Abstract
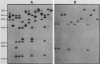
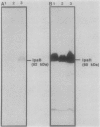
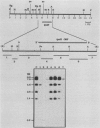
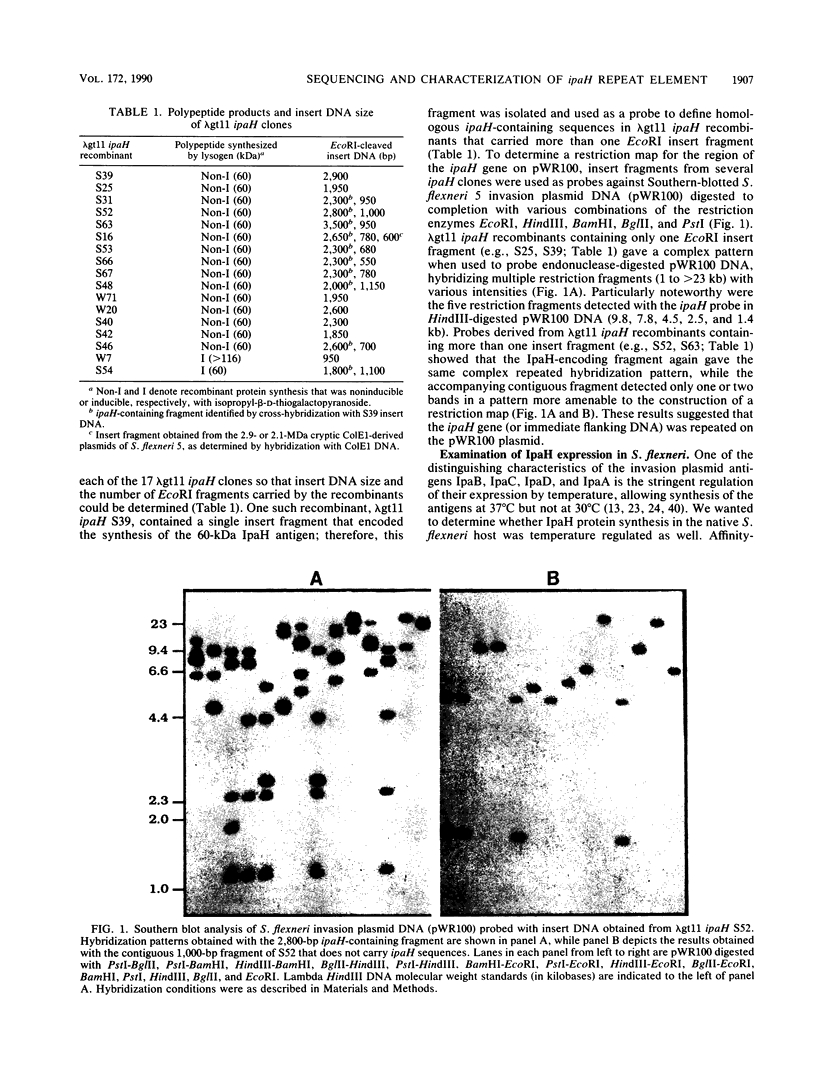
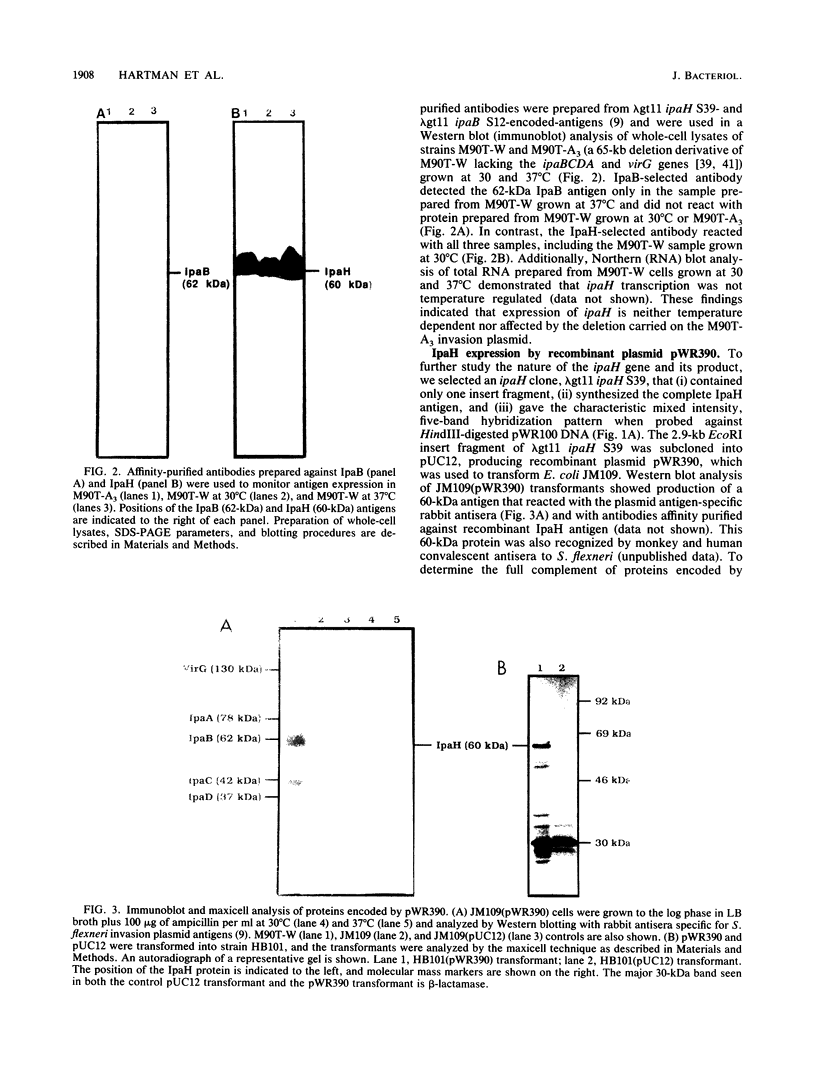
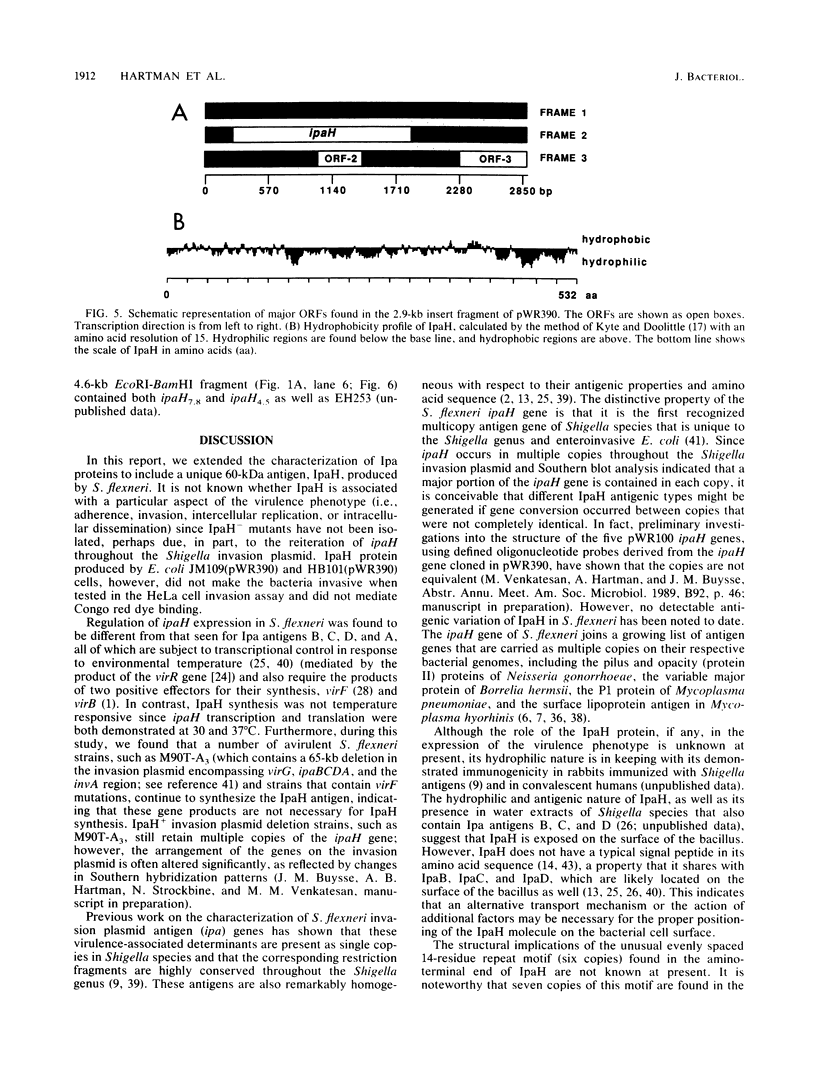
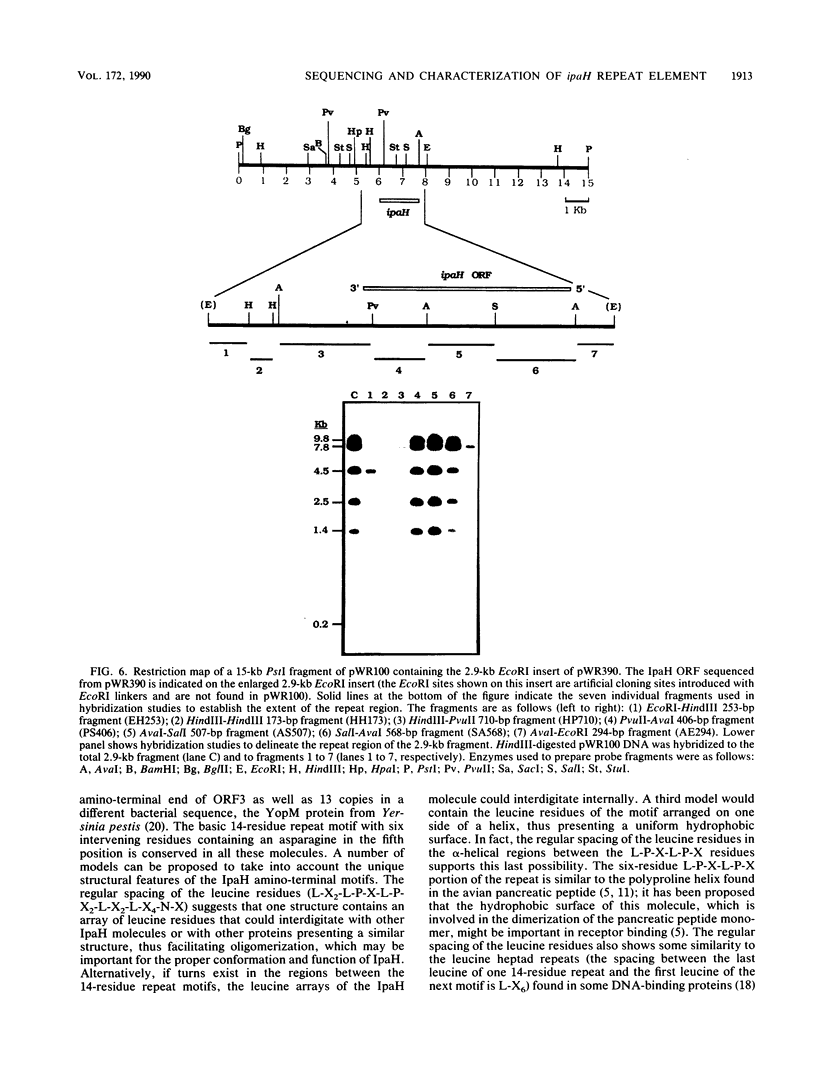
A lambda gt11 expression library of Tn5-tagged invasion plasmid pWR110 (from Shigella flexneri serotype 5, strain M90T-W) contained a set of recombinants encoding a 60-kilodalton protein (designated IpaH) recognized by rabbit antisera raised against S. flexneri invasion plasmid antigens (J. M. Buysse, C. K. Stover, E. V. Oaks, M. M. Venkatesan, and D. J. Kopecko, J. Bacteriol. 169:2561-2569, 1987). Southern blot analysis of wild-type S. flexneri serotype 5 invasion plasmid DNA (pWR100) digested with various combinations of five restriction enzymes and hybridized with defined ipaH probes showed complex hybridization patterns resulting from multiple copies of the ipaH gene on pWR100. DNA sequence analysis of a 2.9-kilobase (kb) EcoRI fragment directing IpaH antigen synthesis in plasmid recombinant pWR390 revealed an open reading frame coding for a 532-amino-acid protein (60.8 kilodaltons); this size matched well with the estimated size of IpaH determined by Western blot analysis of M90T-W cells and maxicell analysis of Escherichia coli HB101(pWR390) transformants. Examination of the amino acid sequence of IpaH revealed a hydrophilic protein with six evenly spaced 14-residue (L-X2-L-P-X-L-P-X2-L-X2-L) repeat motifs in the amino-terminal end of the molecule. Southern blot analysis of HindIII-digested pWR100 DNA probed with defined segments of the pWR390 2.9-kb insert demonstrated that the multiple band hybridization pattern resulted from repeats of a significant portion of the ipaH structural gene in five distinct HindIII fragments (9.8, 7.8, 4.5, 2.5, and 1.4 kb). Affinity-purified IpaH antibody, used to monitor the expression of the antigen in M90T-W cells grown at 30 and 37 degrees C, showed that IpaH synthesis was not regulated by growth temperature.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler B., Sasakawa C., Tobe T., Makino S., Komatsu K., Yoshikawa M. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol. 1989 May;3(5):627–635. doi: 10.1111/j.1365-2958.1989.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Baudry B., Kaczorek M., Sansonetti P. J. Nucleotide sequence of the invasion plasmid antigen B and C genes (ipaB and ipaC) of Shigella flexneri. Microb Pathog. 1988 May;4(5):345–357. doi: 10.1016/0882-4010(88)90062-9. [DOI] [PubMed] [Google Scholar]
- Baudry B., Maurelli A. T., Clerc P., Sadoff J. C., Sansonetti P. J. Localization of plasmid loci necessary for the entry of Shigella flexneri into HeLa cells, and characterization of one locus encoding four immunogenic polypeptides. J Gen Microbiol. 1987 Dec;133(12):3403–3413. doi: 10.1099/00221287-133-12-3403. [DOI] [PubMed] [Google Scholar]
- Bernardini M. L., Mounier J., d'Hauteville H., Coquis-Rondon M., Sansonetti P. J. Identification of icsA, a plasmid locus of Shigella flexneri that governs bacterial intra- and intercellular spread through interaction with F-actin. Proc Natl Acad Sci U S A. 1989 May;86(10):3867–3871. doi: 10.1073/pnas.86.10.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T. L., Pitts J. E., Tickle I. J., Wood S. P., Wu C. W. X-ray analysis (1. 4-A resolution) of avian pancreatic polypeptide: Small globular protein hormone. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4175–4179. doi: 10.1073/pnas.78.7.4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst P., Greaves D. R. Programmed gene rearrangements altering gene expression. Science. 1987 Feb 6;235(4789):658–667. doi: 10.1126/science.3544215. [DOI] [PubMed] [Google Scholar]
- Boyer M. J., Wise K. S. Lipid-modified surface protein antigens expressing size variation within the species Mycoplasma hyorhinis. Infect Immun. 1989 Jan;57(1):245–254. doi: 10.1128/iai.57.1.245-254.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckland R., Wild F. Leucine zipper motif extends. Nature. 1989 Apr 13;338(6216):547–547. doi: 10.1038/338547a0. [DOI] [PubMed] [Google Scholar]
- Buysse J. M., Stover C. K., Oaks E. V., Venkatesan M., Kopecko D. J. Molecular cloning of invasion plasmid antigen (ipa) genes from Shigella flexneri: analysis of ipa gene products and genetic mapping. J Bacteriol. 1987 Jun;169(6):2561–2569. doi: 10.1128/jb.169.6.2561-2569.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover I., Haneef I., Pitts J., Wood S., Moss D., Tickle I., Blundell T. Conformational flexibility in a small globular hormone: x-ray analysis of avian pancreatic polypeptide at 0.98-A resolution. Biopolymers. 1983 Jan;22(1):293–304. doi: 10.1002/bip.360220138. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Formal S. B. Genetics of virulence in Shigella. Microb Pathog. 1986 Dec;1(6):511–518. doi: 10.1016/0882-4010(86)90037-9. [DOI] [PubMed] [Google Scholar]
- Hale T. L., Oaks E. V., Formal S. B. Identification and antigenic characterization of virulence-associated, plasmid-coded proteins of Shigella spp. and enteroinvasive Escherichia coli. Infect Immun. 1985 Dec;50(3):620–629. doi: 10.1128/iai.50.3.620-629.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. N., Silhavy T. J. Genetic analysis of the major outer membrane proteins of Escherichia coli. Annu Rev Genet. 1981;15:91–142. doi: 10.1146/annurev.ge.15.120181.000515. [DOI] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Kopecko D. J., Washington O., Formal S. B. Genetic and physical evidence for plasmid control of Shigella sonnei form I cell surface antigen. Infect Immun. 1980 Jul;29(1):207–214. doi: 10.1128/iai.29.1.207-214.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Lett M. C., Sasakawa C., Okada N., Sakai T., Makino S., Yamada M., Komatsu K., Yoshikawa M. virG, a plasmid-coded virulence gene of Shigella flexneri: identification of the virG protein and determination of the complete coding sequence. J Bacteriol. 1989 Jan;171(1):353–359. doi: 10.1128/jb.171.1.353-359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung K. Y., Straley S. C. The yopM gene of Yersinia pestis encodes a released protein having homology with the human platelet surface protein GPIb alpha. J Bacteriol. 1989 Sep;171(9):4623–4632. doi: 10.1128/jb.171.9.4623-4632.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Sasakawa C., Kamata K., Kurata T., Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in S. flexneri 2a. Cell. 1986 Aug 15;46(4):551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- Maurelli A. T., Baudry B., d'Hauteville H., Hale T. L., Sansonetti P. J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985 Jul;49(1):164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurelli A. T., Sansonetti P. J. Genetic determinants of Shigella pathogenicity. Annu Rev Microbiol. 1988;42:127–150. doi: 10.1146/annurev.mi.42.100188.001015. [DOI] [PubMed] [Google Scholar]
- Mills J. A., Buysse J. M., Oaks E. V. Shigella flexneri invasion plasmid antigens B and C: epitope location and characterization with monoclonal antibodies. Infect Immun. 1988 Nov;56(11):2933–2941. doi: 10.1128/iai.56.11.2933-2941.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oaks E. V., Hale T. L., Formal S. B. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986 Jul;53(1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pál T., Newland J. W., Tall B. D., Formal S. B., Hale T. L. Intracellular spread of Shigella flexneri associated with the kcpA locus and a 140-kilodalton protein. Infect Immun. 1989 Feb;57(2):477–486. doi: 10.1128/iai.57.2.477-486.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T., Sasakawa C., Yoshikawa M. Expression of four virulence antigens of Shigella flexneri is positively regulated at the transcriptional level by the 30 kiloDalton virF protein. Mol Microbiol. 1988 Sep;2(5):589–597. doi: 10.1111/j.1365-2958.1988.tb00067.x. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Makino S., Yamada M., Okada N., Yoshikawa M. Virulence-associated genetic regions comprising 31 kilobases of the 230-kilobase plasmid in Shigella flexneri 2a. J Bacteriol. 1988 Jun;170(6):2480–2484. doi: 10.1128/jb.170.6.2480-2484.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasakawa C., Kamata K., Sakai T., Murayama S. Y., Makino S., Yoshikawa M. Molecular alteration of the 140-megadalton plasmid associated with loss of virulence and Congo red binding activity in Shigella flexneri. Infect Immun. 1986 Feb;51(2):470–475. doi: 10.1128/iai.51.2.470-475.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stoenner H. G., Dodd T., Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982 Nov 1;156(5):1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., Kemper J., Marsh R. C. Molecular cloning and characterization of supQ/newD, a gene substitution system for the leuD gene of Salmonella typhimurium. J Bacteriol. 1988 Jul;170(7):3115–3124. doi: 10.1128/jb.170.7.3115-3124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C. J., Chavoya A., Baseman J. B. Regions of Mycoplasma pneumoniae cytadhesin P1 structural gene exist as multiple copies. Infect Immun. 1988 Dec;56(12):3157–3161. doi: 10.1128/iai.56.12.3157-3161.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Characterization of invasion plasmid antigen genes (ipaBCD) from Shigella flexneri. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9317–9321. doi: 10.1073/pnas.85.23.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M. M., Buysse J. M., Kopecko D. J. Use of Shigella flexneri ipaC and ipaH gene sequences for the general identification of Shigella spp. and enteroinvasive Escherichia coli. J Clin Microbiol. 1989 Dec;27(12):2687–2691. doi: 10.1128/jcm.27.12.2687-2691.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesan M., Buysse J. M., Vandendries E., Kopecko D. J. Development and testing of invasion-associated DNA probes for detection of Shigella spp. and enteroinvasive Escherichia coli. J Clin Microbiol. 1988 Feb;26(2):261–266. doi: 10.1128/jcm.26.2.261-266.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H., Nakamura A. Identification of Shigella sonnei form I plasmid genes necessary for cell invasion and their conservation among Shigella species and enteroinvasive Escherichia coli. Infect Immun. 1986 Aug;53(2):352–358. doi: 10.1128/iai.53.2.352-358.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]