Abstract
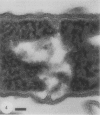
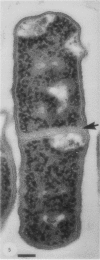
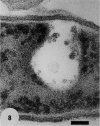
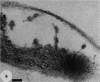
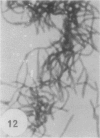
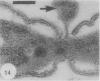
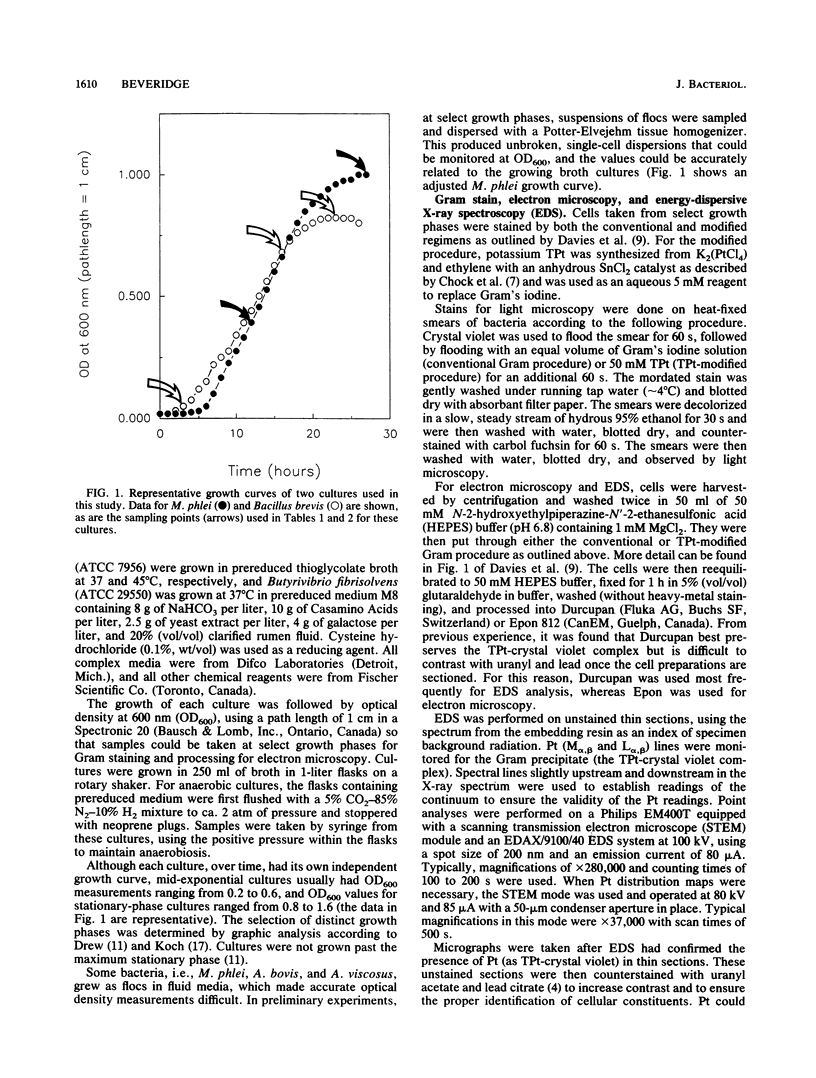
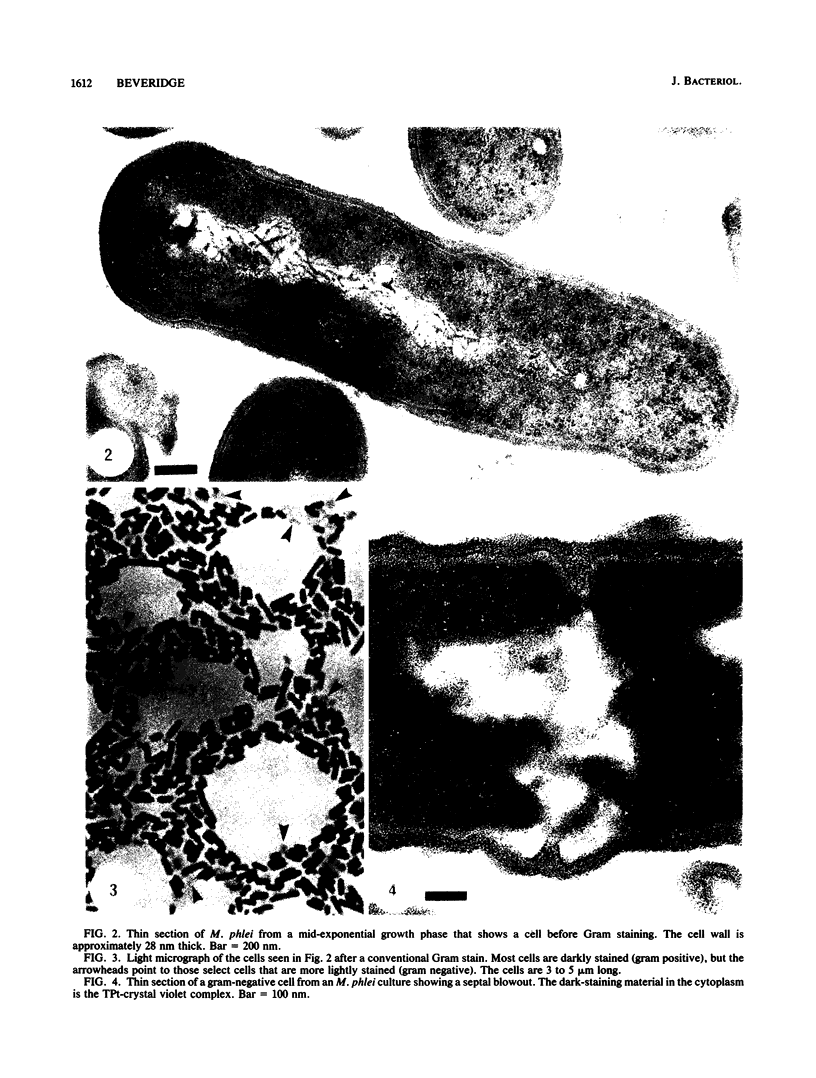
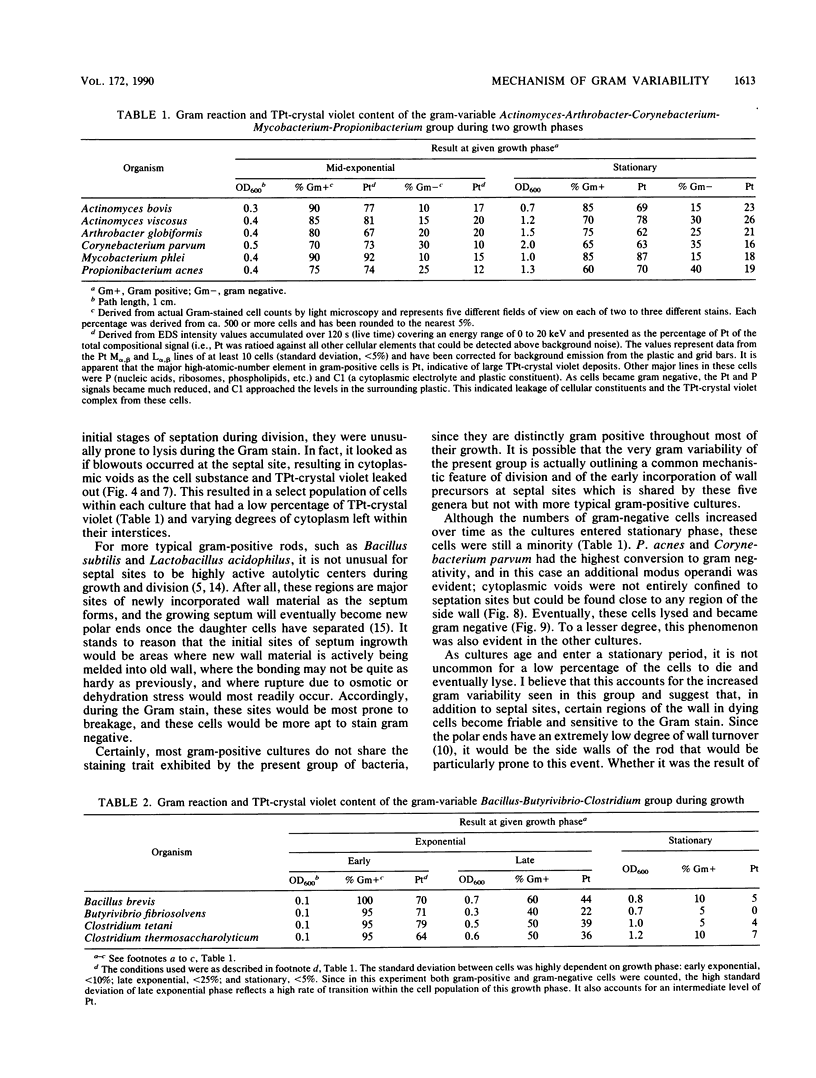
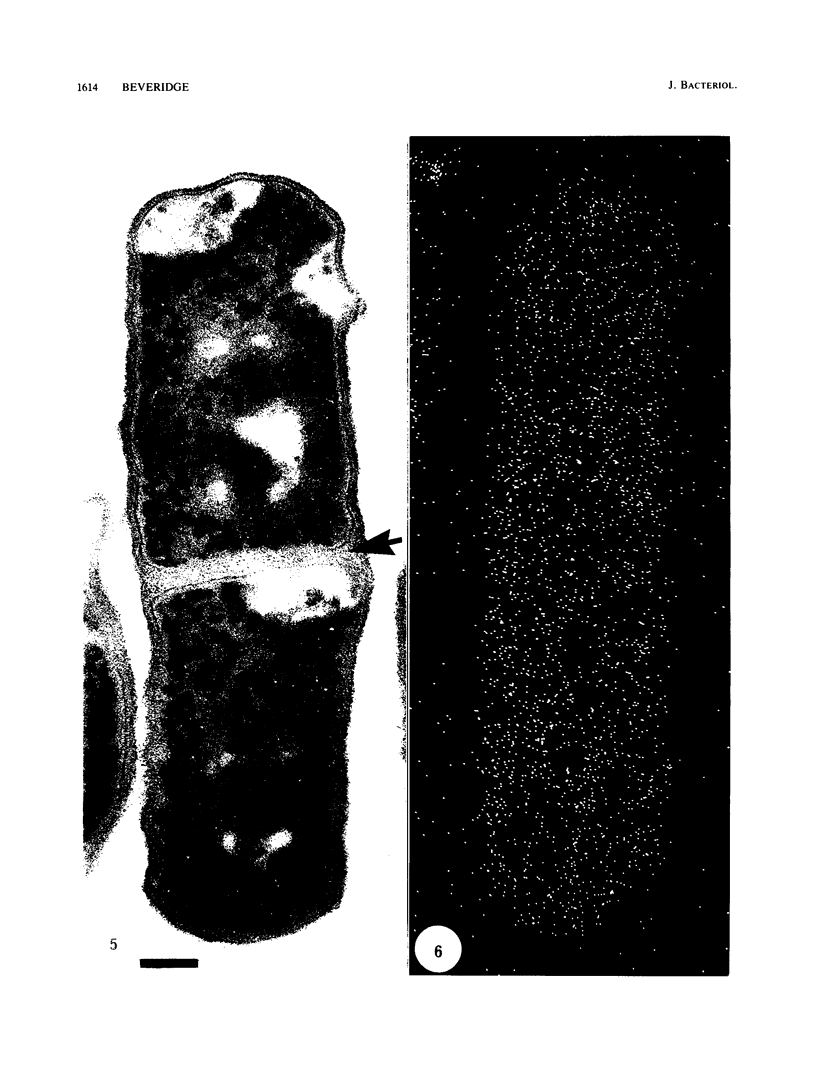
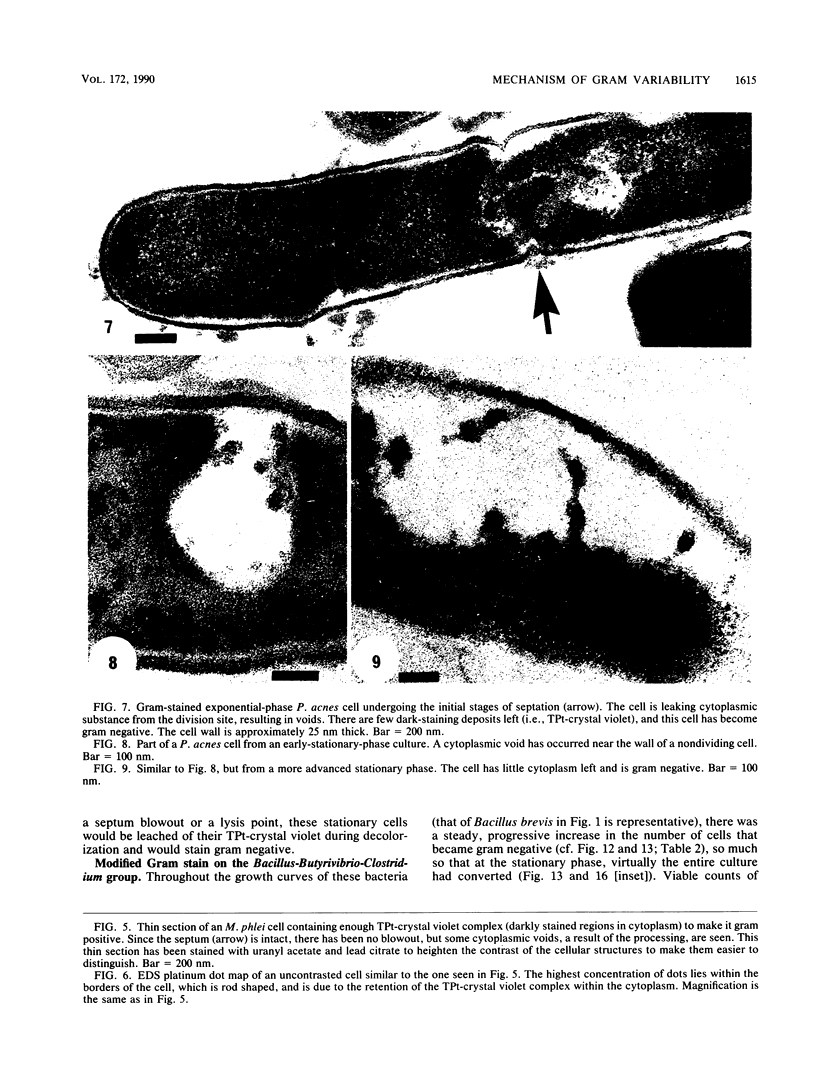
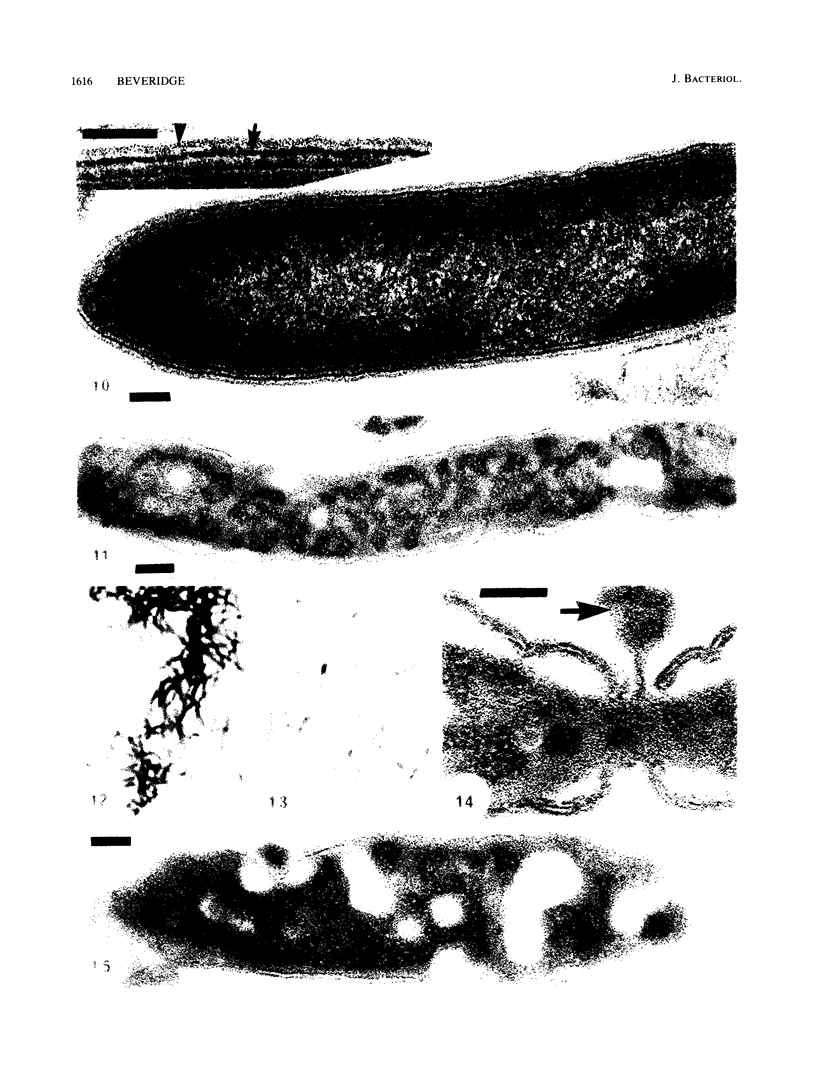
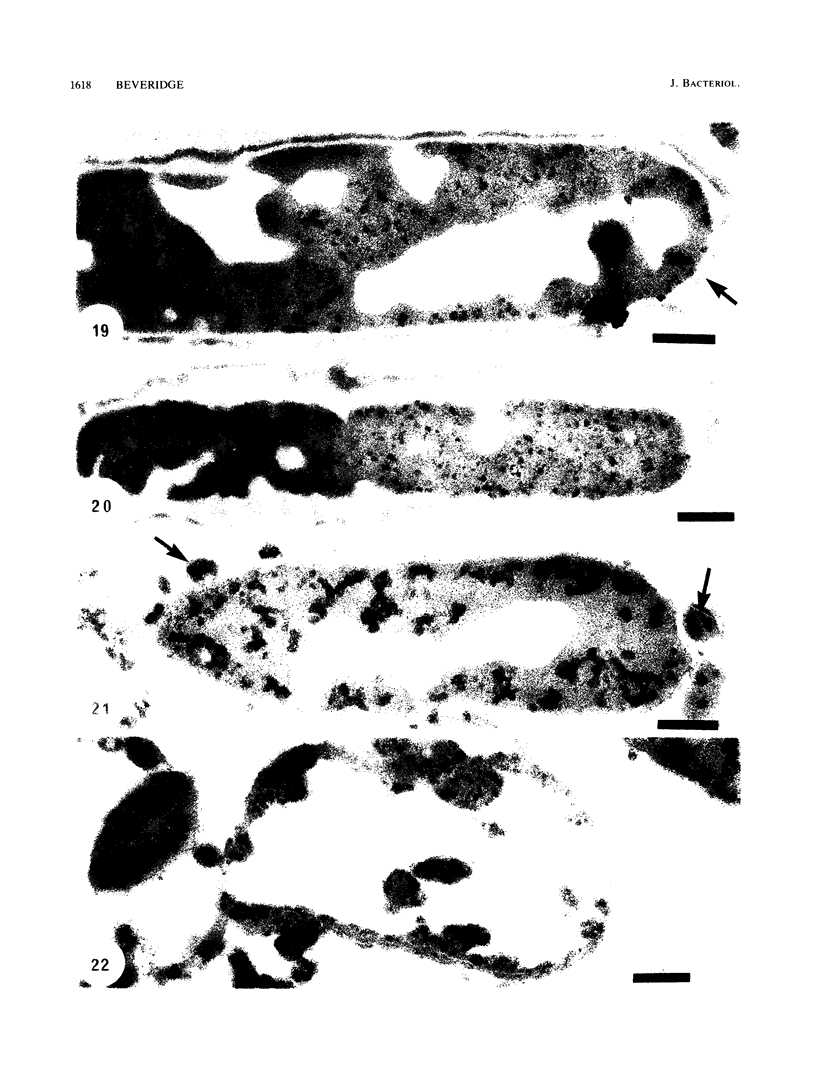
Gram stains were performed on strains of Actinomyces bovis, Actinomyces viscosus, Arthrobacter globiformis, Bacillus brevis, Butyrivibrio fibrisolvens, Clostridium tetani, Clostridium thermosaccharolyticum, Corynebacterium parvum, Mycobacterium phlei, and Propionibacterium acnes, using a modified Gram regimen that allowed the staining process to be observed by electron microscopy (J. A. Davies, G. K. Anderson, T. J. Beveridge, and H. C. Clark, J. Bacteriol. 156:837-845, 1983). Furthermore, since a platinum salt replaced the iodine mordant of the Gram stain, energy-dispersive X-ray spectroscopy could evaluate the stain intensity and location by monitoring the platinum signal. These gram-variable bacteria could be split into two groups on the basis of their staining responses. In the Actinomyces-Arthrobacter-Corynebacterium-Mycobacterium-Propionibacterium group, few cells became gram negative until the exponential growth phase; by mid-exponential phase, 10 to 30% of the cells were gram negative. The cells that became gram negative were a select population of the culture, had initiated septum formation, and were more fragile to the stress of the Gram stain at the division site. As cultures aged to stationary phase, there was a relatively slight increase toward gram negativity (now 15 to 40%) due to the increased lysis of nondividing cells by means of lesions in the side walls; these cells maintained their rod shape but stained gram negative. Those in the Bacillus-Butyrivibrio-Clostridium group also became gram negative as cultures aged but by a separate set of events. These bacteria possessed more complex walls, since they were covered by an S layer. They stained gram positive during lag and the initial exponential growth phases, but as doubling times increased, the wall fabric underlying the S layer became noticeably thinner and diffuse, and the cells became more fragile to the Gram stain. By stationary phase, these cultures were virtually gram negative.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Davies J. A. Cellular responses of Bacillus subtilis and Escherichia coli to the Gram stain. J Bacteriol. 1983 Nov;156(2):846–858. doi: 10.1128/jb.156.2.846-858.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J. The bacterial surface: general considerations towards design and function. Can J Microbiol. 1988 Apr;34(4):363–372. doi: 10.1139/m88-067. [DOI] [PubMed] [Google Scholar]
- Beveridge T. J. Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol. 1981;72:229–317. doi: 10.1016/s0074-7696(08)61198-5. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Ultrastructure of Butyrivibrio fibrisolvens: a gram-positive bacterium. J Bacteriol. 1977 Mar;129(3):1506–1512. doi: 10.1128/jb.129.3.1506-1512.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. A., Anderson G. K., Beveridge T. J., Clark H. C. Chemical mechanism of the Gram stain and synthesis of a new electron-opaque marker for electron microscopy which replaces the iodine mordant of the stain. J Bacteriol. 1983 Nov;156(2):837–845. doi: 10.1128/jb.156.2.837-845.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle R. J., Koch A. L. The functions of autolysins in the growth and division of Bacillus subtilis. Crit Rev Microbiol. 1987;15(2):169–222. doi: 10.3109/10408418709104457. [DOI] [PubMed] [Google Scholar]
- Goodfellow M., Collins M. D., Minnikin D. E. Thin-layer chromatographic analysis of mycolic acid and other long-chain components in whole-organism methanolysates of coryneform and related taxa. J Gen Microbiol. 1976 Oct;96(2):351–358. doi: 10.1099/00221287-96-2-351. [DOI] [PubMed] [Google Scholar]
- Gruber K., Sleytr U. B. Localized insertion of new S-layer during growth of Bacillus stearothermophilus strains. Arch Microbiol. 1988;149(6):485–491. doi: 10.1007/BF00446749. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Coyette J., Shockman G. D. Sites of cellular autolysis in Lactobacillus acidophilus. J Bacteriol. 1973 Dec;116(3):1375–1382. doi: 10.1128/jb.116.3.1375-1382.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Howard L. V., Dalton D. D., McCoubrey W. K., Jr Expansion of the tetragonally arrayed cell wall protein layer during growth of Bacillus sphaericus. J Bacteriol. 1982 Feb;149(2):748–757. doi: 10.1128/jb.149.2.748-757.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckevich M. D., Beveridge T. J. Characterization of a dynamic S layer on Bacillus thuringiensis. J Bacteriol. 1989 Dec;171(12):6656–6667. doi: 10.1128/jb.171.12.6656-6667.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R. The relationship between the nature of the cell wall and the Gram stain. J Gen Microbiol. 1963 Feb;30:223–235. doi: 10.1099/00221287-30-2-223. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Glauert A. M. Ultrastructure of the cell walls of two closely related clostridia that possess different regular arrays of surface subunits. J Bacteriol. 1976 May;126(2):869–882. doi: 10.1128/jb.126.2.869-882.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers in procaryotes. J Bacteriol. 1988 Jul;170(7):2891–2897. doi: 10.1128/jb.170.7.2891-2897.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleytr U. B., Messner P. Crystalline surface layers on bacteria. Annu Rev Microbiol. 1983;37:311–339. doi: 10.1146/annurev.mi.37.100183.001523. [DOI] [PubMed] [Google Scholar]