Abstract
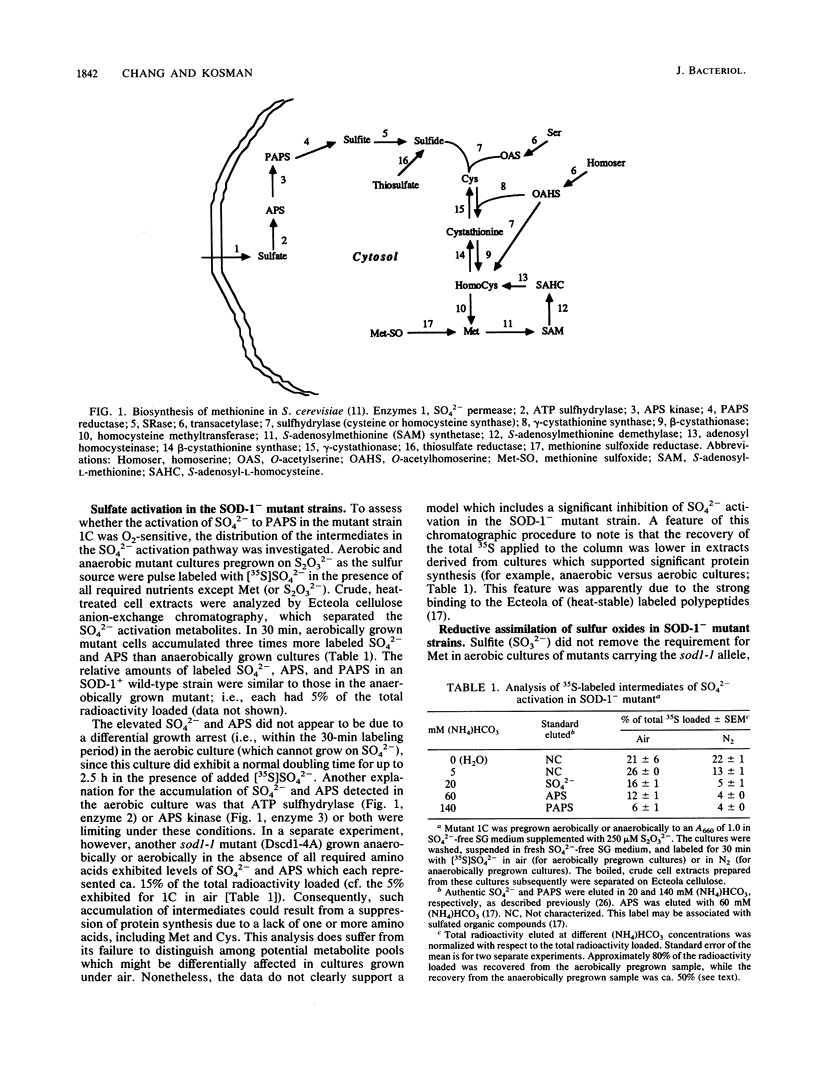
Mutant strains of the yeast Saccharomyces cerevisiae which lack functional Cu,Zn superoxide dismutase (SOD-1) do not grow aerobically unless supplemented with methionine. The molecular basis of this O2-dependent auxotrophy in one of the mutants, Dscd1-1C, has been investigated. Sulfate supported anaerobic but not aerobic mutant growth. On the other hand, cysteine and homocysteine supported aerobic growth while serine, O-acetylserine, and homoserine did not, indicating that the interconversion of cysteine and methionine (and homocysteine) was not impaired. Thiosulfate (S2O3(2-] and sulfide (S2-) also supported aerobic growth; the activities of thiosulfate reductase and sulfhydrylase in the aerobic mutant strain were at wild-type levels. Although the levels of SO4(2-) and adenosine-5'-sulfate (the first intermediate in the SO4(2-) assimilation pathway) were elevated in the aerobically incubated mutant strain, this condition could be attributed to a decrease in protein synthesis caused by the de facto sulfur starvation and not to a block in the pathway. Therefore, the activation of SO4(2-) (to form 3'-phosphoadenosine-5'-phosphosulfate) appeared to be O2 tolerant. Sulfite reductase activity and substrate concentrations [( NADPH] and [SO3(2-)]) were not significantly different in aerobically grown mutant cultures and anaerobic cultures, indicating that SOD-1- mutant strains could reductively assimilate sulfur oxides. However, the mutant strain exhibited an O2-dependent sensitivity to SO3(2-) concentrations of less than 50 microM not exhibited by any SOD-1+ strain or by SOD-1- strains supplemented with a cytosolic O2(-)-scavenging activity. This result suggests that the aerobic reductive assimilation of SO4(2-) at the level of SO3(2-) may generate a cytotoxic compound(s) which persists in SOD-(1-) yeast strains.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermingham-McDonogh O., Gralla E. B., Valentine J. S. The copper, zinc-superoxide dismutase gene of Saccharomyces cerevisiae: cloning, sequencing, and biological activity. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4789–4793. doi: 10.1073/pnas.85.13.4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biliński T., Krawiec Z., Liczmański A., Litwińska J. Is hydroxyl radical generated by the Fenton reaction in vivo? Biochem Biophys Res Commun. 1985 Jul 31;130(2):533–539. doi: 10.1016/0006-291x(85)90449-8. [DOI] [PubMed] [Google Scholar]
- Chang E. C., Kosman D. J. Intracellular Mn (II)-associated superoxide scavenging activity protects Cu,Zn superoxide dismutase-deficient Saccharomyces cerevisiae against dioxygen stress. J Biol Chem. 1989 Jul 25;264(21):12172–12178. [PubMed] [Google Scholar]
- Chauncey T. R., Uhteg L. C., Westley J. Thiosulfate reductase. Methods Enzymol. 1987;143:350–354. doi: 10.1016/0076-6879(87)43062-0. [DOI] [PubMed] [Google Scholar]
- Cooper A. J. Biochemistry of sulfur-containing amino acids. Annu Rev Biochem. 1983;52:187–222. doi: 10.1146/annurev.bi.52.070183.001155. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. An adaptation to a paramagnetic gas. J Biol Chem. 1989 May 15;264(14):7761–7764. [PubMed] [Google Scholar]
- Gonzalez Porqué P., Baldesten A., Reichard P. The involvement of the thioredoxin system in the reduction of methionine sulfoxide and sulfate. J Biol Chem. 1970 May 10;245(9):2371–2374. [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze H., Holzer H. Analysis of the energy metabolism after incubation of Saccharomyces cerevisiae with sulfite or nitrite. Arch Microbiol. 1986 Jun;145(1):27–31. doi: 10.1007/BF00413023. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Leinweber F. J., Monty K. J. Sulfite determination: fuchsin method. Methods Enzymol. 1987;143:15–17. doi: 10.1016/0076-6879(87)43006-1. [DOI] [PubMed] [Google Scholar]
- Masselot M., Surdin-Kerjan Y. Methionine biosynthesis in Saccharomyces cerevisiae. II. Gene-enzyme relationships in the sulfate assimilation pathway. Mol Gen Genet. 1977 Jul 7;154(1):23–30. doi: 10.1007/BF00265572. [DOI] [PubMed] [Google Scholar]
- Neta P., Huie R. E. Free-radical chemistry of sulfite. Environ Health Perspect. 1985 Dec;64:209–217. doi: 10.1289/ehp.8564209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. A., Morphy M., Szymanski H. Formation of adenosine-5'-phosphosulfate from 3'-phosphoadenosine-5'-phosphosulfate in human platelets. Biochem Pharmacol. 1986 Oct 15;35(20):3646–3649. doi: 10.1016/0006-2952(86)90642-8. [DOI] [PubMed] [Google Scholar]
- Schimz K. L. The effect of sulfite on the yeast Saccharomyces cerevisiae. Arch Microbiol. 1980 Mar;125(1-2):89–95. doi: 10.1007/BF00403203. [DOI] [PubMed] [Google Scholar]
- Singh A., Sherman F. Genetic and physiological characterization of met15 mutants of Saccharomyces cerevisiae: a selective system for forward and reverse mutations. Genetics. 1975 Sep;81(1):75–97. doi: 10.1093/genetics/81.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Tonnesen T., Friesen J. D. Inhibitors of ribonucleic acid synthesis in Saccharomyces cerevisiae: decay rate of messenger ribonucleic acid. J Bacteriol. 1973 Sep;115(3):889–896. doi: 10.1128/jb.115.3.889-896.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K., Bandurski R. S. Yeast sulfate-reducing system. 3. An intermediate in the reduction of 3'-phosphoryl-5'-adenosinephosphosulfate to sulfite. Biochim Biophys Acta. 1967 Mar 22;136(2):286–295. doi: 10.1016/0304-4165(67)90074-8. [DOI] [PubMed] [Google Scholar]
- WILSON L. G., ASAHI T., BANDURSKI R. S. Yeast sulfate-reducing system. I. Reduction of sulfate to sulfite. J Biol Chem. 1961 Jun;236:1822–1829. [PubMed] [Google Scholar]
- Westerbeek-Marres C. A., Moore M. M., Autor A. P. Regulation of manganese superoxide dismutase in Saccharomyces cerevisiae. The role of respiratory chain activity. Eur J Biochem. 1988 Jul 1;174(4):611–620. doi: 10.1111/j.1432-1033.1988.tb14142.x. [DOI] [PubMed] [Google Scholar]
- Whittemore R. M., Roth J. A. A modified Ecteola cellulose assay for M and P phenol sulfotransferase. Biochem Pharmacol. 1985 May 15;34(10):1647–1652. doi: 10.1016/0006-2952(85)90629-x. [DOI] [PubMed] [Google Scholar]
- Yamagata S., Takeshima K., Naiki N. Evidence for the identity of O-acetylserine sulfhydrylase with O-acetylhomoserine sulfhydrylase in yeast. J Biochem. 1974 Jun;75(6):1221–1229. doi: 10.1093/oxfordjournals.jbchem.a130505. [DOI] [PubMed] [Google Scholar]
- Yoshimoto A., Sato R. Studies on yeast sulfite reductase. I. Purification and characterization. Biochim Biophys Acta. 1968 Apr 2;153(3):555–575. doi: 10.1016/0005-2728(68)90185-0. [DOI] [PubMed] [Google Scholar]
- van Loon A. P., Pesold-Hurt B., Schatz G. A yeast mutant lacking mitochondrial manganese-superoxide dismutase is hypersensitive to oxygen. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3820–3824. doi: 10.1073/pnas.83.11.3820. [DOI] [PMC free article] [PubMed] [Google Scholar]



