Abstract
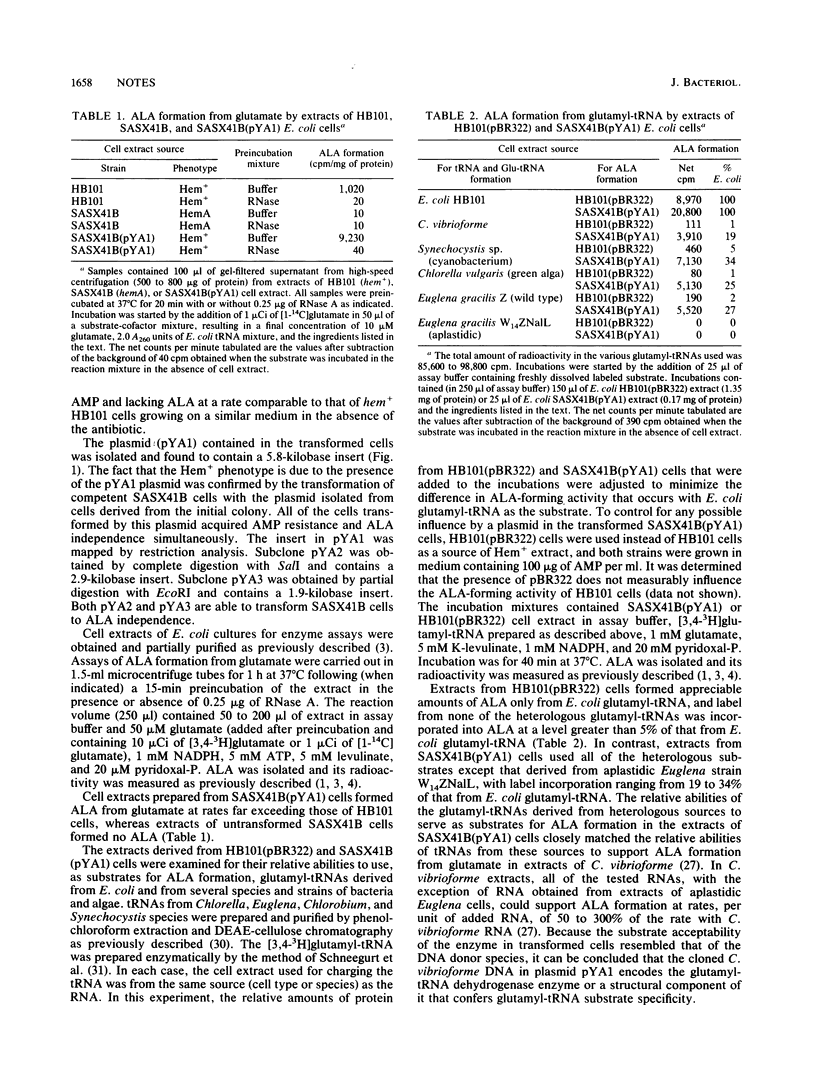
Escherichia coli SASX41B carries the hemA mutation and requires delta-aminolevulinic acid for growth. Strain SASX41B was transformed to prototrophy with pYA1, a plasmid vector carrying a 5.8-kilobase insert of genomic DNA from the green sulfur bacterium Chlorobium vibrioforme. Cell extracts prepared from transformed cells are able to catalyze transfer of label from [1-14C]glutamate or [3,4-3H]glutamyl-tRNA to delta-aminolevullinic acid at rates much higher than extracts of wild-type cells can, whereas extracts prepared from untransformed strain SASX41B cells lack both activities. By comparing the relative abilities of glutamyl-tRNAs derived from several heterologous cell types to function as substrates for the dehydrogenase reaction in extracts of HB101 and SASX41B cells transformed by pYA1, it was determined that the expressed dehydrogenase in the transformed cells resembled that of C. vibrioforme and not that of E. coli. Thus it can be concluded that plasmid pYA1 contains inserted DNA that codes for a structural component of C. vibrioforme glutamyl-tRNA dehydrogenase which confers glutamyl-tRNA substrate specificity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avissar Y. J., Beale S. I. Biosynthesis of Tetrapyrrole Pigment Precursors : Formation and Utilization of Glutamyl-tRNA for delta-Aminolevulinic Acid Synthesis by Isolated Enzyme Fractions from Chlorella Vulgaris. Plant Physiol. 1988 Nov;88(3):879–886. doi: 10.1104/pp.88.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Biosynthesis of Tetrapyrrole Pigment Precursors : Pyridoxal Requirement of the Aminotransferase Step in the Formation of delta-Aminolevulinate from Glutamate in Extracts of Chlorella vulgaris. Plant Physiol. 1989 Mar;89(3):852–859. doi: 10.1104/pp.89.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Beale S. I. Identification of the enzymatic basis for delta-aminolevulinic acid auxotrophy in a hemA mutant of Escherichia coli. J Bacteriol. 1989 Jun;171(6):2919–2924. doi: 10.1128/jb.171.6.2919-2924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avissar Y. J., Ormerod J. G., Beale S. I. Distribution of delta-aminolevulinic acid biosynthetic pathways among phototrophic bacterial groups. Arch Microbiol. 1989;151(6):513–519. doi: 10.1007/BF00454867. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale S. I., Castelfranco P. A. The Biosynthesis of delta-Aminolevulinic Acid in Higher Plants: II. Formation of C-delta-Aminolevulinic Acid from Labeled Precursors in Greening Plant Tissues. Plant Physiol. 1974 Feb;53(2):297–303. doi: 10.1104/pp.53.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet M., Péloquin L., Echelard Y., Cousineau L., Sasarman A. Isolation and nucleotide sequence of the hemA gene of Escherichia coli K12. Mol Gen Genet. 1989 Apr;216(2-3):347–352. doi: 10.1007/BF00334375. [DOI] [PubMed] [Google Scholar]
- Elliott T. Cloning, genetic characterization, and nucleotide sequence of the hemA-prfA operon of Salmonella typhimurium. J Bacteriol. 1989 Jul;171(7):3948–3960. doi: 10.1128/jb.171.7.3948-3960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott T., Roth J. R. Heme-deficient mutants of Salmonella typhimurium: two genes required for ALA synthesis. Mol Gen Genet. 1989 Apr;216(2-3):303–314. doi: 10.1007/BF00334369. [DOI] [PubMed] [Google Scholar]
- Guerinot M. L., Chelm B. K. Bacterial delta-aminolevulinic acid synthase activity is not essential for leghemoglobin formation in the soybean/Bradyrhizobium japonicum symbiosis. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1837–1841. doi: 10.1073/pnas.83.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddock B. A., Schairer H. U. Electron-transport chains of Escherichia coli. Reconstitution of respiration in a 5-aminolaevulinic acid-requiring mutant. Eur J Biochem. 1973 May;35(1):34–45. doi: 10.1111/j.1432-1033.1973.tb02806.x. [DOI] [PubMed] [Google Scholar]
- Hoober J. K., Kahn A., Ash D. E., Gough S., Kannangara C. G. Biosynthesis of delta-aminolevulinate in greening barley leaves. IX. Structure of the substrate, mode of gabaculine inhibition, and the catalytic mechanism of glutamate 1-semialdehyde aminotransferase. Carlsberg Res Commun. 1988;53(1):11–25. doi: 10.1007/BF02908411. [DOI] [PubMed] [Google Scholar]
- Leong S. A., Ditta G. S., Helinski D. R. Heme biosynthesis in Rhizobium. Identification of a cloned gene coding for delta-aminolevulinic acid synthetase from Rhizobium meliloti. J Biol Chem. 1982 Aug 10;257(15):8724–8730. [PubMed] [Google Scholar]
- Li J. M., Brathwaite O., Cosloy S. D., Russell C. S. 5-Aminolevulinic acid synthesis in Escherichia coli. J Bacteriol. 1989 May;171(5):2547–2552. doi: 10.1128/jb.171.5.2547-2552.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. M., Russell C. S., Cosloy S. D. Cloning and structure of the hem A gene of Escherichia coli K-12. Gene. 1989 Oct 30;82(2):209–217. doi: 10.1016/0378-1119(89)90046-2. [DOI] [PubMed] [Google Scholar]
- Mat-Jan F., Williams C. R., Clark D. P. Anaerobic growth defects resulting from gene fusions affecting succinyl-CoA synthetase in Escherichia coli K12. Mol Gen Genet. 1989 Jan;215(2):276–280. doi: 10.1007/BF00339728. [DOI] [PubMed] [Google Scholar]
- Mayer S. M., Beale S. I., Weinstein J. D. Enzymatic conversion of glutamate to delta-aminolevulinic acid in soluble extracts of Euglena gracilis. J Biol Chem. 1987 Sep 15;262(26):12541–12549. [PubMed] [Google Scholar]
- O'Neill G. P., Chen M. W., Söll D. delta-Aminolevulinic acid biosynthesis in Escherichia coli and Bacillus subtilis involves formation of glutamyl-tRNA. FEMS Microbiol Lett. 1989 Aug;51(3):255–259. doi: 10.1016/0378-1097(89)90406-0. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Seto H., Miyachi S. 13C-NMR evidence of bacteriochlorophyll a formation by the C5 pathway in Chromatium. Arch Biochem Biophys. 1986 Apr;246(1):192–198. doi: 10.1016/0003-9861(86)90463-7. [DOI] [PubMed] [Google Scholar]
- Oh-hama T., Stolowich N. J., Scott A. I. 5-Aminolevulinic acid formation from glutamate via the C5 pathway in Clostridium thermoaceticum. FEBS Lett. 1988 Feb 8;228(1):89–93. doi: 10.1016/0014-5793(88)80591-x. [DOI] [PubMed] [Google Scholar]
- Rieble S., Beale S. I. Transformation of glutamate to delta-aminolevulinic acid by soluble extracts of Synechocystis sp. PCC 6803 and other oxygenic prokaryotes. J Biol Chem. 1988 Jun 25;263(18):8864–8871. [PubMed] [Google Scholar]
- Rieble S., Ormerod J. G., Beale S. I. Transformation of glutamate to delta-aminolevulinic acid by soluble extracts of Chlorobium vibrioforme. J Bacteriol. 1989 Jul;171(7):3782–3787. doi: 10.1128/jb.171.7.3782-3787.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneegurt M. A., Beale S. I. Characterization of the RNA Required for Biosynthesis of delta-Aminolevulinic Acid from Glutamate : Purification by Anticodon-Based Affinity Chromatography and Determination That the UUC Glutamate Anticodon Is a General Requirement for Function in ALA Biosynthesis. Plant Physiol. 1988 Feb;86(2):497–504. doi: 10.1104/pp.86.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneegurt M. A., Rieble S., Beale S. I. The tRNA Required for in Vitro delta-Aminolevulinic Acid Formation from Glutamate in Synechocystis Extracts : Determination of Activity in a Synechocystis in Vitro Protein Synthesizing System. Plant Physiol. 1988 Dec;88(4):1358–1366. doi: 10.1104/pp.88.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenhaut D. S., Curtis P. J. Nucleotide sequence of mouse 5-aminolevulinic acid synthase cDNA and expression of its gene in hepatic and erythroid tissues. Gene. 1986;48(1):55–63. doi: 10.1016/0378-1119(86)90351-3. [DOI] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Horodniceanu T. Locus determining the synthesis of delta-aminolevulinic acid in Escherichia coli K-12. J Bacteriol. 1968 Nov;96(5):1882–1884. doi: 10.1128/jb.96.5.1882-1884.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Săsărman A., Surdeanu M., Szégli G., Horodniceanu T., Greceanu V., Dumitrescu A. Hemin-deficient mutants of Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):570–572. doi: 10.1128/jb.96.2.570-572.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai T. N., Moore M. D., Kaplan S. Cloning and characterization of the 5-aminolevulinate synthase gene(s) from Rhodobacter sphaeroides. Gene. 1988 Oct 15;70(1):139–151. doi: 10.1016/0378-1119(88)90112-6. [DOI] [PubMed] [Google Scholar]
- Verkamp E., Chelm B. K. Isolation, nucleotide sequence, and preliminary characterization of the Escherichia coli K-12 hemA gene. J Bacteriol. 1989 Sep;171(9):4728–4735. doi: 10.1128/jb.171.9.4728-4735.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. Y., Huang D. D., Stachon D., Gough S. P., Kannangara C. G. Purification, Characterization, and Fractionation of the delta-Aminolevulinic Acid Synthesizing Enzymes from Light-Grown Chlamydomonas reinhardtii Cells. Plant Physiol. 1984 Mar;74(3):569–575. doi: 10.1104/pp.74.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]



