Abstract
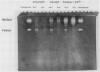
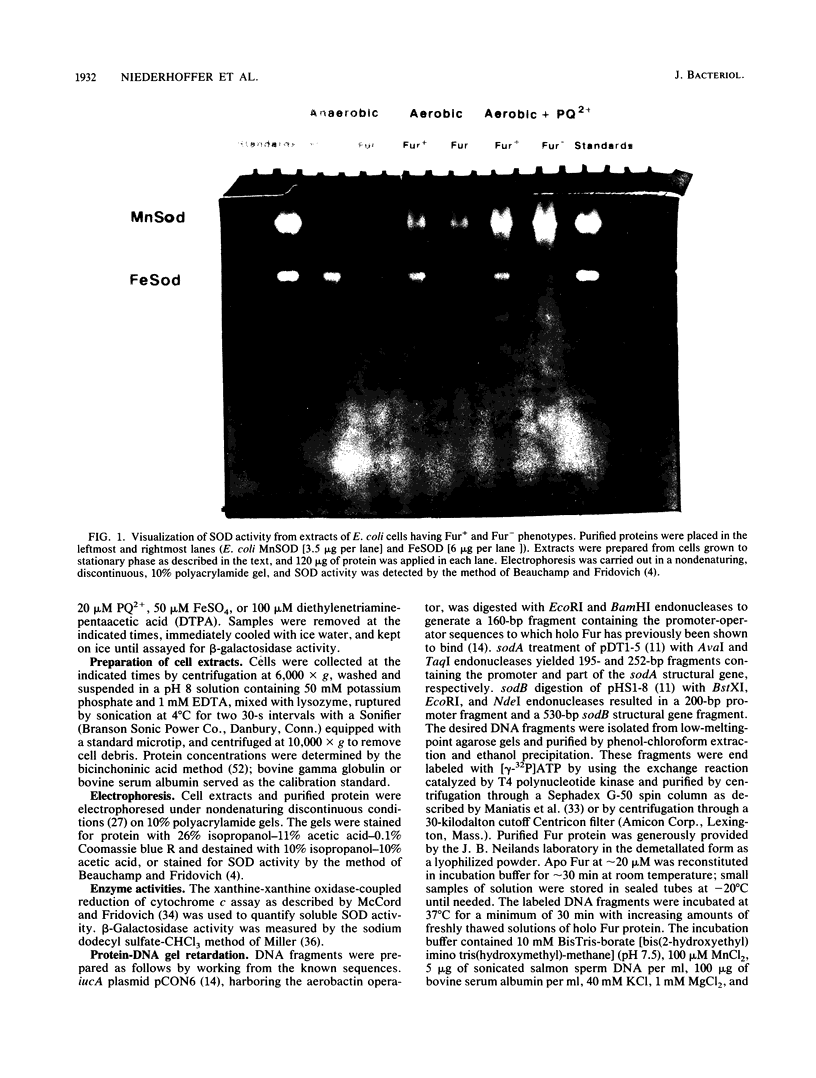
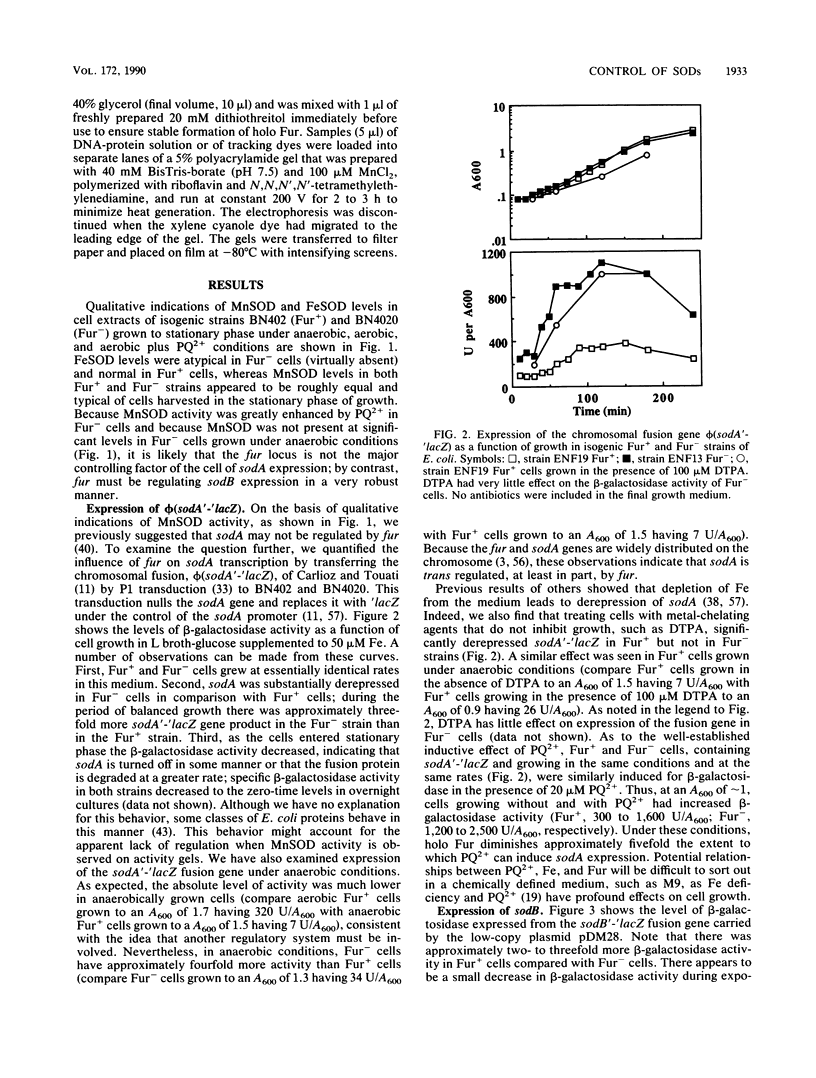
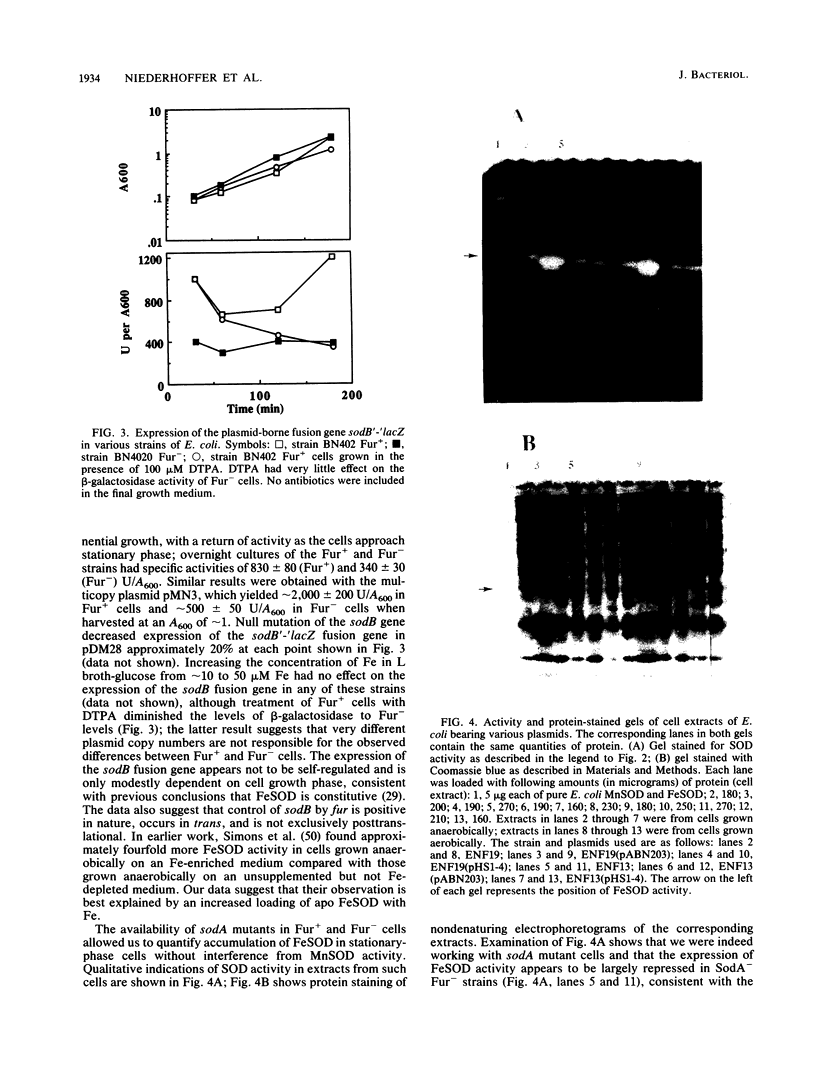
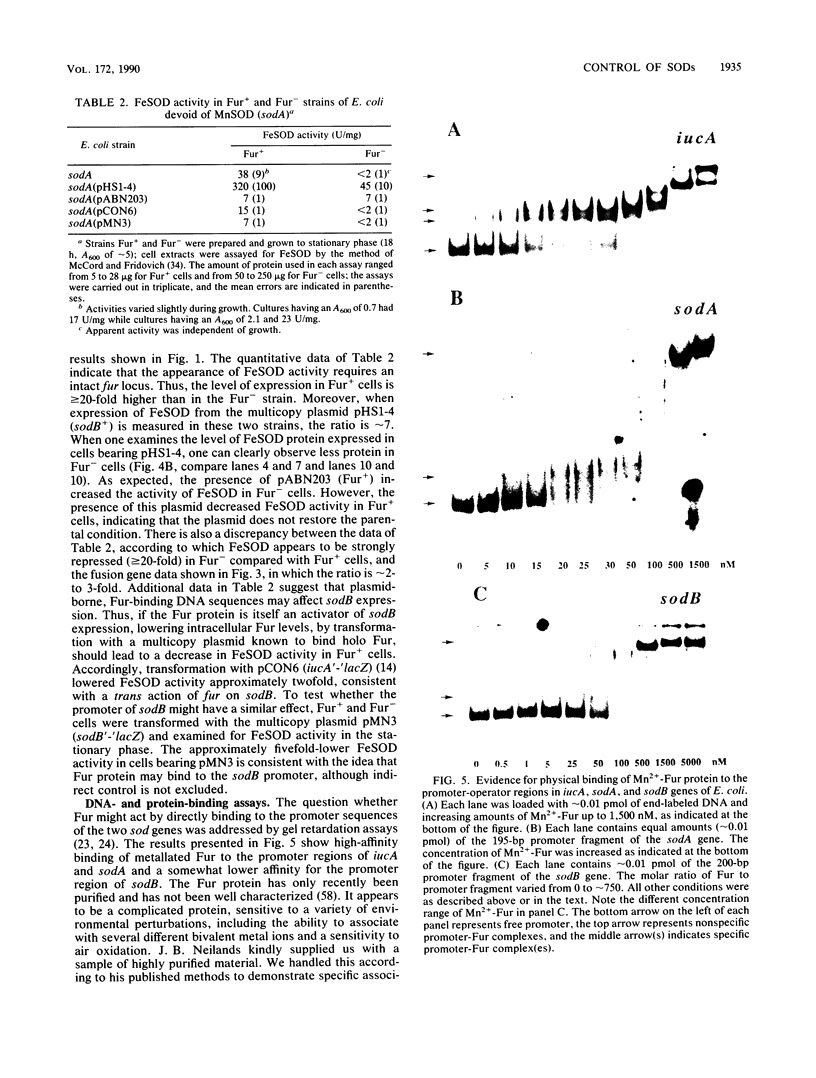
The ferric uptake regulation (fur) gene product participates in regulating expression of the manganese- and iron-containing superoxide dismutase genes of Escherichia coli. Examination of beta-galactosidase activity coded from a chromosomal phi(sodA'-'lacZ) fusion suggests that metallated Fur protein acts as a transcriptional repressor of sodA (manganese superoxide dismutase [MnSOD]). Gel retardation assays demonstrate high-affinity binding of pure, Mn2(+)-Fur protein to DNA fragments containing the sodA promoter. These data and the presence of an iron box sequence in its promoter strongly suggest that sodA is part of the iron uptake regulon. An sodB'-'lacZ fusion gene borne on either a low- or high-copy plasmid yielded approximately two- to threefold more beta-galactosidase activity in Fur+ compared with Fur- cells; the levels of activity depended only weakly on the growth phase and did not change during an extended stationary phase. Measurement of FeSOD activity in logarithmic growth phase and in overnight cultures of sodA and fur sodA backgrounds revealed that almost no FeSOD activity was expressed in Fur- strains, whereas wild-type levels were expressed in Fur+ cells. Fur+ and Fur- cells bearing the multicopy plasmid pHS1-4 (sodB+) expressed approximately sevenfold less FeSOD activity in the fur background, and staining of nondenaturing electrophoretic gels indicates that synthesis of FeSOD protein was greatly reduced in Fur- cells. Gel retardation assays show that Mn2(+)-Fur had a significantly higher affinity for the promoter fragment of sodB compared with that of random DNA sequences but significantly lower than for the promoter fragment of sodA. These observations suggest that the apparent positive regulation of sodB does not result exclusively from a direct interaction of holo (metallated) Fur itself with the sodB promoter. Nevertheless, the sodB gene also appears to be part of the iron uptake regulon but not in the classical manner of Fe-dependent repression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987 Aug 25;26(17):5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Sleeter D. D., Noller H. F. Gene organization and primary structure of a ribosomal RNA operon from Escherichia coli. J Mol Biol. 1981 May 15;148(2):107–127. doi: 10.1016/0022-2836(81)90508-8. [DOI] [PubMed] [Google Scholar]
- Calderwood S. B., Mekalanos J. J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlioz A., Ludwig M. L., Stallings W. C., Fee J. A., Steinman H. M., Touati D. Iron superoxide dismutase. Nucleotide sequence of the gene from Escherichia coli K12 and correlations with crystal structures. J Biol Chem. 1988 Jan 25;263(3):1555–1562. [PubMed] [Google Scholar]
- Carlioz A., Touati D. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 1986 Mar;5(3):623–630. doi: 10.1002/j.1460-2075.1986.tb04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Lactose genes fused to exogenous promoters in one step using a Mu-lac bacteriophage: in vivo probe for transcriptional control sequences. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4530–4533. doi: 10.1073/pnas.76.9.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D. N., Armstrong G. A., Hearst J. E. Induction of anaerobic gene expression in Rhodobacter capsulatus is not accompanied by a local change in chromosomal supercoiling as measured by a novel assay. J Bacteriol. 1989 Sep;171(9):4836–4843. doi: 10.1128/jb.171.9.4836-4843.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lorenzo V., Herrero M., Giovannini F., Neilands J. B. Fur (ferric uptake regulation) protein and CAP (catabolite-activator protein) modulate transcription of fur gene in Escherichia coli. Eur J Biochem. 1988 May 2;173(3):537–546. doi: 10.1111/j.1432-1033.1988.tb14032.x. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner M. M., Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981 Jul 10;9(13):3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. W., Konisky J. Mechanism for iron-regulated transcription of the Escherichia coli cir gene: metal-dependent binding of fur protein to the promoters. J Bacteriol. 1989 Feb;171(2):1048–1054. doi: 10.1128/jb.171.2.1048-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs D. W., Tharp B. B., Konisky J. Cloning and promoter identification of the iron-regulated cir gene of Escherichia coli. J Bacteriol. 1987 Dec;169(12):5343–5352. doi: 10.1128/jb.169.12.5343-5352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol Gen Genet. 1987 Nov;210(1):135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Regulation of the synthesis of superoxide dismutase in Escherichia coli. Induction by methyl viologen. J Biol Chem. 1977 Nov 10;252(21):7667–7672. [PubMed] [Google Scholar]
- Hassan H. M., Fridovich I. Superoxide radical and the oxygen enhancement of the toxicity of paraquat in Escherichia coli. J Biol Chem. 1978 Nov 25;253(22):8143–8148. [PubMed] [Google Scholar]
- Hassan H. M., Moody C. S. Regulation of manganese-containing superoxide dismutase in Escherichia coli. Anaerobic induction by nitrate. J Biol Chem. 1987 Dec 15;262(35):17173–17177. [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- McCord J. M., Keele B. B., Jr, Fridovich I. An enzyme-based theory of obligate anaerobiosis: the physiological function of superoxide dismutase. Proc Natl Acad Sci U S A. 1971 May;68(5):1024–1027. doi: 10.1073/pnas.68.5.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody C. S., Hassan H. M. Anaerobic biosynthesis of the manganese-containing superoxide dismutase in Escherichia coli. J Biol Chem. 1984 Oct 25;259(20):12821–12825. [PubMed] [Google Scholar]
- Nau C. D., Konisky J. Evolutionary relationship between the TonB-dependent outer membrane transport proteins: nucleotide and amino acid sequences of the Escherichia coli colicin I receptor gene. J Bacteriol. 1989 Feb;171(2):1041–1047. doi: 10.1128/jb.171.2.1041-1047.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozenberger B. A., Brickman T. J., McIntosh M. A. Nucleotide sequence of Escherichia coli isochorismate synthetase gene entC and evolutionary relationship of isochorismate synthetase and other chorismate-utilizing enzymes. J Bacteriol. 1989 Feb;171(2):775–783. doi: 10.1128/jb.171.2.775-783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Crystal structure of manganese superoxide dismutase from Bacillus stearothermophilus at 2.4 A resolution. J Mol Biol. 1988 Feb 20;199(4):649–661. doi: 10.1016/0022-2836(88)90308-7. [DOI] [PubMed] [Google Scholar]
- Pedersen S., Bloch P. L., Reeh S., Neidhardt F. C. Patterns of protein synthesis in E. coli: a catalog of the amount of 140 individual proteins at different growth rates. Cell. 1978 May;14(1):179–190. doi: 10.1016/0092-8674(78)90312-4. [DOI] [PubMed] [Google Scholar]
- Pressler U., Staudenmaier H., Zimmermann L., Braun V. Genetics of the iron dicitrate transport system of Escherichia coli. J Bacteriol. 1988 Jun;170(6):2716–2724. doi: 10.1128/jb.170.6.2716-2724.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle C. T., Beyer W. F., Jr, Fridovich I. Anaerobic induction of ProMn-superoxide dismutase in Escherichia coli. J Biol Chem. 1989 Feb 15;264(5):2758–2763. [PubMed] [Google Scholar]
- Sauer M., Hantke K., Braun V. Ferric-coprogen receptor FhuE of Escherichia coli: processing and sequence common to all TonB-dependent outer membrane receptor proteins. J Bacteriol. 1987 May;169(5):2044–2049. doi: 10.1128/jb.169.5.2044-2049.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavone J. R., Hassan H. M. The role of redox in the regulation of manganese-containing superoxide dismutase biosynthesis in Escherichia coli. J Biol Chem. 1988 Mar 25;263(9):4269–4273. [PubMed] [Google Scholar]
- Simons R. S., Jackett P. S., Carroll M. E., Lowrie D. B. Superoxide independence of paraquat toxicity in Escherichia coli. Toxicol Appl Pharmacol. 1976 Aug;37(2):271–280. doi: 10.1016/0041-008x(76)90090-9. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Neidhardt F. C. Proteins induced by aerobiosis in Escherichia coli. J Bacteriol. 1983 Apr;154(1):344–350. doi: 10.1128/jb.154.1.344-350.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita S., Sato M., Toba M., Masahashi W., Hashimoto-Gotoh T. High-copy-number and low-copy-number plasmid vectors for lacZ alpha-complementation and chloramphenicol- or kanamycin-resistance selection. Gene. 1987;61(1):63–74. doi: 10.1016/0378-1119(87)90365-9. [DOI] [PubMed] [Google Scholar]
- Terech A., Pucheault J., Ferradini C. Saturation behavior of the manganese-containing superoxide dismutase from Paracoccus denitrificans. Biochem Biophys Res Commun. 1983 May 31;113(1):114–120. doi: 10.1016/0006-291x(83)90439-4. [DOI] [PubMed] [Google Scholar]
- Touati D. Molecular genetics of superoxide dismutases. Free Radic Biol Med. 1988;5(5-6):393–402. doi: 10.1016/0891-5849(88)90113-x. [DOI] [PubMed] [Google Scholar]
- Touati D. Transcriptional and posttranscriptional regulation of manganese superoxide dismutase biosynthesis in Escherichia coli, studied with operon and protein fusions. J Bacteriol. 1988 Jun;170(6):2511–2520. doi: 10.1128/jb.170.6.2511-2520.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S., Neilands J. B., Bittner M. L., Hemming B. C., Haymore B. L., Seetharam R. Expression, isolation and properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Met. 1988;1(1):62–68. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Giovannini F., Herrero M., Neilands J. B. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988 Oct 20;203(4):875–884. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- de Lorenzo V., Wee S., Herrero M., Neilands J. B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987 Jun;169(6):2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]