Abstract
Escherichia coli contains two L-asparaginase isozymes: L-asparaginase I, a low-affinity enzyme located in the cytoplasm, and L-asparaginase II, a high-affinity secreted enzyme. A molecular genetic analysis of the gene (ansA) encoding the former enzyme has previously been reported. We now present a molecular study of the gene, ansB, encoding L-asparaginase II. This gene was isolated by using oligonucleotide probes, whose sequences were based on the previously determined amino acid sequence. The nucleotide sequence of ansB, including 5'- and 3'-untranslated regions, was determined. The amino acid sequence of L-asparaginase II, deduced from this nucleotide sequence, contains differences at 11 positions when compared with the previously determined amino acid sequence. The deduced amino acid sequence also reveals a typical secretory signal peptide of 22 residues. A single region of sequence similarity is observed when ansA and ansB are compared. The transcriptional start site in ansB was determined, allowing the identification of the promoter region. The regulation of ansB was studied by using ansB'-'lacZ fusions, together with a deletion analysis of the 5' region upstream of the promoter. Regulation by cyclic AMP receptor protein and anaerobiosis (FNR protein) was confirmed, and the presence of nucleotide sequence motifs, with homology to cyclic AMP receptor protein and FNR protein-binding sites, investigated.
Full text
PDF

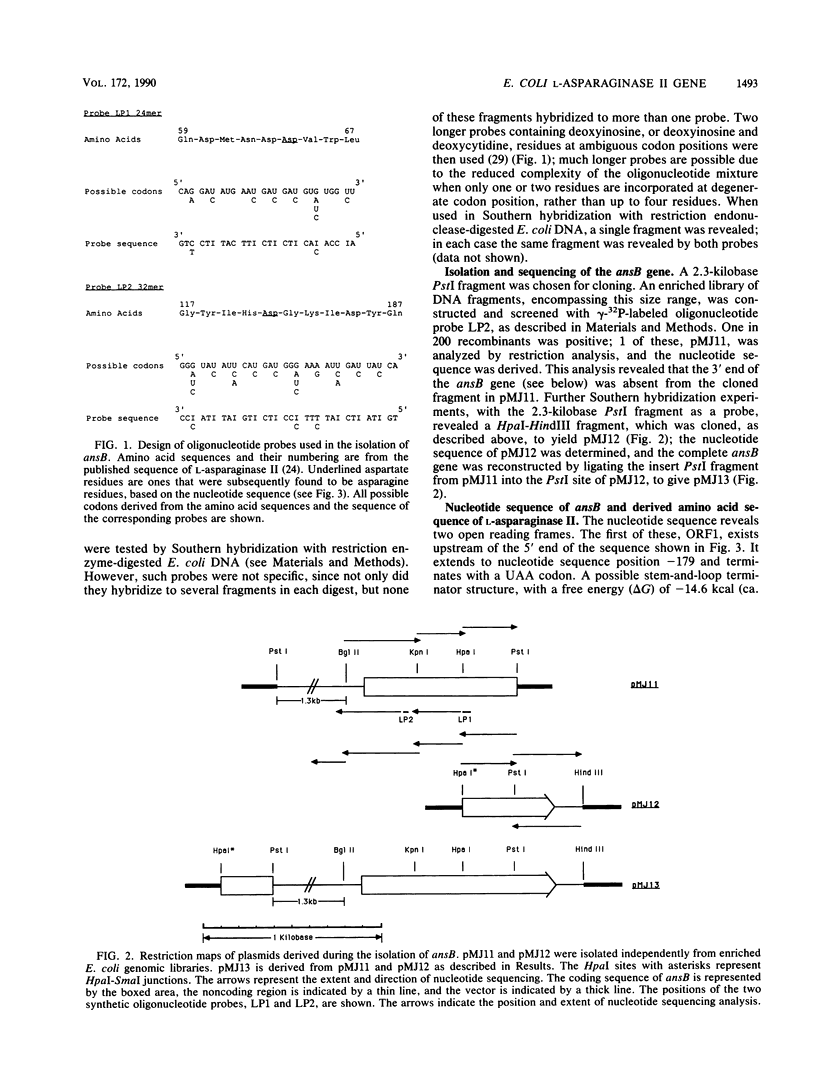
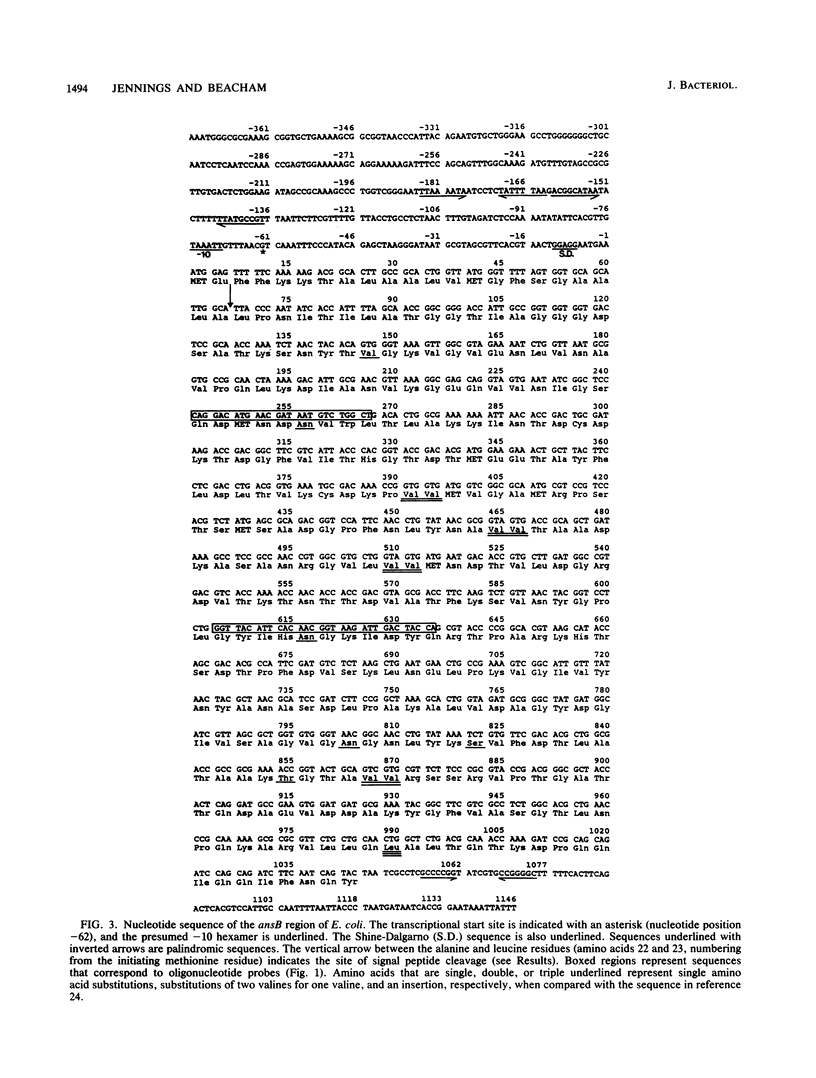
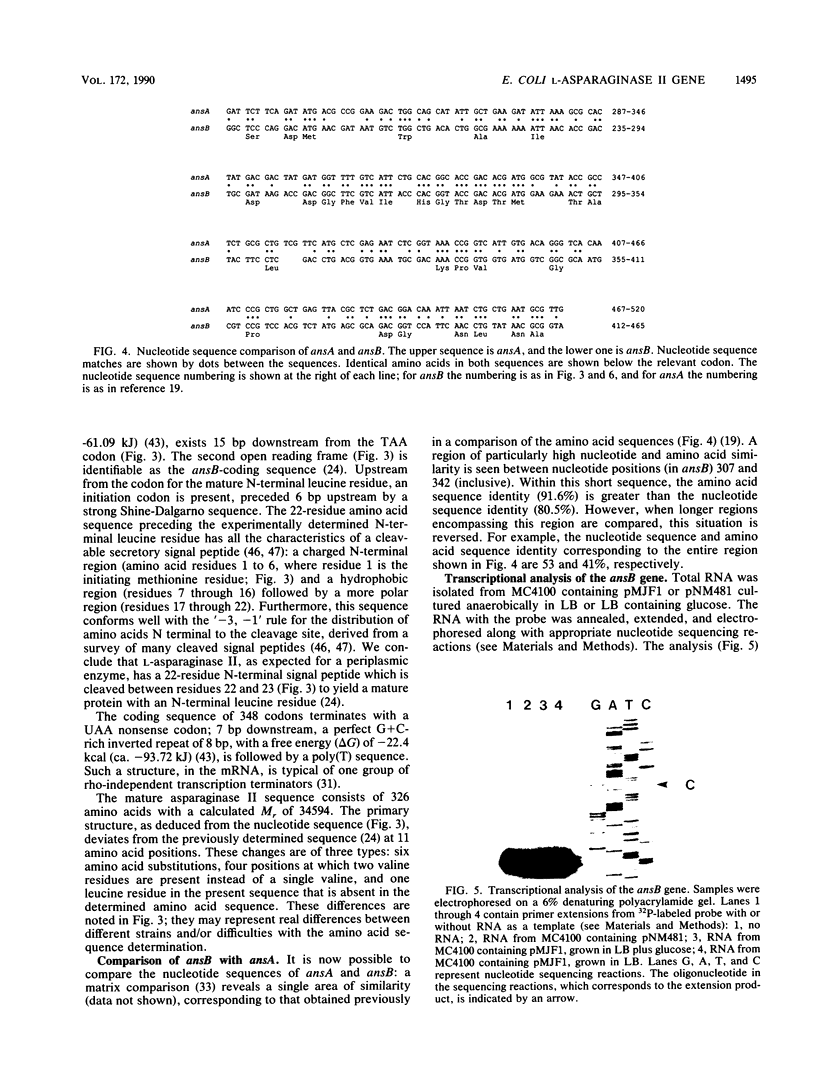


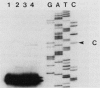
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Ammon H. L., Weber I. T., Wlodawer A., Harrison R. W., Gilliland G. L., Murphy K. C., Sjölin L., Roberts J. Preliminary crystal structure of Acinetobacter glutaminasificans glutaminase-asparaginase. J Biol Chem. 1988 Jan 5;263(1):150–156. [PubMed] [Google Scholar]
- Burns D. M., Abraham L. J., Beacham I. R. Characterization of the ush gene of Escherichia coli and its protein products. Gene. 1983 Nov;25(2-3):343–353. doi: 10.1016/0378-1119(83)90239-1. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. A method for the ligation of DNA following isolation from low melting temperature agarose. Anal Biochem. 1983 Nov;135(1):48–51. doi: 10.1016/0003-2697(83)90728-5. [DOI] [PubMed] [Google Scholar]
- Cedar H., Schwartz J. H. Localization of the two-L-asparaginases in anaerobically grown Escherichia coli. J Biol Chem. 1967 Aug 25;242(16):3753–3755. [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Crowther D. L-asparaginase and human malignant disease. Nature. 1971 Jan 15;229(5281):168–171. doi: 10.1038/229168a0. [DOI] [PubMed] [Google Scholar]
- Ebright R. H., Beckwith J. The catabolite gene activator protein (CAP) is not required for indole-3-acetic acid to activate transcription of the araBAD operon of Escherichia coli K-12. Mol Gen Genet. 1985;201(1):51–55. doi: 10.1007/BF00397986. [DOI] [PubMed] [Google Scholar]
- Ho P. P., Milikin E. B., Bobbitt J. L., Grinnan E. L., Burck P. J., Frank B. H., Boeck L. D., Squires R. W. Crystalline L-asparaginase from Escherichia coli B. I. Purification and chemical characterization. J Biol Chem. 1970 Jul 25;245(14):3708–3715. [PubMed] [Google Scholar]
- Hudson G. S., Davidson B. E. Nucleotide sequence and transcription of the phenylalanine and tyrosine operons of Escherichia coli K12. J Mol Biol. 1984 Dec 25;180(4):1023–1051. doi: 10.1016/0022-2836(84)90269-9. [DOI] [PubMed] [Google Scholar]
- Jansen C., Gronenborn A. M., Clore G. M. The binding of the cyclic AMP receptor protein to synthetic DNA sites containing permutations in the consensus sequence TGTGA. Biochem J. 1987 Aug 15;246(1):227–232. doi: 10.1042/bj2460227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman P. S., Gaston K. L., Cole J. A., Busby S. J. The nirB promoter of Escherichia coli: location of nucleotide sequences essential for regulation by oxygen, the FNR protein and nitrite. Mol Microbiol. 1988 Jul;2(4):527–530. doi: 10.1111/j.1365-2958.1988.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Jayaraman P. S., Peakman T. C., Busby S. J., Quincey R. V., Cole J. A. Location and sequence of the promoter of the gene for the NADH-dependent nitrite reductase of Escherichia coli and its regulation by oxygen, the Fnr protein and nitrite. J Mol Biol. 1987 Aug 20;196(4):781–788. doi: 10.1016/0022-2836(87)90404-9. [DOI] [PubMed] [Google Scholar]
- Jerlström P. G., Bezjak D. A., Jennings M. P., Beacham I. R. Structure and expression in Escherichia coli K-12 of the L-asparaginase I-encoding ansA gene and its flanking regions. Gene. 1989 May 15;78(1):37–46. doi: 10.1016/0378-1119(89)90312-0. [DOI] [PubMed] [Google Scholar]
- Jones H. M., Gunsalus R. P. Regulation of Escherichia coli fumarate reductase (frdABCD) operon expression by respiratory electron acceptors and the fnr gene product. J Bacteriol. 1987 Jul;169(7):3340–3349. doi: 10.1128/jb.169.7.3340-3349.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. F., DeMoss J. A. Location of sequences in the nar promoter of Escherichia coli required for regulation by Fnr and NarL. J Biol Chem. 1988 Sep 25;263(27):13700–13705. [PubMed] [Google Scholar]
- Li S. F., DeMoss J. A. Promoter region of the nar operon of Escherichia coli: nucleotide sequence and transcription initiation signals. J Bacteriol. 1987 Oct;169(10):4614–4620. doi: 10.1128/jb.169.10.4614-4620.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maita T., Matsuda G. The primary structure of L-asparaginase from Escherichia coli. Hoppe Seylers Z Physiol Chem. 1980;361(2):105–117. doi: 10.1515/bchm2.1980.361.1.105. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Minton N. P. Improved plasmid vectors for the isolation of translational lac gene fusions. Gene. 1984 Nov;31(1-3):269–273. doi: 10.1016/0378-1119(84)90220-8. [DOI] [PubMed] [Google Scholar]
- Ohtsuka E., Matsuki S., Ikehara M., Takahashi Y., Matsubara K. An alternative approach to deoxyoligonucleotides as hybridization probes by insertion of deoxyinosine at ambiguous codon positions. J Biol Chem. 1985 Mar 10;260(5):2605–2608. [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Raibaud O., Schwartz M. Positive control of transcription initiation in bacteria. Annu Rev Genet. 1984;18:173–206. doi: 10.1146/annurev.ge.18.120184.001133. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Bucholtz C. A. The MTX package of computer programmes for the comparison of sequences of nucleotides and amino acid residues. Nucleic Acids Res. 1986 Jan 10;14(1):233–238. doi: 10.1093/nar/14.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L., Yamazaki H. The dependence of Escherichia coli asparaginase II formation on cyclic AMP and cyclic AMP receptor protein. Can J Microbiol. 1978 May;24(5):629–631. doi: 10.1139/m78-104. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. H., Reeves J. Y., Broome J. D. Two L-asparaginases from E. coli and their action against tumors. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1516–1519. doi: 10.1073/pnas.56.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro S., Guest J. R. Regulation and over-expression of the fnr gene of Escherichia coli. J Gen Microbiol. 1987 Dec;133(12):3279–3288. doi: 10.1099/00221287-133-12-3279. [DOI] [PubMed] [Google Scholar]
- Spring K. J., Jerlström P. G., Burns D. M., Beacham I. R. L-asparaginase genes in Escherichia coli: isolation of mutants and characterization of the ansA gene and its protein product. J Bacteriol. 1986 Apr;166(1):135–142. doi: 10.1128/jb.166.1.135-142.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes H. W., Betts P. W., Hall B. G. Sequence of the ebgA gene of Escherichia coli: comparison with the lacZ gene. Mol Biol Evol. 1985 Nov;2(6):469–477. doi: 10.1093/oxfordjournals.molbev.a040372. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Unden G., Guest J. R. Cyclic AMP and anaerobic gene expression in E. coli. FEBS Lett. 1984 May 21;170(2):321–325. doi: 10.1016/0014-5793(84)81336-8. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Webster C., Gaston K., Busby S. Transcription from the Escherichia coli melR promoter is dependent on the cyclic AMP receptor protein. Gene. 1988 Sep 7;68(2):297–305. doi: 10.1016/0378-1119(88)90032-7. [DOI] [PubMed] [Google Scholar]
- Willis R. C., Woolfolk C. A. Asparagine utilization in Escherichia coli. J Bacteriol. 1974 Apr;118(1):231–241. doi: 10.1128/jb.118.1.231-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crombrugghe B., Busby S., Buc H. Cyclic AMP receptor protein: role in transcription activation. Science. 1984 May 25;224(4651):831–838. doi: 10.1126/science.6372090. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. How signal sequences maintain cleavage specificity. J Mol Biol. 1984 Feb 25;173(2):243–251. doi: 10.1016/0022-2836(84)90192-x. [DOI] [PubMed] [Google Scholar]