Abstract
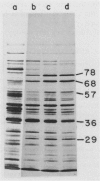
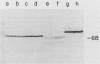
We have measured the effect of heat shock on three mycoplasmas (Acholeplasma laidlawii K2 and JA1 and Mycoplasma capricolum Kid) and demonstrated the induction of mycoplasma heat shock proteins under these conditions. Increased synthesis of at least 5 heat shock proteins in A. laidlawii K2, 11 heat shock proteins in A. laidlawii JA1, and 7 heat shock proteins in M. capricolum was observed by electrophoretic analysis of proteins from heat-shocked cells in sodium dodecyl sulfate-polyacrylamide gels. In all three strains, major heat shock proteins (66 to 68 and 26 to 29 kilodaltons [kDa]) were found. The 66- to 68-kDa protein cross-reacted with antibody to Escherichia coli DnaK protein, suggesting that this heat shock protein has been conserved in spite of major reductions in genetic complexity during mycoplasma evolution. A. laidlawii also contained a 60-kDa protein that cross-reacted with eubacterial GroEL protein and a 40-kDa protein that cross-reacted with E. coli RecA protein. Unlike with coliphages, the mycoplasma virus L2 progeny yield was not increased when virus was plated on heat-shocked A. laidlawii host cells. However, UV-irradiated L2 virus could be host cell reactivated by both A. laidlawii SOS repair and heat shock systems.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardwell J. C., Craig E. A. Major heat shock gene of Drosophila and the Escherichia coli heat-inducible dnaK gene are homologous. Proc Natl Acad Sci U S A. 1984 Feb;81(3):848–852. doi: 10.1073/pnas.81.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Das J., Nowak J. A., Maniloff J. Host cell and ultraviolet reactivation of ultraviolet-irradiated Mycoplasmaviruses. J Bacteriol. 1977 Mar;129(3):1424–1427. doi: 10.1128/jb.129.3.1424-1427.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C., Tilly K., Drahos D., Hendrix R. The B66.0 protein of Escherichia coli is the product of the dnaK+ gene. J Bacteriol. 1982 Mar;149(3):1175–1177. doi: 10.1128/jb.149.3.1175-1177.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Das J., Maniloff J. Lack of repair of ultraviolet light damage in Mycoplasma gallisepticum. J Mol Biol. 1977 Oct 25;116(2):337–344. doi: 10.1016/0022-2836(77)90221-2. [DOI] [PubMed] [Google Scholar]
- Haberer K., Klotz G., Maniloff J., Kleinschmidt A. K. Structural and biological properties of mycoplasmavirus MVL3: an unusual virus-procaryote interaction. J Virol. 1979 Oct;32(1):268–275. doi: 10.1128/jvi.32.1.268-275.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberer K., Maniloff J. Virus and host cell DNA syntheses during infection of Acholeplasma laidlawii by MVL3, a nonlytic cytocidal mycoplasmavirus. J Virol. 1980 Feb;33(2):671–679. doi: 10.1128/jvi.33.2.671-679.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger J. H., Walker G. C. groEL and dnaK genes of Escherichia coli are induced by UV irradiation and nalidixic acid in an htpR+-dependent fashion. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1499–1503. doi: 10.1073/pnas.81.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S., Craig E. A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Liss A., Maniloff J. Infection of Acholeplasma laidlawii by MVL51 virus. Virology. 1973 Sep;55(1):118–126. doi: 10.1016/s0042-6822(73)81013-x. [DOI] [PubMed] [Google Scholar]
- Maniloff J. Evolution of wall-less prokaryotes. Annu Rev Microbiol. 1983;37:477–499. doi: 10.1146/annurev.mi.37.100183.002401. [DOI] [PubMed] [Google Scholar]
- Maniloff J., Morowitz H. J. Cell biology of the mycoplasmas. Bacteriol Rev. 1972 Sep;36(3):263–290. doi: 10.1128/br.36.3.263-290.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J. Mycoplasma viruses. Crit Rev Microbiol. 1988;15(4):339–389. doi: 10.3109/10408418809104462. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A., Vaughn V. The genetics and regulation of heat-shock proteins. Annu Rev Genet. 1984;18:295–329. doi: 10.1146/annurev.ge.18.120184.001455. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Nowak J. A., Maniloff J. Physical characterization of the superhelical DNA genome of an enveloped mycoplasmavirus. J Virol. 1979 Jan;29(1):374–380. doi: 10.1128/jvi.29.1.374-380.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyström S., Johansson K. E., Wieslander A. Selective acylation of membrane proteins in Acholeplasma laidlawii. Eur J Biochem. 1986 Apr 1;156(1):85–94. doi: 10.1111/j.1432-1033.1986.tb09552.x. [DOI] [PubMed] [Google Scholar]
- Poddar S. K., Cadden S. P., Das J., Maniloff J. Heterogeneous progeny viruses are produced by a budding enveloped phage. Intervirology. 1985;23(4):208–221. doi: 10.1159/000149607. [DOI] [PubMed] [Google Scholar]
- Poddar S. K., Maniloff J. Chromosome analysis by two-dimensional fingerprinting. Gene. 1986;49(1):93–102. doi: 10.1016/0378-1119(86)90388-4. [DOI] [PubMed] [Google Scholar]
- Poddar S. K., Maniloff J. Determination of microbial genome sizes by two-dimensional denaturing gradient gel electrophoresis. Nucleic Acids Res. 1989 Apr 25;17(8):2889–2895. doi: 10.1093/nar/17.8.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razin S., Barile M. F., Harasawa R., Amikam D., Glaser G. Characterization of the mycoplasma genome. Yale J Biol Med. 1983 Sep-Dec;56(5-6):357–366. [PMC free article] [PubMed] [Google Scholar]
- Rogers M. J., Simmons J., Walker R. T., Weisburg W. G., Woese C. R., Tanner R. S., Robinson I. M., Stahl D. A., Olsen G., Leach R. H. Construction of the mycoplasma evolutionary tree from 5S rRNA sequence data. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1160–1164. doi: 10.1073/pnas.82.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan J. L., Morowitz H. J. Partial purification of native rRNA and tRNA cistrons from mycoplasma sp. (Kid). Proc Natl Acad Sci U S A. 1969 Aug;63(4):1282–1289. doi: 10.1073/pnas.63.4.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins: the search for functions. J Cell Biol. 1986 Aug;103(2):321–325. doi: 10.1083/jcb.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek T. L., Maniloff J. Polyethylene glycol-dependent transfection of Acholeplasma laidlawii with mycoplasma virus L2 DNA. J Bacteriol. 1983 Aug;155(2):734–741. doi: 10.1128/jb.155.2.734-741.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waghorne C., Fuerst C. R. Involvement of the htpR gene product of Escherichia coli in phage lambda development. Virology. 1985 Feb;141(1):51–64. doi: 10.1016/0042-6822(85)90182-5. [DOI] [PubMed] [Google Scholar]
- Weisburg W. G., Tully J. G., Rose D. L., Petzel J. P., Oyaizu H., Yang D., Mandelco L., Sechrest J., Lawrence T. G., Van Etten J. A phylogenetic analysis of the mycoplasmas: basis for their classification. J Bacteriol. 1989 Dec;171(12):6455–6467. doi: 10.1128/jb.171.12.6455-6467.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg J. S., Mowrey-McKee M. F., Stevens E. J. Induction of the heat shock regulon of Escherichia coli markedly increases production of bacterial viruses at high temperatures. J Virol. 1988 Jan;62(1):234–245. doi: 10.1128/jvi.62.1.234-245.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Maniloff J., Zablen L. B. Phylogenetic analysis of the mycoplasmas. Proc Natl Acad Sci U S A. 1980 Jan;77(1):494–498. doi: 10.1073/pnas.77.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]