Abstract
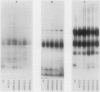
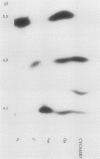
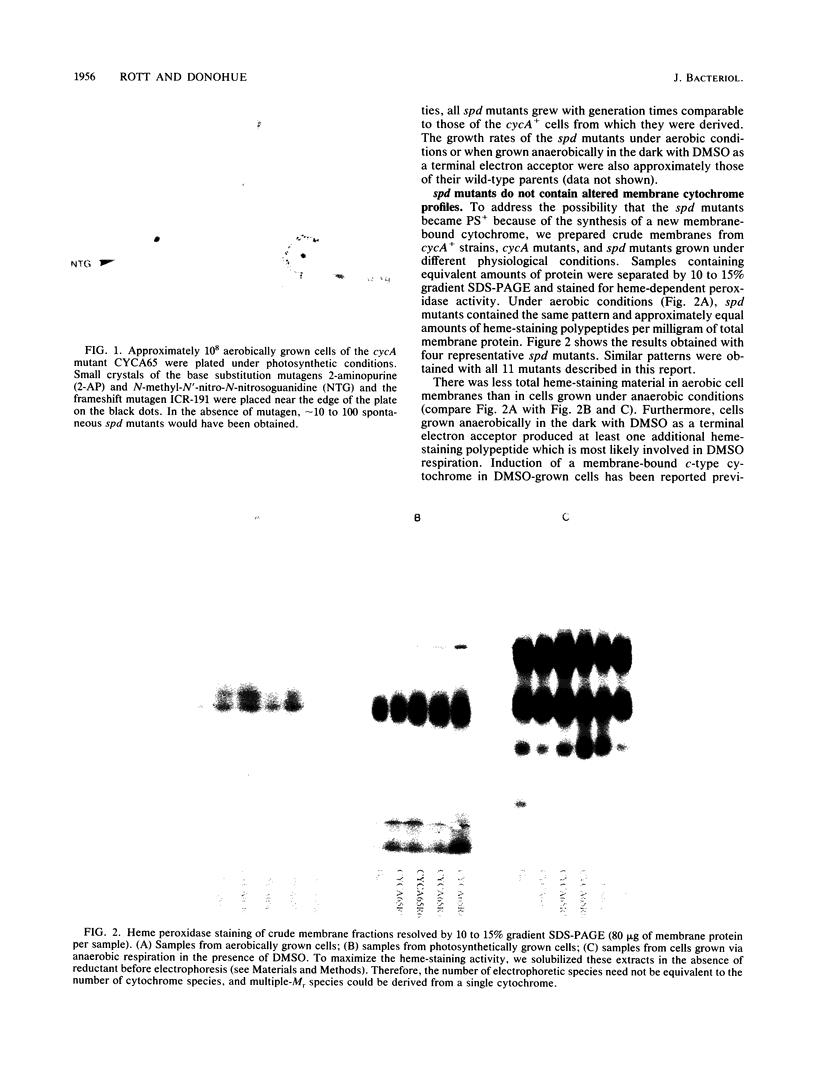
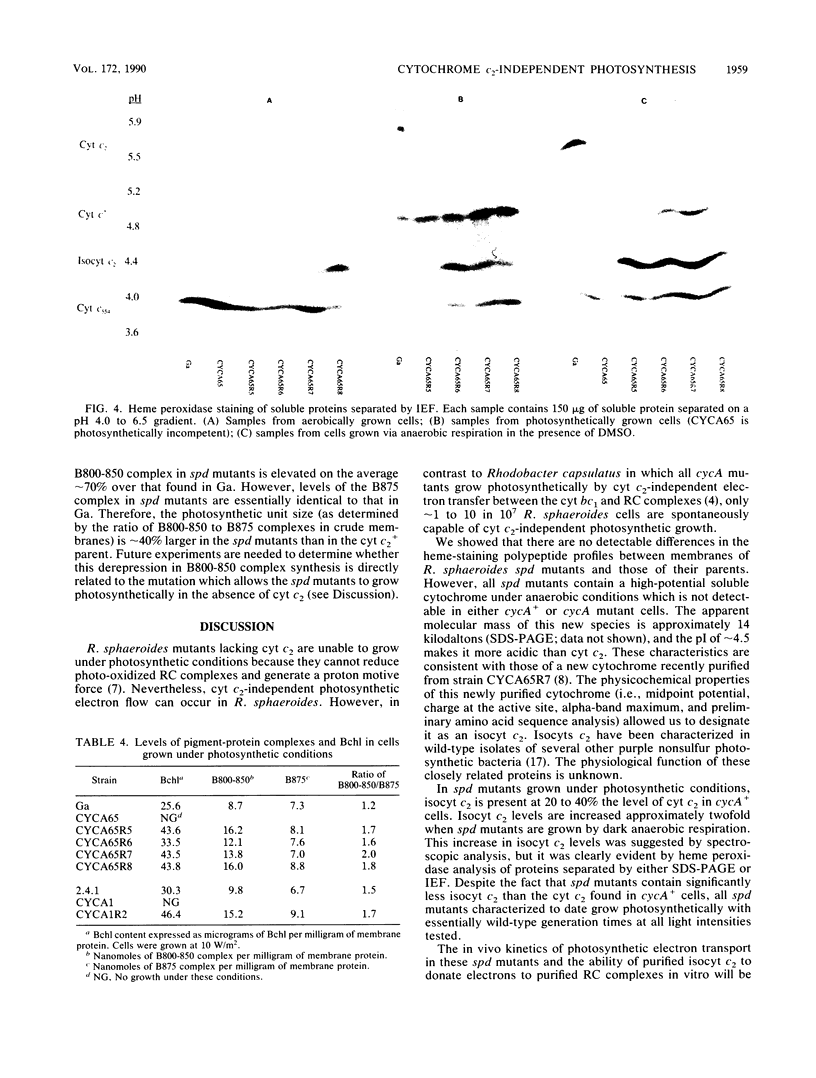
In Rhodobacter sphaeroides, cytochrome c2 (cyt c2) is a periplasmic redox protein required for photosynthetic electron transfer. cyt c2-deficient mutants created by replacing the gene encoding the apoprotein for cyt c2 (cycA) with a kanamycin resistance cartridge are photosynthetically incompetent. Spontaneous mutations that suppress this photosynthesis deficiency (spd mutants) arise at a frequency of 1 to 10 in 10(7). We analyzed the cytochrome content of several spd mutants spectroscopically and by heme peroxidase assays. These suppressors lacked detectable cyt c2, but they contained a new soluble cytochrome which was designated isocytochrome c2 (isocyt c2) that was not detectable in either cycA+ or cycA mutant cells. When spd mutants were grown photosynthetically, isocyt c2 was present at approximately 20 to 40% of the level of cyt c2 found in photosynthetically grown wild type cells, and it was found in the periplasm with cytochromes c' and c554. These spd mutants also had several other pleiotropic phenotypes. Although photosynthetic growth rates of the spd mutants were comparable to those of wild-type strains at all light intensities tested, they contained elevated levels of B800-850 pigment-protein complexes. Several spd mutants contained detectable amounts of isocyt c2 under aerobic conditions. Finally, heme peroxidase assays indicated that, under anaerobic conditions, the spd mutants may contain another new cytochrome in addition to isocyt c2. These pleiotropic phenotypes, the frequency at which the spd mutants arise, and the fact that a frameshift mutagen is very effective in generating the spd phenotype suggest that some spd mutants contain a mutation in loci which regulate cytochrome synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandner J. P., McEwan A. G., Kaplan S., Donohue T. J. Expression of the Rhodobacter sphaeroides cytochrome c2 structural gene. J Bacteriol. 1989 Jan;171(1):360–368. doi: 10.1128/jb.171.1.360-368.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN-BAZIRE G., SISTROM W. R., STANIER R. Y. Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol. 1957 Feb;49(1):25–68. doi: 10.1002/jcp.1030490104. [DOI] [PubMed] [Google Scholar]
- Daldal F., Cheng S., Applebaum J., Davidson E., Prince R. C. Cytochrome c(2) is not essential for photosynthetic growth of Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2012–2016. doi: 10.1073/pnas.83.7.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMartini M., Inouye M. Interaction between two major outer membrane proteins of Escherichia coli: the matrix protein and the lipoprotein. J Bacteriol. 1978 Jan;133(1):329–335. doi: 10.1128/jb.133.1.329-335.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., McEwan A. G., Kaplan S. Cloning, DNA sequence, and expression of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1986 Nov;168(2):962–972. doi: 10.1128/jb.168.2.962-972.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue T. J., McEwan A. G., Van Doren S., Crofts A. R., Kaplan S. Phenotypic and genetic characterization of cytochrome c2 deficient mutants of Rhodobacter sphaeroides. Biochemistry. 1988 Mar 22;27(6):1918–1925. doi: 10.1021/bi00406a018. [DOI] [PubMed] [Google Scholar]
- Fitch J., Cannac V., Meyer T. E., Cusanovich M. A., Tollin G., Van Beeumen J., Rott M. A., Donohue T. J. Expression of a cytochrome c2 isozyme restores photosynthetic growth of Rhodobacter sphaeroides mutants lacking the wild-type cytochrome c2 gene. Arch Biochem Biophys. 1989 Jun;271(2):502–507. doi: 10.1016/0003-9861(89)90301-9. [DOI] [PubMed] [Google Scholar]
- Hendry G. A., Houghton J. D., Jones O. T. The cytochromes in microsomal fractions of germinating mung beans. Biochem J. 1981 Mar 15;194(3):743–751. doi: 10.1042/bj1940743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Kaplan S. Molecular genetics of photosynthetic membrane biosynthesis in Rhodobacter sphaeroides. Microbiol Rev. 1988 Mar;52(1):50–69. doi: 10.1128/mr.52.1.50-69.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley P. J., Varga A., Kaplan S. Physiological and structural analysis of light-harvesting mutants of Rhodobacter sphaeroides. J Bacteriol. 1988 Mar;170(3):1103–1115. doi: 10.1128/jb.170.3.1103-1115.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueking D. R., Fraley R. T., Kaplan S. Intracytoplasmic membrane synthesis in synchronous cell populations of Rhodopseudomonas sphaeroides. Fate of "old" and "new" membrane. J Biol Chem. 1978 Jan 25;253(2):451–457. [PubMed] [Google Scholar]
- Markwell J. P., Lascelles J. Membrane-bound, pyridine nucleotide-independent L-lactate dehydrogenase of Rhodopseudomonas sphaeroides. J Bacteriol. 1978 Feb;133(2):593–600. doi: 10.1128/jb.133.2.593-600.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meinhardt S. W., Kiley P. J., Kaplan S., Crofts A. R., Harayama S. Characterization of light-harvesting mutants of Rhodopseudomonas sphaeroides. I. Measurement of the efficiency of energy transfer from light-harvesting complexes to the reaction center. Arch Biochem Biophys. 1985 Jan;236(1):130–139. doi: 10.1016/0003-9861(85)90612-5. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Cusanovich M. A. Soluble cytochrome composition of the purple phototrophic bacterium, Rhodopseudomonas sphaeroides ATCC 17023. Biochim Biophys Acta. 1985 May 31;807(3):308–319. doi: 10.1016/0005-2728(85)90263-4. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Kamen M. D. New perspectives on c-type cytochromes. Adv Protein Chem. 1982;35:105–212. doi: 10.1016/s0065-3233(08)60469-6. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Baccarini-Melandri A., Hauska G. A., Melandri B. A., Crofts A. R. Asymmetry of an energy transducing membrane the location of cytochrome c2 in Rhodopseudomonas spheroides and Rhodopseudomonas capsulata. Biochim Biophys Acta. 1975 May 15;387(2):212–227. doi: 10.1016/0005-2728(75)90104-8. [DOI] [PubMed] [Google Scholar]
- SISTROM W. R. A requirement for sodium in the growth of Rhodopseudomonas spheroides. J Gen Microbiol. 1960 Jun;22:778–785. doi: 10.1099/00221287-22-3-778. [DOI] [PubMed] [Google Scholar]
- Tai S. P., Kaplan S. Intracellular localization of phospholipid transfer activity in Rhodopseudomonas sphaeroides and a possible role in membrane biogenesis. J Bacteriol. 1985 Oct;164(1):181–186. doi: 10.1128/jb.164.1.181-186.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. E., Ryan D., Levin W. An improved staining procedure for the detection of the peroxidase activity of cytochrome P-450 on sodium dodecyl sulfate polyacrylamide gels. Anal Biochem. 1976 Sep;75(1):168–176. doi: 10.1016/0003-2697(76)90067-1. [DOI] [PubMed] [Google Scholar]
- Ward J. A., Hunter C. N., Jones O. T. Changes in the cytochrome composition of Rhodopseudomonas sphaeroides grown aerobically, photosynthetically and on dimethyl sulphoxide. Biochem J. 1983 Jun 15;212(3):783–790. doi: 10.1042/bj2120783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. L. Protoplast formation in Escherichia coli. J Bacteriol. 1976 Nov;128(2):668–670. doi: 10.1128/jb.128.2.668-670.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]