Abstract
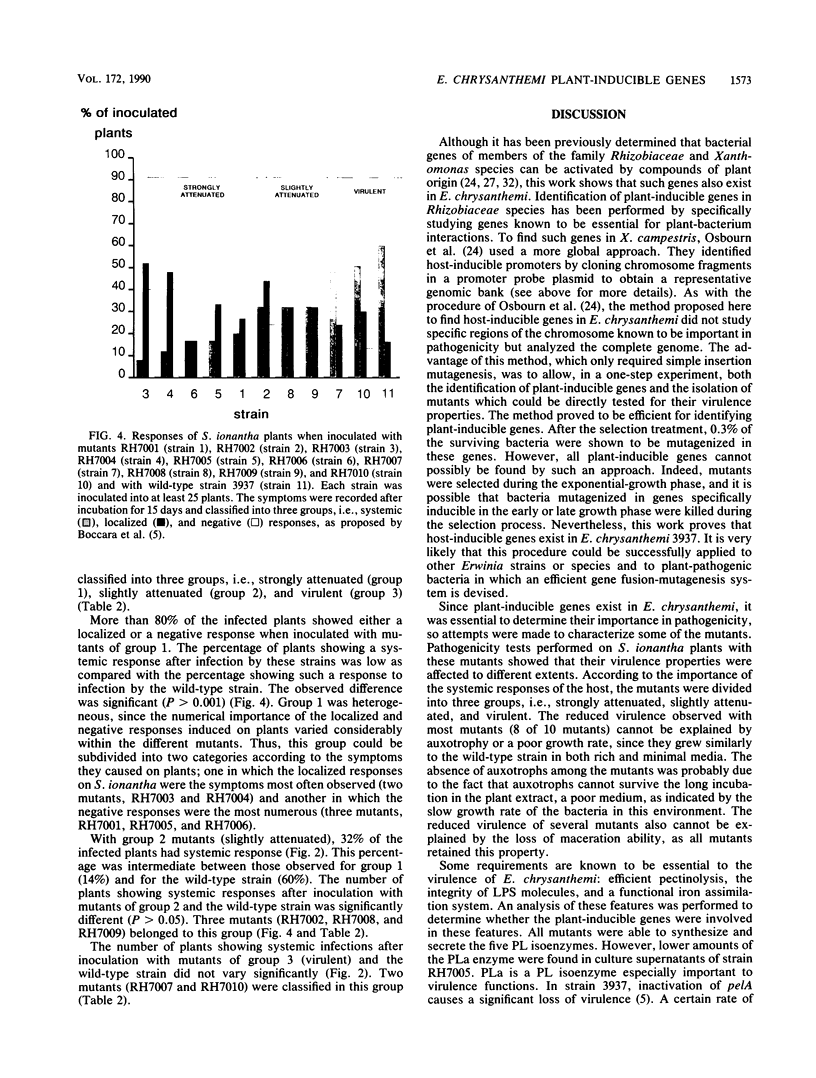
We present a method for identifying plant-inducible genes of Erwinia chrysanthemi 3937. Mutagenesis was done with the Mu dIIPR3 transposon, which carries a promoterless neomycin phosphotransferase gene (nptI), so upon insertion, the truncated gene can fuse to E. chrysanthemi promoters. Mutants containing insertions in plant-inducible genes were selected for their sensitivity to kanamycin on minimal plates and for their acquired resistance to this antibiotic when an S. ionantha plant extract was added to kanamycin minimal plates. The selection allowed the identification of E. chrysanthemi promoters inducible by host factors present in the S. ionantha plant extract. Using this method, we isolated 30 mutants and characterized 10 of them. Two mutants were defective in cation uptake, one was defective in the galacturonate degradation pathway, and another was altered in the production of the acidic pectate lyase. The functions of the other mutated genes are still unknown, but we show that most of them are involved in pathogenicity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertheau Y., Madgidi-Hervan E., Kotoujansky A., Nguyen-The C., Andro T., Coleno A. Detection of depolymerase isoenzymes after electrophoresis or electrofocusing, or in titration curves. Anal Biochem. 1984 Jun;139(2):383–389. doi: 10.1016/0003-2697(84)90022-8. [DOI] [PubMed] [Google Scholar]
- Boccara M., Chatain V. Regulation and role in pathogenicity of Erwinia chrysanthemi 3937 pectin methylesterase. J Bacteriol. 1989 Jul;171(7):4085–4087. doi: 10.1128/jb.171.7.4085-4087.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher C. A., Van Gijsegem F., Barberis P. A., Arlat M., Zischek C. Pseudomonas solanacearum genes controlling both pathogenicity on tomato and hypersensitivity on tobacco are clustered. J Bacteriol. 1987 Dec;169(12):5626–5632. doi: 10.1128/jb.169.12.5626-5632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmet L., Faelen M., Gama M. J., Ferhat A., Toussaint A. Characterization of amber mutations in bacteriophage Mu transposase: a functional analysis of the protein. Mol Microbiol. 1989 Sep;3(9):1145–1158. doi: 10.1111/j.1365-2958.1989.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987 May;6(5):1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Halperin W., Nester E. W. Agrobacterium tumefaciens mutants affected in attachment to plant cells. J Bacteriol. 1982 Dec;152(3):1265–1275. doi: 10.1128/jb.152.3.1265-1275.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enard C., Diolez A., Expert D. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J Bacteriol. 1988 Jun;170(6):2419–2426. doi: 10.1128/jb.170.6.2419-2426.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert D., Toussaint A. Bacteriocin-resistant mutants of Erwinia chrysanthemi: possible involvement of iron acquisition in phytopathogenicity. J Bacteriol. 1985 Jul;163(1):221–227. doi: 10.1128/jb.163.1.221-227.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLANSDORFF N. TOPOGRAPHY OF COTRANSDUCIBLE ARGININE MUTATIONS IN ESCHERICHIA COLI K-12. Genetics. 1965 Feb;51:167–179. doi: 10.1093/genetics/51.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux-Cotte-Pattat N., Robert-Baudouy J. Hexuronate catabolism in Erwinia chrysanthemi. J Bacteriol. 1987 Mar;169(3):1223–1231. doi: 10.1128/jb.169.3.1223-1231.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles A. A., Khimji P. L. Enterobacterial chelators of iron: their occurrence, detection, and relation to pathogenicity. J Med Microbiol. 1975 Nov;8(4):477–490. doi: 10.1099/00222615-8-4-477. [DOI] [PubMed] [Google Scholar]
- Nester E. W., Kosuge T. Plasmids specifying plant hyperplasias. Annu Rev Microbiol. 1981;35:531–565. doi: 10.1146/annurev.mi.35.100181.002531. [DOI] [PubMed] [Google Scholar]
- Osbourn A. E., Barber C. E., Daniels M. J. Identification of plant-induced genes of the bacterial pathogen Xanthomonas campestris pathovar campestris using a promoter-probe plasmid. EMBO J. 1987 Jan;6(1):23–28. doi: 10.1002/j.1460-2075.1987.tb04713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratet P., Richaud F. Construction and uses of a new transposable element whose insertion is able to produce gene fusions with the neomycin-phosphotransferase-coding region of Tn903. Gene. 1986;42(2):185–192. doi: 10.1016/0378-1119(86)90295-7. [DOI] [PubMed] [Google Scholar]
- Reverchon S., Robert-Baudouy J. Regulation of expression of pectate lyase genes pelA, pelD, and pelE in Erwinia chrysanthemi. J Bacteriol. 1987 Jun;169(6):2417–2423. doi: 10.1128/jb.169.6.2417-2423.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverchon S., Van Gijsegem F., Rouve M., Kotoujansky A., Robert-Baudouy J. Organization of a pectate lyase gene family in Erwinia chrysanthemi. Gene. 1986;49(2):215–224. doi: 10.1016/0378-1119(86)90282-9. [DOI] [PubMed] [Google Scholar]
- Schoonejans E., Expert D., Toussaint A. Characterization and virulence properties of Erwinia chrysanthemi lipopolysaccharide-defective, phi EC2-resistant mutants. J Bacteriol. 1987 Sep;169(9):4011–4017. doi: 10.1128/jb.169.9.4011-4017.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W., Zambryski P. C. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci U S A. 1986 Jan;83(2):379–383. doi: 10.1073/pnas.83.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]
- van Gijsegem F. Relationship between the pel genes of the pelADE cluster in Erwinia chrysanthemi strain B374. Mol Microbiol. 1989 Oct;3(10):1415–1424. doi: 10.1111/j.1365-2958.1989.tb00124.x. [DOI] [PubMed] [Google Scholar]