Abstract
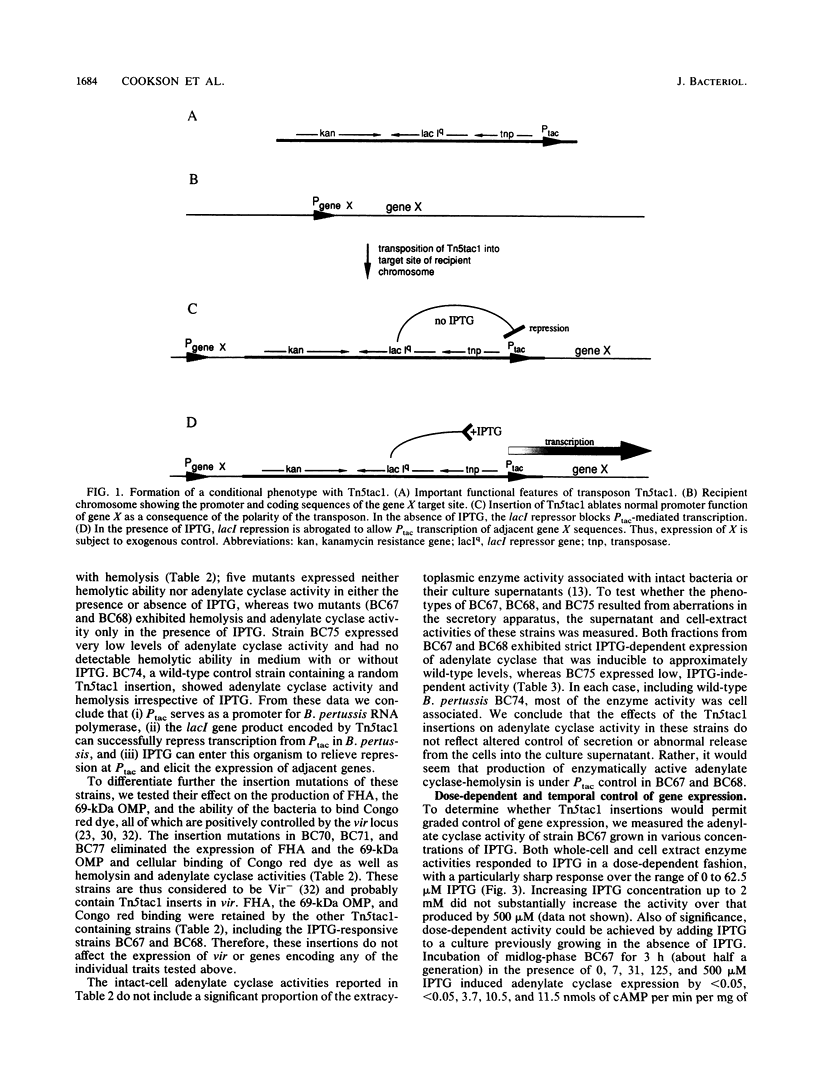
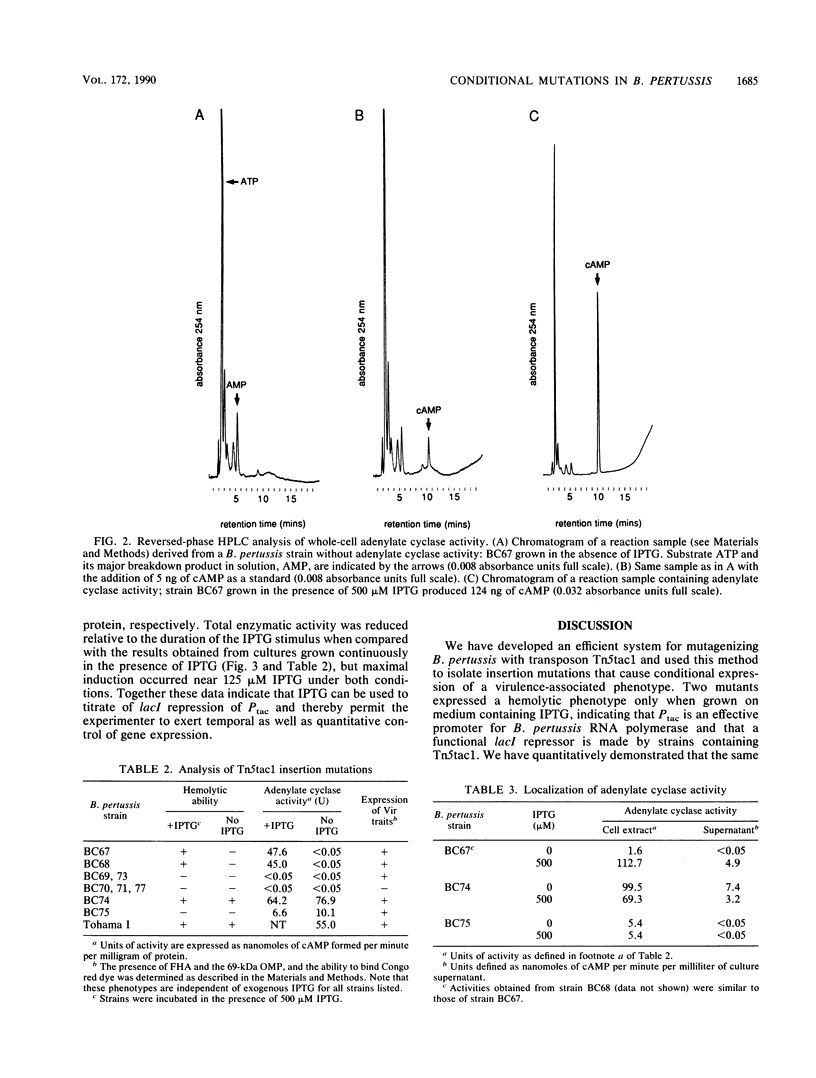
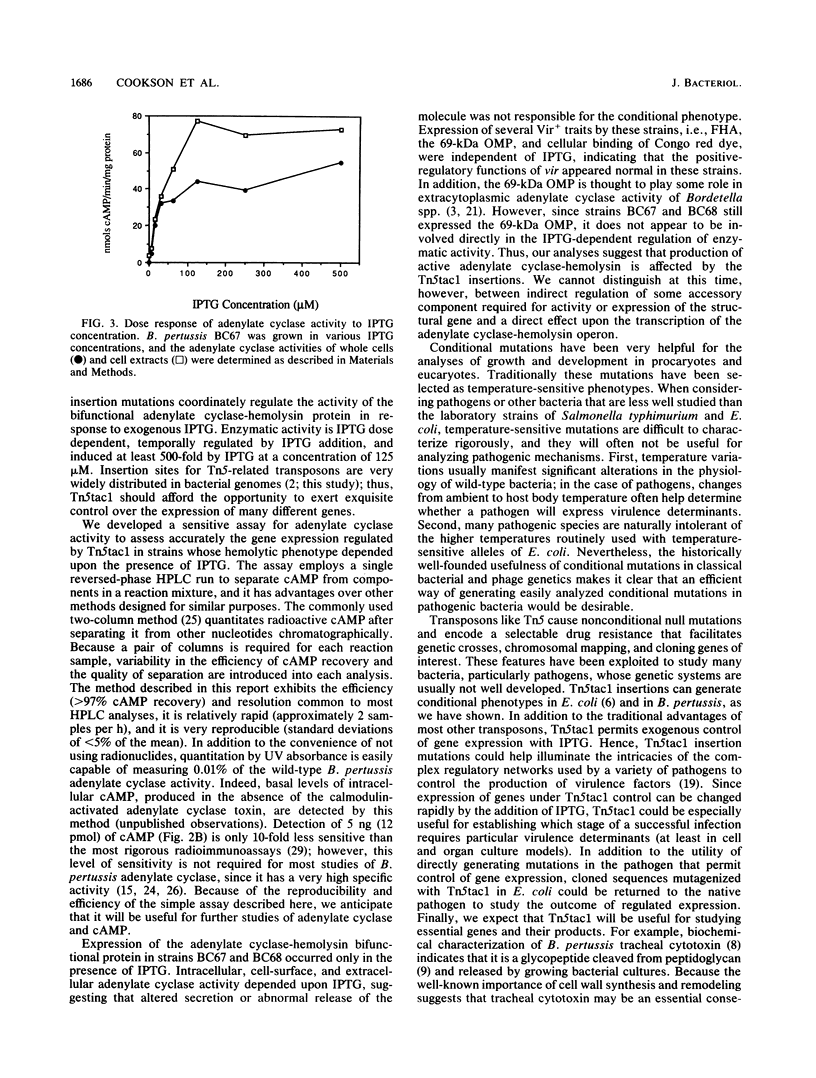
The Tn5tac1 transposon contains a strong outward-facing promoter, Ptac, a lacI repressor gene, and a selectable Kanr gene. Transcription from Ptac is repressed by the lacI protein unless an inducer (isopropyl-beta-D-thiogalactopyranoside [IPTG]) is present. Thus, Tn5tac1 generates insertion mutations in Escherichia coli with conditional phenotypes because it is polar on distal gene expression when IPTG is absent and directs transcription of these genes when the inducer is present. To test the usefulness of Tn5tac1 in Bordetella pertussis, a nonenteric gram-negative bacterial pathogen, we chose the bifunctional adenylate cyclase-hemolysin determinant as an easily scored marker to monitor insertional mutagenesis. Tn5tac1 delivered to B. pertussis on conjugal suicide plasmids resulted in Kanr exconjugants at a frequency of 10(-3) per donor cell, and nonhemolytic (Hly-) mutants were found among the Kanr colonies at a frequency of about 1%. Of eight independent Kanr Hly- mutants, two were conditional and exhibited an Hly+ phenotype only in the presence of IPTG. Using a new quantitative assay for adenylate cyclase based on high-pressure liquid chromatography, we found that enzymatic activity in these two strains was specifically induced at least 500-fold in a dose-dependent fashion over the range of 0 to 125 microM IPTG. These data show that Ptac serves as a promoter, lacI is expressed and is functional, and IPTG can induce Ptac transcription in B. pertussis. Adenylate cyclase expression in whole cells, culture supernatants, and cell extracts from these strains depended upon IPTG, suggesting that the insertions do not merely alter secretion of adenylate cyclase-hemolysin. Other virulence determinants under control of the vir locus are expressed normally, implying that these Tn5tac1 insertions specifically regulate adenylate cyclase-hemolysin expression. We conclude that Tn5tac1 insertion mutations permit sensitive, exogenous control over the expression of genes of interest, providing a useful tool for studying virulence and other important traits of diverse bacterial species.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brennan M. J., Li Z. M., Cowell J. L., Bisher M. E., Steven A. C., Novotny P., Manclark C. R. Identification of a 69-kilodalton nonfimbrial protein as an agglutinogen of Bordetella pertussis. Infect Immun. 1988 Dec;56(12):3189–3195. doi: 10.1128/iai.56.12.3189-3195.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Chow W. Y., Berg D. E. Tn5tac1, a derivative of transposon Tn5 that generates conditional mutations. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6468–6472. doi: 10.1073/pnas.85.17.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Peterson L. P., Baseman J. B. Pathogenesis of infection with Bordetella pertussis in hamster tracheal organ culture. J Infect Dis. 1977 Aug;136 (Suppl):S196–S203. doi: 10.1093/infdis/136.supplement.s196. [DOI] [PubMed] [Google Scholar]
- Cookson B. T., Cho H. L., Herwaldt L. A., Goldman W. E. Biological activities and chemical composition of purified tracheal cytotoxin of Bordetella pertussis. Infect Immun. 1989 Jul;57(7):2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cookson B. T., Tyler A. N., Goldman W. E. Primary structure of the peptidoglycan-derived tracheal cytotoxin of Bordetella pertussis. Biochemistry. 1989 Feb 21;28(4):1744–1749. doi: 10.1021/bi00430a048. [DOI] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Glaser P., Sakamoto H., Bellalou J., Ullmann A., Danchin A. Secretion of cyclolysin, the calmodulin-sensitive adenylate cyclase-haemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988 Dec 1;7(12):3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E. L., Urban M. A., Manclark C. R., Wolff J. Extracytoplasmic adenylate cyclase of Bordetella pertussis. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1926–1930. doi: 10.1073/pnas.73.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewlett E., Wolff J. Soluble adenylate cyclase from the culture medium of Bordetella pertussis: purification and characterization. J Bacteriol. 1976 Aug;127(2):890–898. doi: 10.1128/jb.127.2.890-898.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladant D., Brezin C., Alonso J. M., Crenon I., Guiso N. Bordetella pertussis adenylate cyclase. Purification, characterization, and radioimmunoassay. J Biol Chem. 1986 Dec 5;261(34):16264–16269. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Nag D. K., Huang H. V., Berg D. E. Bidirectional chain-termination nucleotide sequencing: transposon Tn5seq1 as a mobile source of primer sites. Gene. 1988 Apr 15;64(1):135–145. doi: 10.1016/0378-1119(88)90487-8. [DOI] [PubMed] [Google Scholar]
- Novotny P., Chubb A. P., Cownley K., Montaraz J. A. Adenylate cyclase activity of a 68,000-molecular-weight protein isolated from the outer membrane of Bordetella bronchiseptica. Infect Immun. 1985 Oct;50(1):199–206. doi: 10.1128/iai.50.1.199-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Parton R. Differentiation of phase I and variant strains of Bordetella pertussis on Congo red media. J Med Microbiol. 1988 Aug;26(4):301–306. doi: 10.1099/00222615-26-4-301. [DOI] [PubMed] [Google Scholar]
- Rogel A., Farfel Z., Goldschmidt S., Shiloach J., Hanski E. Bordetella pertussis adenylate cyclase. Identification of multiple forms of the enzyme by antibodies. J Biol Chem. 1988 Sep 15;263(26):13310–13316. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Shattuck R. L., Oldenburg D. J., Storm D. R. Purification and characterization of a calmodulin-sensitive adenylate cyclase from Bordetella pertussis. Biochemistry. 1985 Nov 5;24(23):6356–6362. doi: 10.1021/bi00344a006. [DOI] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Wehmann R. E., Parker C. W., Kipnis D. M. Radioimmunoassay for the measurement of cyclic nucleotides. Adv Cyclic Nucleotide Res. 1972;2:51–61. [PubMed] [Google Scholar]
- Stibitz S., Weiss A. A., Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988 Jul;170(7):2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]



