. J Biol Chem. 1978 Dec 25;253(24):8708–8720. [PubMed] [Google Scholar]
- Tsunasawa S., Kondo J., Sakiyama F. Isocratic separation of PTH-amino acids at picomole level by reverse-phase HPLC in the presence of sodium dodecylsulfate. J Biochem. 1985 Feb;97(2):701–704. doi: 10.1093/oxfordjournals.jbchem.a135108. [DOI] [PubMed] [Google Scholar]