Abstract
Background
External apical root resorption is a pathologic consequence of orthodontic tooth movement. Cementum and dentin are removed from the root surface while active force is present.
Objective
The aim of this study was to identify and quantify extracellular matrix proteins, dentin matrix protein 1 (DMP1), dentin phosphophoryn (PP), and dentin sialoprotein (DSP) in the gingival crevicular fluid (GCF) of subjects undergoing orthodontic treatment.
Methods
Subjects with mild (less than 2mm) and severe (more than 2mm) root resorption during orthodontic treatment were identified by radiographs. A control group of subjects with neither signs of root loss nor undergoing orthodontic treatment was also identified. GCF was collected from the upper incisors by using filter paper strips (Periopaper). The absorbed GCF was eluted and the proteins were separated by SDS–PAGE analysis and stained. Western blot and ELISA were also performed. One Way Anova and Scheffé test were used for statistical analysis.
Results
SDS-PAGE analysis identified proteins at 77, 66, 55, 50 and 26 kDa. Immunoblotting did not show any differential expression pattern between control and study groups. ELISA results revealed a significant difference in the concentrations of DMP1, PP and DSP between control and root resorption groups. Concentration of PP and DSP in severe root resorption group was also statistically higher than in mild root resorption group.
Conclusion
DSP and PP could be suitable biological markers for monitoring root resorption during orthodontic treatment, since a significant difference in the level of these dentin specific proteins is detected in all groups.
Introduction
External apical root resorption is a common and undesirable sequela of orthodontic treatment.1 Generally root resorption may be described as mild, moderate and severe.2 Usually it is mild and clinically insignificant; however it can occur in large amounts, i.e. loss of over one third of the root length, in some patients. Close radiographic examination of orthodontically treated individuals show some loss of root length in nearly every patient.3,4 The incisors are the most susceptible while the molars seem to be the least affected.5
Early detection of root resorption during orthodontic treatment is essential for identifying teeth at risk of severe resorption.6 At present, detection of root resorption is obtained using radiographic techniques. However, radiographs are technique sensitive and can detect resorption only after 60-70% of the mineralized tissue is lost and they only provide two-dimensional information primarily identifying apical change. In addition, radiographs cannot indicate if the process of root resorption is still active. Monitoring the progress of root resorption requires additional radiation exposure to the patient. The initial resorption lacunae are small and can be identified only by histological methods. Orthodontically-induced root resorption areas after 7 weeks of treatment, verified histologically, are not visible in periapical radiographs.7 Thus, using film-based radiography, the diagnosis is uncertain during the first months of treatment. After 5-6 months a reliable radiographic diagnosis of apical root resorption can be performed.8 Published results demonstrate the presence of DMP1 and PP in the GCF of patients undergoing orthodontic treatment.9
The purpose of this study is to determine if an alternate molecular method is effective in assessing ongoing resorption in active orthodontic patients by identifying and quantifying extracellular matrix proteins associated with dentin mineralization like dentin matrix protein 1 (DMP1), dentin phosphophoryn (PP) and dentin sialoprotein (DSP) in the gingival crevicular fluid (GCF) of subjects undergoing orthodontic treatment. PP and DSP are non-collagenous dentin-specific matrix proteins postulated to be involved in the mineralization of pre-dentin into dentin10-13, while DMP1 is present in dentin as well as in bone.14, 15 Dentin undergoes continuous deposition throughout life as a secondary dentin only on the pulpal surface. Therefore, these proteins are not routinely released into the surrounding space as dentin does not undergo the process of remodeling as in bone. It is only in the presence of active external root resorption that these proteins could be freed into the periodontal ligament space. Therefore, we hypothesize that a significant qualitative and quantitative difference of levels of these proteins exists between orthodontic patients with radiographic signs of root resorption and non-treated patients and between patients with mild and severe root resorption.
Materials and methods
Subject selection
Sixty subjects were selected from patients seeking treatment in the Department of Orthodontics at the University of Illinois at Chicago. Two study groups and one control group were set up. One “study” group included 20 subjects in treatment for at least one year and with radiographic signs of mild root resorption, less than 2 mm (1.34 ± 0.39 mm) of root loss (Fig. 1a) and the other “study” group included 20 subjects with radiographic signs of severe root resorption, more than 2 mm (2.78 ± 0.48 mm) of root loss (Fig. 1b) and were undergoing orthodontic treatment. The “control” group included 20 subjects who had not started treatment yet and demonstrated no radiographic evidence for root shortening. All subjects were free of systemic disease, periodontal disease and bleeding on probing, had good oral hygiene and had not received any anti-inflammatory drugs in the month preceding the study or antibiotic therapy within the past 6 months. An informed consent was obtained from each patient. This project was approved by the Institutional Review Board (IRB # 2001-0328) prior to collection of samples.
Figure 1.
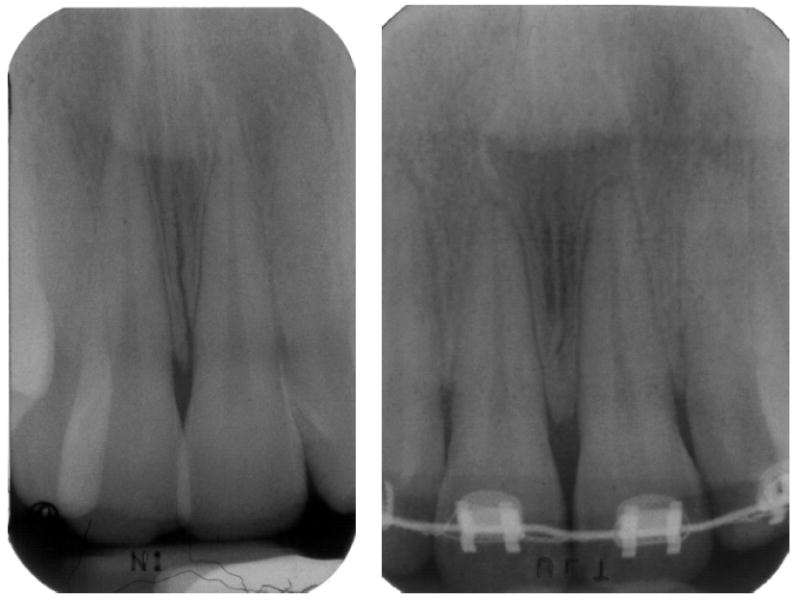
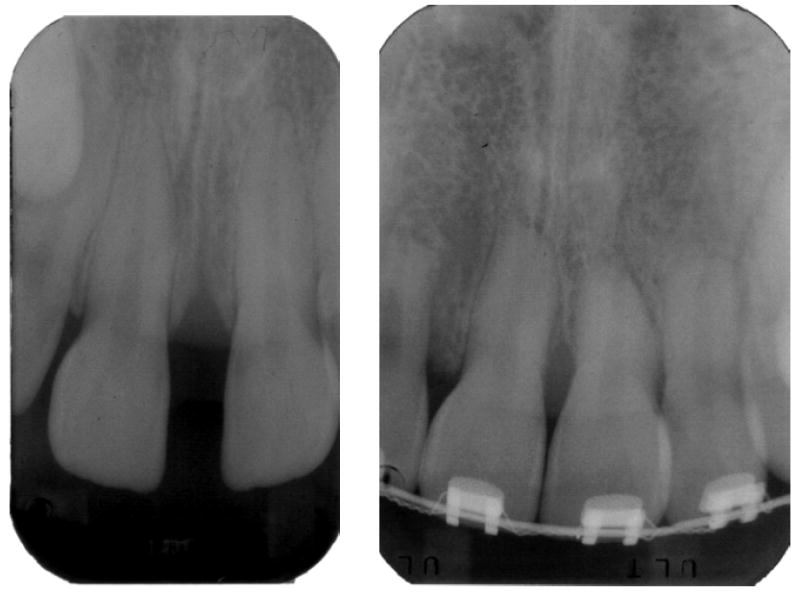
Periapical radiograph of a subject presenting mild root resorption (a) and severe root resorption (b).
The control group included 13 females and 7 males; age ranging from 12 to 34 years. The mild root resorption group had 11 females and 9 males; age ranging from 14 to 40 years. The severe root resorption group included 15 females and 5 males, with ages from 16 to 44 years.
Gingival crevicular fluid collection
Gingival crevicular fluid was collected from the mesial and distal side of the upper central and lateral incisors by using filter paper strips (Periopaper®, Oraflow, Plainview, NY) inserted 1-2 mm into the gingival sulcus for 30 sec. The same procedure was repeated twice at 1 minute interval. Cotton rolls and saliva ejector were used to isolate the teeth from saliva contamination. After removal, the strips were immediately sealed in a micro centrifuge tube with 1 X phosphate buffered saline solution containing the protease inhibitor, 0.1 mM phenylmethylsulphonylfluoride. To retrieve the sample from the paper strip, the GCF was eluted by centrifugal filtration at 15 000 g for 5 minutes with 100 μL aliquots of buffer. GCF from two strips was pooled to give a total volume of 200 μL and then stored at -80°C.
SDS-polyacrylamide gel electrophoresis
Protein content of each sample was determined according to the Bradford method (Bio-Rad protein assay, Bio-Rad, Hercules, CA, USA) at 4°C to avoid protein degradation. The same amount of protein from each subject was loaded onto a first-dimensional separation 10% polyacrylamide gel run at 90 V along with molecular weight marker (Rainbow mix code RPN 756, Bio-Rad, Hercules, CA). The gels were then stained by Coomassie, Silver Staining and Stains All technique (Bio-Rad, Hercules, CA).
Western blotting
Western blot analysis was performed to confirm the specificity of the antibodies. The GCF proteins were transferred overnight at 4°C to nitrocellulose membranes at 70 V and incubated with 3% bovine serum albumin in phosphate-buffered saline (PBS) as a blocking agent, followed by primary antibodies against the proteins under investigation. The antibodies and dilutions used were 1:250 for DMP116 and PP17, 1:1000 for DSP (Dr. Jianjun Hao). The blots were washed with PBS and secondary anti-rabbit immunoglobulins G (IgG) 1:2000 conjugated to alkaline phosphatase and adsorbed with human serum proteins developed in goat were used. Color development was carried out using alkaline phosphatase conjugate substrate kit (Bio-Rad, Hercules, CA).
Enzyme-linked immunosorbent assay
Indirect ELISA technique was used for detecting and quantitating the amount of proteins (ng/μL) in the crevicular fluid. For each sample three dilutions in duplicate were used. The standard proteins were purified in the laboratory. The primary antibody dilutions used in this assay were: for DMP1, 1: 500, for PP, 1:400 and for DSP, 1: 2000. The secondary anti-rabbit IgG antibody was used at a 1:2000 dilution.
Statistical analysis
Statistical analysis among the groups was performed using one-way ANOVA and Scheffé test to evaluate the statistical difference between each pair of groups. Shapiro-Wilk statistics was used to assess the normal distribution of the concentration values of the dentin matrix proteins.
Results
SDS-PAGE analysis
SDS-PAGE analysis identified proteins at 77, 66, 55, 50 and 26 kDa. The concentration of the total protein in GCF from the study group was higher when compared with those in the control sample (Fig. 2). This could be attributed to the degradation of the matrix proteins during root resorption.
Figure 2.
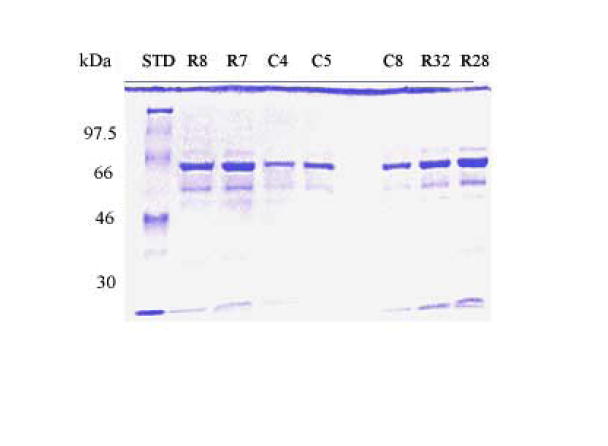
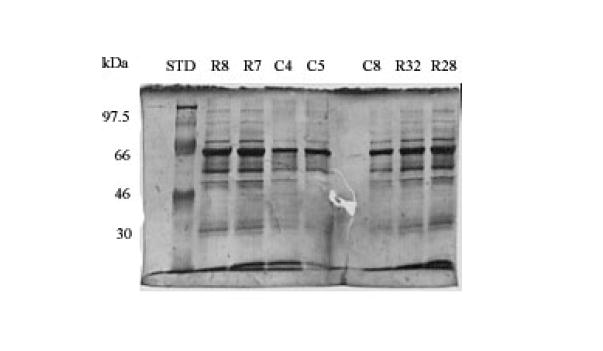
(a) Coomassie-stained SDS-PAGE gel (10%) showing the total proteins present in the crevicular fluid; (b) Silver stained SDS-PAGE gel (10%). C4, C5, C8 are control samples. R7, R8 are mild root resorption samples. R32, R28 are severe root resorption samples. STD is the molecular weight marker.
Western blot analysis
Immunoblotting with antibodies against DMP1, PP and DSP showed the same sized bands in both the control and study group subjects but with different intensities. Control had less intense bands and the severe cases had high intense bands (Fig. 3). With DMP1 antibody 2 bands were identified between 55 and 66 kDa. PP antibody recognized a band between 55-60 kDa and DSP recognized two bands between 50 and 70kDa.
Figure 3.
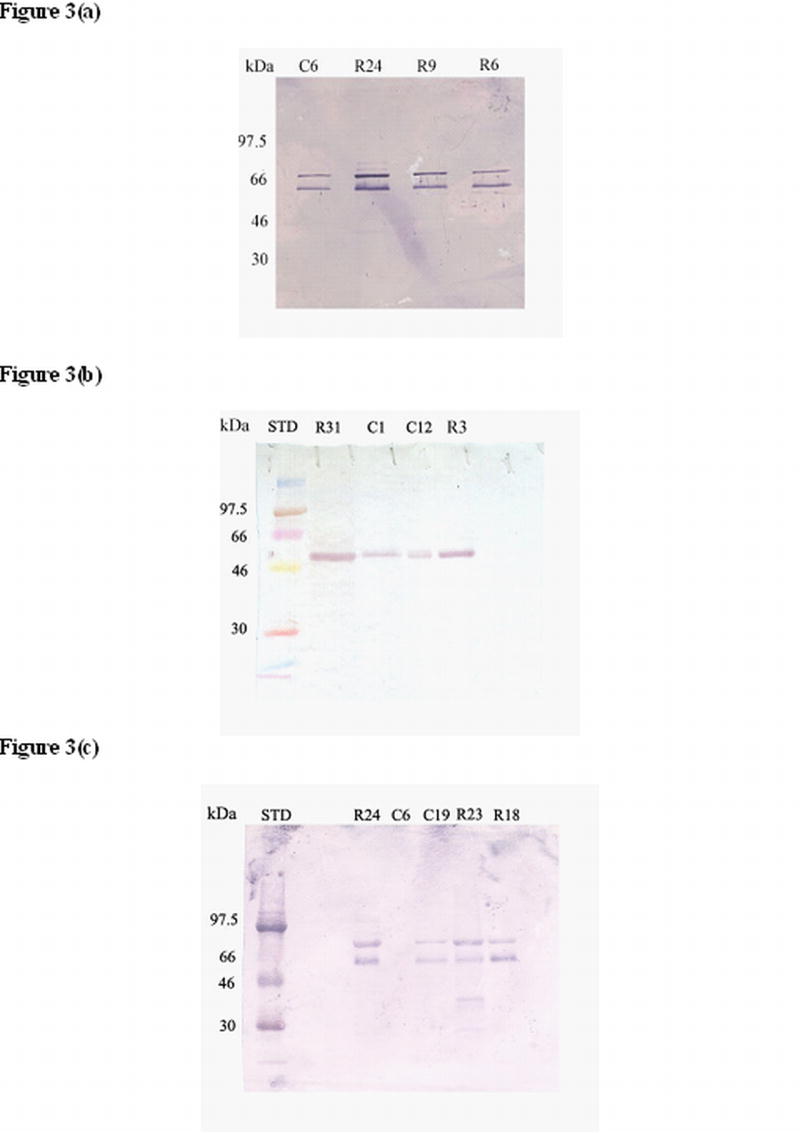
Western blot analysis using anti-DMP1 antibody (a) anti-PP antibody (b) and anti-DSP antibody (c) to identify these proteins in the gingival crevicular fluid of control, mild and severe root resorption group subjects. C1, C12, C6 and C19 are control samples; R3, R6, R9 and R18 are mild root resorption samples; R23, R24 and R31 are severe root resorption samples. STD is the molecular weight marker.
ELISA
The Shapiro-Wilk statistics showed normal distribution of the concentration values for DMP1, PP and DSP within all groups.
ELISA with DMP1 antibody showed a higher concentration in the study groups than in the control group that is statistically significant. No statistically significant difference was found between the mild and severe root resorption group (Fig. 4a).
Figure 4.
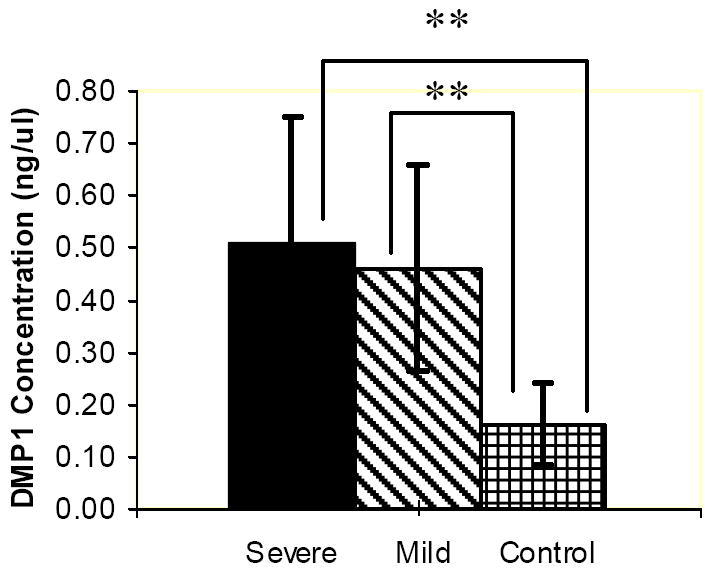
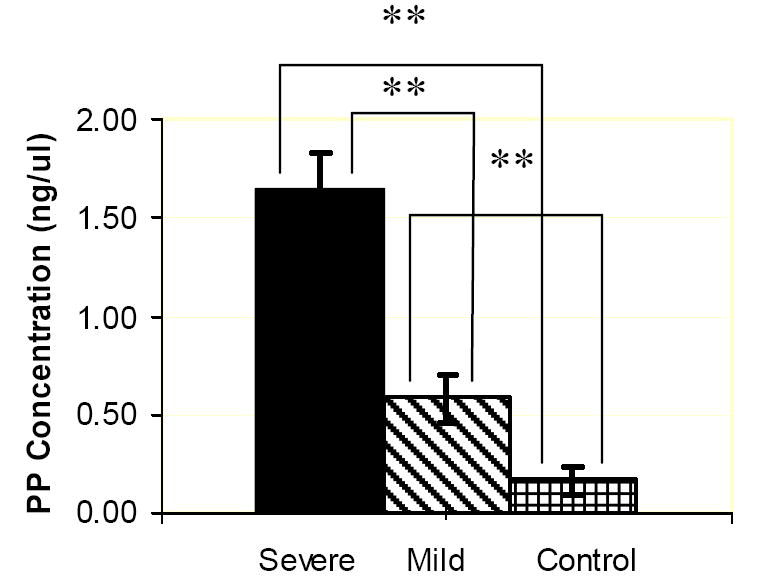
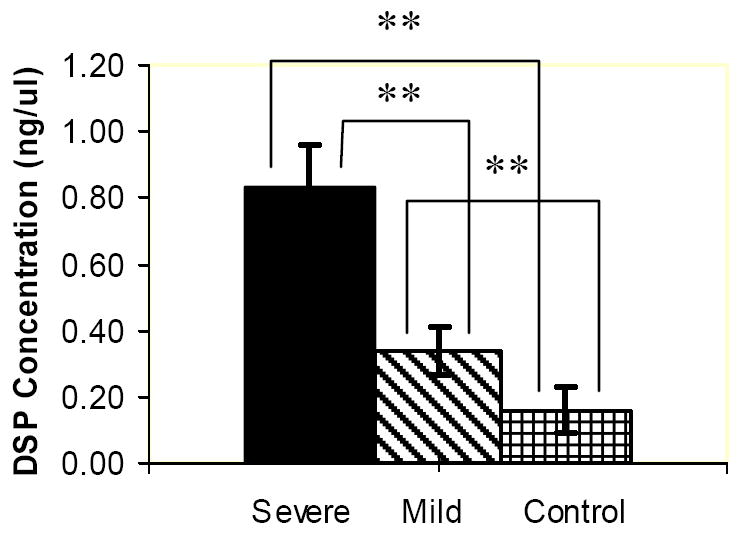
ELISA charts demonstrating the concentrations (ng/μL) of DMP1 (a) PP (b), DSP (c) proteins in the gingival crevicular fluid of control, severe and mild root resorption group subjects. Data are expressed as the mean ± SEM. ** P < 0.05.
PP concentration in the three groups is statistically different, the highest being in the severe root resorption group, followed by the mild root resorption group and control group with the least amount of protein (Fig. 4b).
DSP concentration in the severe root resorption group is statistically higher than in the mild root resorption group. The control group showed the least amount of protein compared to the other groups and this was statistically different (Fig. 4c).
Discussion and conclusions
External apical root resorption is a common, yet unexplained phenomenon associated with orthodontic tooth movement. Early detection of small root resorptions during orthodontic treatment is essential for identifying teeth at risk of severe resorption. This study was performed to identify dentin proteins in the crevicular fluid during the resoptive process.
The presence of dentin matrix proteins in the gingival crevicular fluid was an astounding finding. Dentin matrix protein 1, a non-collagenous protein identified in the mineral matrix of bone and dentin, was found in large amounts in the crevicular fluid. This could be attributed to the presence of protein that leached out from the bone and dentin during the resorption process. Western blot analysis with anti-DMP1 antibody indicated the presence of two bands, one around 55 kDa and the other at 66 kDa in both control and study groups in agreement with the previous report.9 The recombinant protein was used in earlier experiments as a positive control16 and found to be specific to primary anti-DMP1 antibody. The size of the band detected on the western blot matched to the expected size of the human DMP1 protein. However, the ELISA assay performed with DMP1 antibody showed a statistically significant difference between the amount of proteins present in the control and study group samples but no statistically significant difference was found between the mild and the severe root resorption group.
Although there is a statistically significant difference between the control and study groups, DMP1 is not specific to dentin. Its presence in the crevicular fluid may not be entirely a result of ongoing resorption activity, but also due to increased bone remodeling during orthodontic tooth movement. Thus, DMP1 is not a good marker for root resorption since it is not possible to distinguish between normal and pathologic activity.
Dentin phosphophoryn is an intense negatively charged protein that is a key regulatory protein found in the dentin matrix. Due to its high negative charge it is not readily transferred onto the membrane during western blot analysis. This difficulty was circumvented by using the ELISA technique. Using this method, ELISA results showed a significant quantitative difference between the three groups. Severe root resorption group had the highest range of absorbance measurements indicating a higher protein concentration, and conversely the absorbance measurements had lower values indicating lower protein concentrations. These results are in good agreement with a previous study18 that found elevated levels of PP in mild root resorption group relative to the control group; the amount of proteins found in this study is, however, larger and this could have been influenced by subject selection and degree of root resorption. In fact, the results of this study showed that there is also a different amount of PP and DSP released from roots with mild and severe degree of resorption.
The presence of small amounts of DSP and PP in the untreated group (negative control) was not anticipated as these teeth were not undergoing any clinically visible tooth structural changes. The antibodies used in this study were polyclonal antibodies and might have reacted to proteins with a similar epitope. The presence of PP in the untreated group could be suggestive of more subtle changes taking place at a structural level due to physiological root resorption. Odontoblasts and odontoclasts might have a similar function as osteoblasts and osteoclasts of bone to resorb, remodel and maintain the root surface. Histological studies have demonstrated, in fact, that even untreated teeth are affected by root resorption, especially apical root areas. From the ages of 16 to 32 years, the incidence of physiologic root resorption was 86.4%, and from 32 to 50 years was 96.4 %.19
These results for PP and DSP suggest that these two proteins could be potential markers for root resorption, as their concentration in the study groups were significantly higher than the control group. Moreover, since the concentration found in the severe and mild root resorption groups is statistically different, PP and DSP could be suitable markers for dynamic monitoring of root resorption before its appearance on radiographs.
A longitudinal study to further confirm these results is needed using PP and DSP as biological markers to monitor root resorption from its onset. Three dimensional radiology may be used for a better assessment of the degree of root resorption.
The ultimate goal is to develop a screen test based on biological markers that could be used easily and non-invasively by the orthodontist at chairside to detect early root resorption and monitor the ongoing process.
Acknowledgments
This work was supported by NIH-NIDCR grant DE11657 and the Department of Orthodontics. The authors thank Dr. Ellen BeGole for the statistical analysis and Dr. Arthur Veis for the antibodies to phosphophoryn.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 1. Literature review. Am J Orthod Dentofacial Orthop. 1993;103:62–66. doi: 10.1016/0889-5406(93)70106-X. [DOI] [PubMed] [Google Scholar]
- 2.Kaley J, Phillips C. Factors related to root resorption in edgewise practice. Angle Orthod. 1991;61:125–132. doi: 10.1043/0003-3219(1991)061<0125:FRTRRI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 3.Massler M, Perreault JG. Root resorption in the permanent teeth of young adults. J Dent Child. 1954;21:158–164. [Google Scholar]
- 4.Harry MR, Sims MR. Root resorption in bicuspid intrusion: a scanning electron microscopic study. Angle Orthod. 1982;52:235–258. doi: 10.1043/0003-3219(1982)052<0235:RRIBI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Baumrind S, Korn EL, Boyd RL. Apical root resorption in orthodontically treated adults. Am J Orthod Dentofacial Orthop. 1996;110:311–320. doi: 10.1016/s0889-5406(96)80016-3. [DOI] [PubMed] [Google Scholar]
- 6.Levander E, Malmgren O. Evaluation of the risk of root resorption during orthodontic treatment: a study of upper incisors. Eur J Orthod. 1988;10:30–38. doi: 10.1093/ejo/10.1.30. [DOI] [PubMed] [Google Scholar]
- 7.Owman-Moll P, Kurol J, Lundgren D. Repair of orthodontically induced root resorption in adolescents. Angle Orthod. 1995;95:403–408. doi: 10.1043/0003-3219(1995)065<0403:ROOIRR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 8.Levander E, Bajka R, Malmgren O. Early radiographic diagnosis of apical root resorption during orthodontic treatment: a study of maxillary incisors. Eur J Orthod. 1998;16:223–228. doi: 10.1093/ejo/20.1.57. [DOI] [PubMed] [Google Scholar]
- 9.Evans CA, Srinivasan R, George A. Detection of dentin proteins in human gingival crevicular fluid. In: Davidovitch Z, Mah J, editors. Biological Mechanisms of Tooth Movement and Craniofacial Adaptation. Harvard Society for the Advancement of Orthodontics. 2000. pp. 201–205. [Google Scholar]
- 10.Takagi Y, Fujisawa R, Sasaki S. Identification of dentin phosphophoryn localization by histochemical stainings. Connect Tissue Res. 1986;14:279–292. doi: 10.3109/03008208609017471. [DOI] [PubMed] [Google Scholar]
- 11.MacDougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu TT. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- 12.Ritchie HH, Berry JE, Somerman MJ, Hanks JT, Bronckers AL, Hotton D, et al. Dentin sialoprotein (DSP) transcripts: developmentally-sustained expression in odontoblasts and transient expression in pre-ameloblasts. Eur J Oral Sci. 1997;105:405–413. doi: 10.1111/j.1600-0722.1997.tb02137.x. [DOI] [PubMed] [Google Scholar]
- 13.Begue-Kirn C, Ruch JV, Ridall AL, Butler WT. Comparative analysis of mouse DSP and DPP expression in odontoblasts, preameloblasts, and experimentally induced odontoblast-like cells. Eur J Oral Sci. 1998;1:254–259. doi: 10.1111/j.1600-0722.1998.tb02184.x. [DOI] [PubMed] [Google Scholar]
- 14.George A, Sabsay B, Simonian PAL, Veis A. Characterization of a novel dentin matrix acid phosphoprotein. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- 15.George A. Dentin matrix proteins. In: Rabie AM, Urist MR, editors. Bone Formation and Repair. Elsevier Science BV; 1997. pp. 125–132. [Google Scholar]
- 16.Srinivasan R, Chen B, Gorski JP, George A. Recombinant expression and characterization of dentin matrix protein 1. Conn Tissue Res. 1999;40:251–258. doi: 10.3109/03008209909000703. [DOI] [PubMed] [Google Scholar]
- 17.Rabie AM, Veis A. An immunohistochemical study of the routes of secretion of collagen and phosphophoryn form odontoblasts into dentin. Connect Tissue Res. 1995;31:197–210. doi: 10.3109/03008209509010811. [DOI] [PubMed] [Google Scholar]
- 18.Mah J, Prasad N. Dentine phosphoproteins in gingival crevicular fluid during root resorption. Eur J Orthod. 2004;26:25–30. doi: 10.1093/ejo/26.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Wehrbein H, Fuhrmann RAW, Diedrich PR. Human histological tissue response after long-term orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1995;107:360–371. doi: 10.1016/s0889-5406(95)70088-9. [DOI] [PubMed] [Google Scholar]


