Abstract
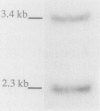
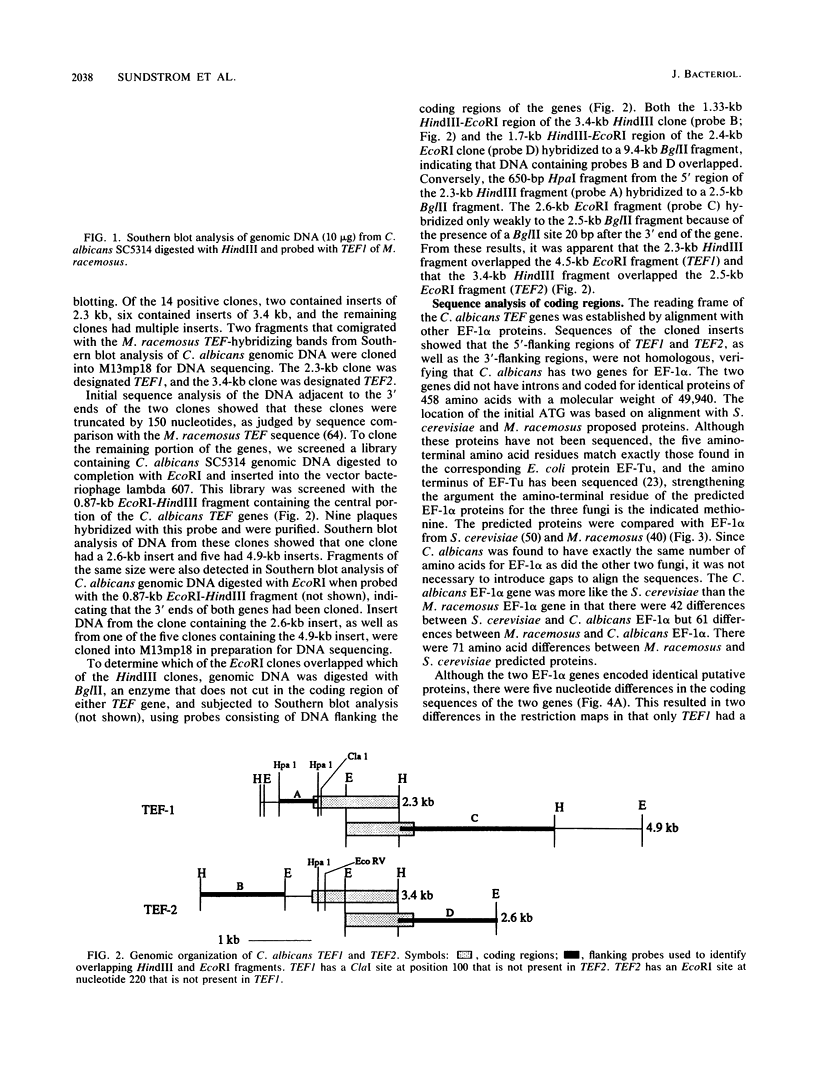
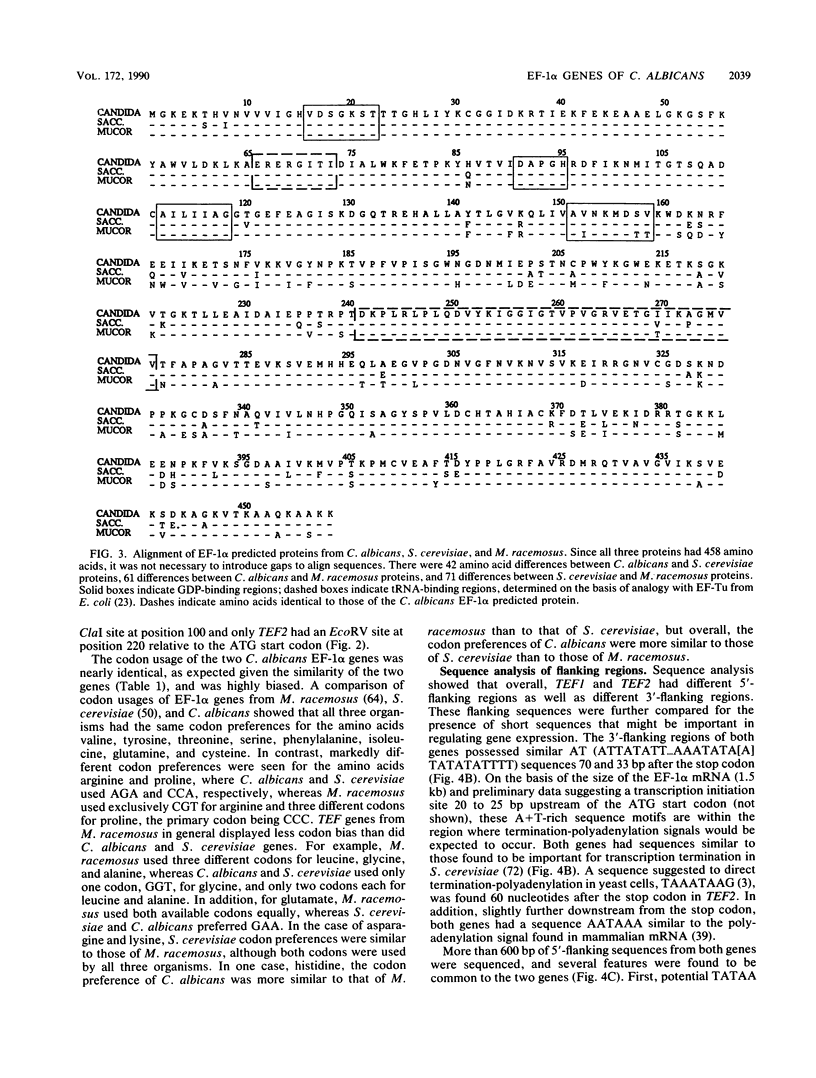
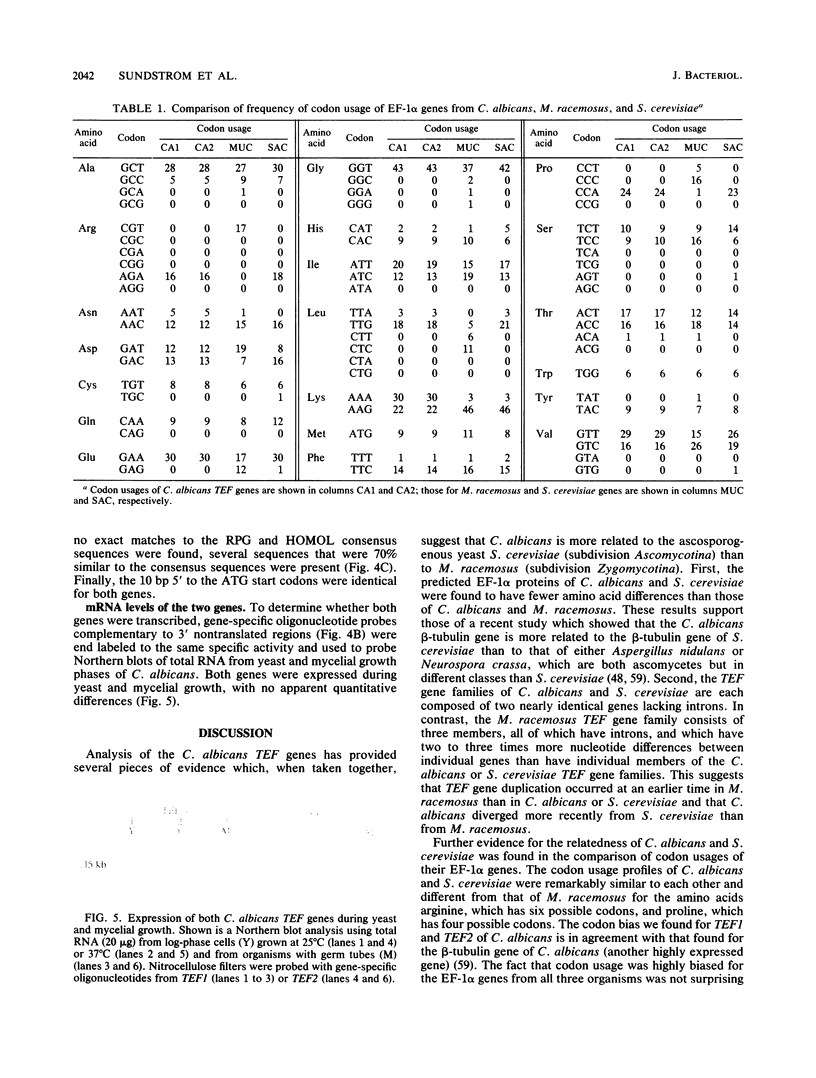
Two Candida albicans genes that encode the protein synthesis factor elongation factor 1 alpha (EF-1 alpha) were cloned by using a heterologous TEF1 probe from Mucor racemosus to screen libraries of C. albicans genomic DNA. Sequence analysis of the two clones showed that regions of DNA flanking the coding regions of the two genes were not homologous, verifying the presence of two genes, called TEF1 and TEF2, for EF-1 alpha in C. albicans. The coding regions of TEF1 and TEF2 differed by only five nucleotides and encoded identical EF-1 alpha proteins of 458 amino acids. Both genes were transcribed into mRNA in vivo, as shown by hybridization of oligonucleotide probes, which bound specifically to the 3' nontranslated regions of TEF1 and TEF2, respectively, to C. albicans total RNA in Northern (RNA) blot analysis. The predicted EF-1 alpha protein of C. albicans was more similar to Saccharomyces cerevisiae EF-1 alpha than to M. racemosus EF-1 alpha. Furthermore, codon bias and the promoter and termination signals of the C. albicans EF-1 alpha proteins were remarkably similar to those of S. cerevisiae EF-1 alpha. Taken together, these results suggest that C. albicans is more closely related to the ascomycete S. cerevisiae than to the zygomycete M. racemosus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachleitner M., Ludwig W., Stetter K. O., Schleifer K. H. Nucleotide sequence of the gene coding for the elongation factor Tu from the extremely thermophilic eubacterium Thermotoga maritima. FEMS Microbiol Lett. 1989 Jan 1;48(1):115–120. doi: 10.1016/0378-1097(89)90157-2. [DOI] [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Bennetzen J. L., Hall B. D. The primary structure of the Saccharomyces cerevisiae gene for alcohol dehydrogenase. J Biol Chem. 1982 Mar 25;257(6):3018–3025. [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Boeke J. D., Garfinkel D. J., Styles C. A., Fink G. R. Ty elements transpose through an RNA intermediate. Cell. 1985 Mar;40(3):491–500. doi: 10.1016/0092-8674(85)90197-7. [DOI] [PubMed] [Google Scholar]
- Brands J. H., Maassen J. A., van Hemert F. J., Amons R., Möller W. The primary structure of the alpha subunit of human elongation factor 1. Structural aspects of guanine-nucleotide-binding sites. Eur J Biochem. 1986 Feb 17;155(1):167–171. doi: 10.1111/j.1432-1033.1986.tb09472.x. [DOI] [PubMed] [Google Scholar]
- Brummel M., Soll D. R. The temporal regulation of protein synthesis during synchronous bud or mycelium formation in the dimorphic yeast Candida albicans. Dev Biol. 1982 Jan;89(1):211–224. doi: 10.1016/0012-1606(82)90308-6. [DOI] [PubMed] [Google Scholar]
- Buchman A. R., Lue N. F., Kornberg R. D. Connections between transcriptional activators, silencers, and telomeres as revealed by functional analysis of a yeast DNA-binding protein. Mol Cell Biol. 1988 Dec;8(12):5086–5099. doi: 10.1128/mcb.8.12.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrelle P., Thiele D., Price V. L., Memet S., Micouin J. Y., Marck C., Buhler J. M., Sentenac A., Fromageot P. Cloning, nucleotide sequence, and expression of one of two genes coding for yeast elongation factor 1 alpha. J Biol Chem. 1985 Mar 10;260(5):3090–3096. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillum A. M., Tsay E. Y., Kirsch D. R. Isolation of the Candida albicans gene for orotidine-5'-phosphate decarboxylase by complementation of S. cerevisiae ura3 and E. coli pyrF mutations. Mol Gen Genet. 1984;198(2):179–182. doi: 10.1007/BF00328721. [DOI] [PubMed] [Google Scholar]
- Goldstein B. P., Zaffaroni G., Tiboni O., Amiri B., Denaro M. Determination of the number of tuf genes in Chlamydia trachomatis and Neisseria gonorrhoeae. FEMS Microbiol Lett. 1989 Aug;51(3):305–309. doi: 10.1016/0378-1097(89)90415-1. [DOI] [PubMed] [Google Scholar]
- Hiatt W. R., Garcia R., Merrick W. C., Sypherd P. S. Methylation of elongation factor 1 alpha from the fungus Mucor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3433–3437. doi: 10.1073/pnas.79.11.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovemann B., Richter S., Walldorf U., Cziepluch C. Two genes encode related cytoplasmic elongation factors 1 alpha (EF-1 alpha) in Drosophila melanogaster with continuous and stage specific expression. Nucleic Acids Res. 1988 Apr 25;16(8):3175–3194. doi: 10.1093/nar/16.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Sentenac A. TUF, the yeast DNA-binding factor specific for UASrpg upstream activating sequences: identification of the protein and its DNA-binding domain. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3648–3652. doi: 10.1073/pnas.84.11.3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemura T. Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol. 1982 Jul 15;158(4):573–597. doi: 10.1016/0022-2836(82)90250-9. [DOI] [PubMed] [Google Scholar]
- Jones M. D., Petersen T. E., Nielsen K. M., Magnusson S., Sottrup-Jensen L., Gausing K., Clark B. F. The complete amino-acid sequence of elongation factor Tu from Escherichia coli. Eur J Biochem. 1980 Jul;108(2):507–526. doi: 10.1111/j.1432-1033.1980.tb04748.x. [DOI] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Kurtz M. B., Kirsch D. R. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987 Jan;7(1):199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Miller S. M., Kurtz M. B. One-step gene disruption by cotransformation to isolate double auxotrophs in Candida albicans. Mol Gen Genet. 1988 Sep;214(1):24–31. doi: 10.1007/BF00340174. [DOI] [PubMed] [Google Scholar]
- Kimura L. H., Pearsall N. N. Relationship between germination of Candida albicans and increased adherence to human buccal epithelial cells. Infect Immun. 1980 May;28(2):464–468. doi: 10.1128/iai.28.2.464-468.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King R. D., Lee J. C., Morris A. L. Adherence of Candida albicans and other Candida species to mucosal epithelial cells. Infect Immun. 1980 Feb;27(2):667–674. doi: 10.1128/iai.27.2.667-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch D. R., Lai M. H., O'Sullivan J. Isolation of the gene for cytochrome P450L1A1 (lanosterol 14 alpha-demethylase) from Candida albicans. Gene. 1988 Sep 7;68(2):229–237. doi: 10.1016/0378-1119(88)90025-x. [DOI] [PubMed] [Google Scholar]
- Kurtz M. B., Cortelyou M. W., Kirsch D. R. Integrative transformation of Candida albicans, using a cloned Candida ADE2 gene. Mol Cell Biol. 1986 Jan;6(1):142–149. doi: 10.1128/mcb.6.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz M. B., Kirsch D. R., Kelly R. The molecular genetics of Candida albicans. Microbiol Sci. 1988 Feb;5(2):58–63. [PubMed] [Google Scholar]
- Kurtz M. B., Marrinan J. Isolation of hem3 mutants from Candida albicans by sequential gene disruption. Mol Gen Genet. 1989 May;217(1):47–52. doi: 10.1007/BF00330941. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Gallwitz D. Evidence for an intron-contained sequence required for the splicing of yeast RNA polymerase II transcripts. Cell. 1983 Jun;33(2):519–527. doi: 10.1016/0092-8674(83)90433-6. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Buckley H. R., Campbell C. C. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida Albicans. Sabouraudia. 1975 Jul;13(2):148–153. doi: 10.1080/00362177585190271. [DOI] [PubMed] [Google Scholar]
- Leer R. J., Van Raamsdonk-Duin M. M., Mager W. H., Planta R. J. Conserved sequences upstream of yeast ribosomal protein genes. Curr Genet. 1985;9(4):273–277. doi: 10.1007/BF00419955. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lehrer N., Segal E., Cihlar R. L., Calderone R. A. Pathogenesis of vaginal candidiasis: studies with a mutant which has reduced ability to adhere in vitro. J Med Vet Mycol. 1986 Apr;24(2):127–131. doi: 10.1080/02681218680000191. [DOI] [PubMed] [Google Scholar]
- Lenstra J. A., Van Vliet A., Arnberg A. C., Van Hemert F. J., Möller W. Genes coding for the elongation factor EF-1 alpha in Artemia. Eur J Biochem. 1986 Mar 17;155(3):475–483. doi: 10.1111/j.1432-1033.1986.tb09514.x. [DOI] [PubMed] [Google Scholar]
- Linz J. E., Katayama C., Sypherd P. S. Three genes for the elongation factor EF-1 alpha in Mucor racemosus. Mol Cell Biol. 1986 Feb;6(2):593–600. doi: 10.1128/mcb.6.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz J. E., Lira L. M., Sypherd P. S. The primary structure and the functional domains of an elongation factor-1 alpha from Mucor racemosus. J Biol Chem. 1986 Nov 15;261(32):15022–15029. [PubMed] [Google Scholar]
- Linz J. E., Sypherd P. S. Expression of three genes for elongation factor 1 alpha during morphogenesis of Mucor racemosus. Mol Cell Biol. 1987 May;7(5):1925–1932. doi: 10.1128/mcb.7.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. A., Werner D. The complete cDNA sequence of mouse elongation factor 1 alpha (EF 1 alpha) mRNA. Nucleic Acids Res. 1989 Jan 11;17(1):442–442. doi: 10.1093/nar/17.1.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue N. F., Buchman A. R., Kornberg R. D. Activation of yeast RNA polymerase II transcription by a thymidine-rich upstream element in vitro. Proc Natl Acad Sci U S A. 1989 Jan;86(2):486–490. doi: 10.1073/pnas.86.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisch P. A., Calderone R. A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980 Feb;27(2):650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil J. B. Functional characterization of a pyrimidine-rich element in the 5'-noncoding region of the yeast iso-1-cytochrome c gene. Mol Cell Biol. 1988 Mar;8(3):1045–1054. doi: 10.1128/mcb.8.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K., Kasai M., Nagata S., Kaziro Y. Structure of the two genes coding for polypeptide chain elongation factor 1 alpha (EF-1 alpha) from Saccharomyces cerevisiae. Gene. 1986;45(3):265–273. doi: 10.1016/0378-1119(86)90024-7. [DOI] [PubMed] [Google Scholar]
- Nagata S., Nagashima K., Tsunetsugu-Yokota Y., Fujimura K., Miyazaki M., Kaziro Y. Polypeptide chain elongation factor 1 alpha (EF-1 alpha) from yeast: nucleotide sequence of one of the two genes for EF-1 alpha from Saccharomyces cerevisiae. EMBO J. 1984 Aug;3(8):1825–1830. doi: 10.1002/j.1460-2075.1984.tb02053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer K., Kim K. S., Kogan S., Guarente L. Functional dissection and sequence of yeast HAP1 activator. Cell. 1989 Jan 27;56(2):291–301. doi: 10.1016/0092-8674(89)90903-3. [DOI] [PubMed] [Google Scholar]
- Pokalsky A. R., Hiatt W. R., Ridge N., Rasmussen R., Houck C. M., Shewmaker C. K. Structure and expression of elongation factor 1 alpha in tomato. Nucleic Acids Res. 1989 Jun 26;17(12):4661–4673. doi: 10.1093/nar/17.12.4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosset R., Gorini L. A ribosomal ambiguity mutation. J Mol Biol. 1969 Jan 14;39(1):95–112. doi: 10.1016/0022-2836(69)90336-2. [DOI] [PubMed] [Google Scholar]
- Rotenberg M. O., Woolford J. L., Jr Tripartite upstream promoter element essential for expression of Saccharomyces cerevisiae ribosomal protein genes. Mol Cell Biol. 1986 Feb;6(2):674–687. doi: 10.1128/mcb.6.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandbaken M. G., Culbertson M. R. Mutations in elongation factor EF-1 alpha affect the frequency of frameshifting and amino acid misincorporation in Saccharomyces cerevisiae. Genetics. 1988 Dec;120(4):923–934. doi: 10.1093/genetics/120.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975 May 25;94(3):441–448. doi: 10.1016/0022-2836(75)90213-2. [DOI] [PubMed] [Google Scholar]
- Seidler L., Peter M., Meissner F., Sprinzl M. Sequence and identification of the nucleotide binding site for the elongation factor Tu from Thermus thermophilus HB8. Nucleic Acids Res. 1987 Nov 25;15(22):9263–9277. doi: 10.1093/nar/15.22.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. C., Richards C. A., Ferone R., Benedict D., Ray P. Cloning, purification, and properties of Candida albicans thymidylate synthase. J Bacteriol. 1989 Mar;171(3):1372–1378. doi: 10.1128/jb.171.3.1372-1378.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. A., Allaudeen H. S., Whitman M. H., Koltin Y., Gorman J. A. Isolation and characterization of a beta-tubulin gene from Candida albicans. Gene. 1988;63(1):53–63. doi: 10.1016/0378-1119(88)90545-8. [DOI] [PubMed] [Google Scholar]
- Smith I., Paress P. Genetic and biochemical characterization of kirromycin resistance mutations in Bacillus subtilis. J Bacteriol. 1978 Sep;135(3):1107–1117. doi: 10.1128/jb.135.3.1107-1117.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel J. D., Muller G., Buckley H. R. Critical role of germ tube formation in the pathogenesis of candidal vaginitis. Infect Immun. 1984 Jun;44(3):576–580. doi: 10.1128/iai.44.3.576-580.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Struhl K. Promoters, activator proteins, and the mechanism of transcriptional initiation in yeast. Cell. 1987 May 8;49(3):295–297. doi: 10.1016/0092-8674(87)90277-7. [DOI] [PubMed] [Google Scholar]
- Sundstrom P., Lira L. M., Choi D., Linz J. E., Sypherd P. S. Sequence analysis of the EF-1 alpha gene family of Mucor racemosus. Nucleic Acids Res. 1987 Dec 10;15(23):9997–10006. doi: 10.1093/nar/15.23.9997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapio S., Kurland C. G. Mutant EF-Tu increases missense error in vitro. Mol Gen Genet. 1986 Oct;205(1):186–188. doi: 10.1007/BF02428051. [DOI] [PubMed] [Google Scholar]
- Thompson R. C. EFTu provides an internal kinetic standard for translational accuracy. Trends Biochem Sci. 1988 Mar;13(3):91–93. doi: 10.1016/0968-0004(88)90047-3. [DOI] [PubMed] [Google Scholar]
- Vignais M. L., Woudt L. P., Wassenaar G. M., Mager W. H., Sentenac A., Planta R. J. Specific binding of TUF factor to upstream activation sites of yeast ribosomal protein genes. EMBO J. 1987 May;6(5):1451–1457. doi: 10.1002/j.1460-2075.1987.tb02386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgenboom E., Nilsson L., Bosch L. The elongation factor EF-Tu from E. coli binds to the upstream activator region of the tRNA-tufB operon. Nucleic Acids Res. 1988 Nov 11;16(21):10183–10197. doi: 10.1093/nar/16.21.10183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudt L. P., Mager W. H., Nieuwint R. T., Wassenaar G. M., van der Kuyl A. C., Murre J. J., Hoekman M. F., Brockhoff P. G., Planta R. J. Analysis of upstream activation sites of yeast ribosomal protein genes. Nucleic Acids Res. 1987 Aug 11;15(15):6037–6048. doi: 10.1093/nar/15.15.6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woudt L. P., Smit A. B., Mager W. H., Planta R. J. Conserved sequence elements upstream of the gene encoding yeast ribosomal protein L25 are involved in transcription activation. EMBO J. 1986 May;5(5):1037–1040. doi: 10.1002/j.1460-2075.1986.tb04319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zaret K. S., Sherman F. DNA sequence required for efficient transcription termination in yeast. Cell. 1982 Mar;28(3):563–573. doi: 10.1016/0092-8674(82)90211-2. [DOI] [PubMed] [Google Scholar]
- van Hemert F. J., Amons R., Pluijms W. J., van Ormondt H., Möller W. The primary structure of elongation factor EF-1 alpha from the brine shrimp Artemia. EMBO J. 1984 May;3(5):1109–1113. doi: 10.1002/j.1460-2075.1984.tb01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]