Abstract
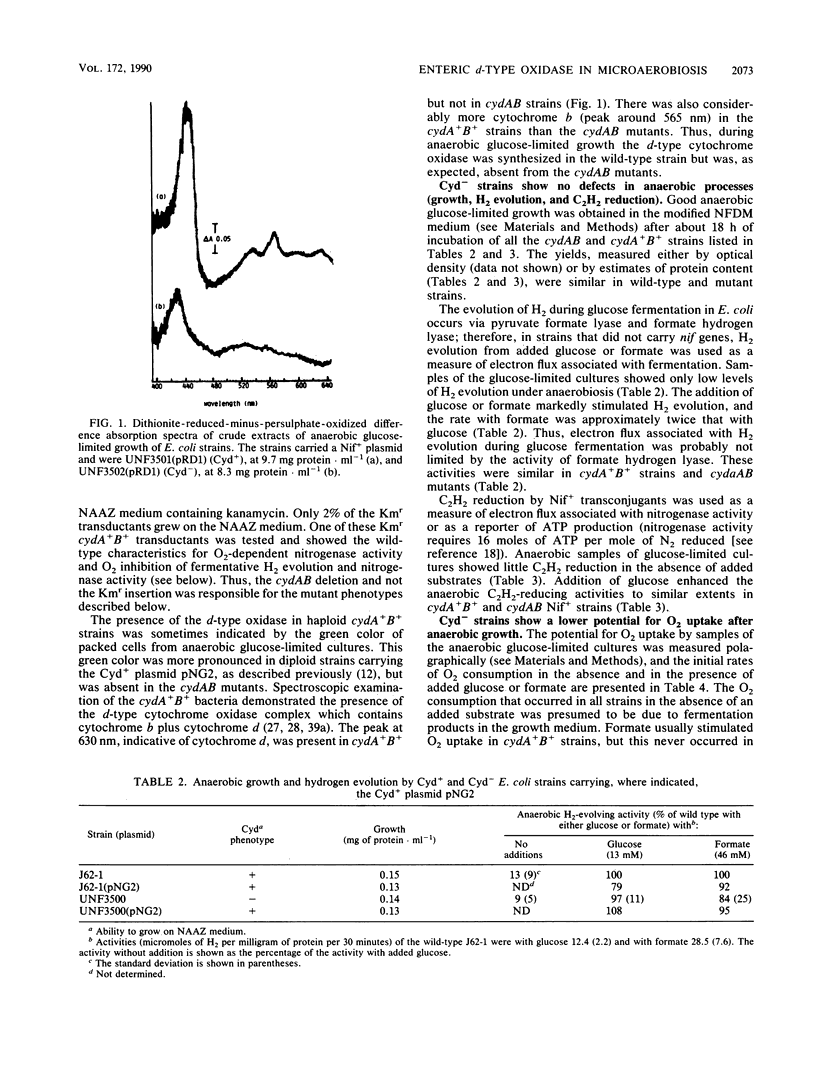
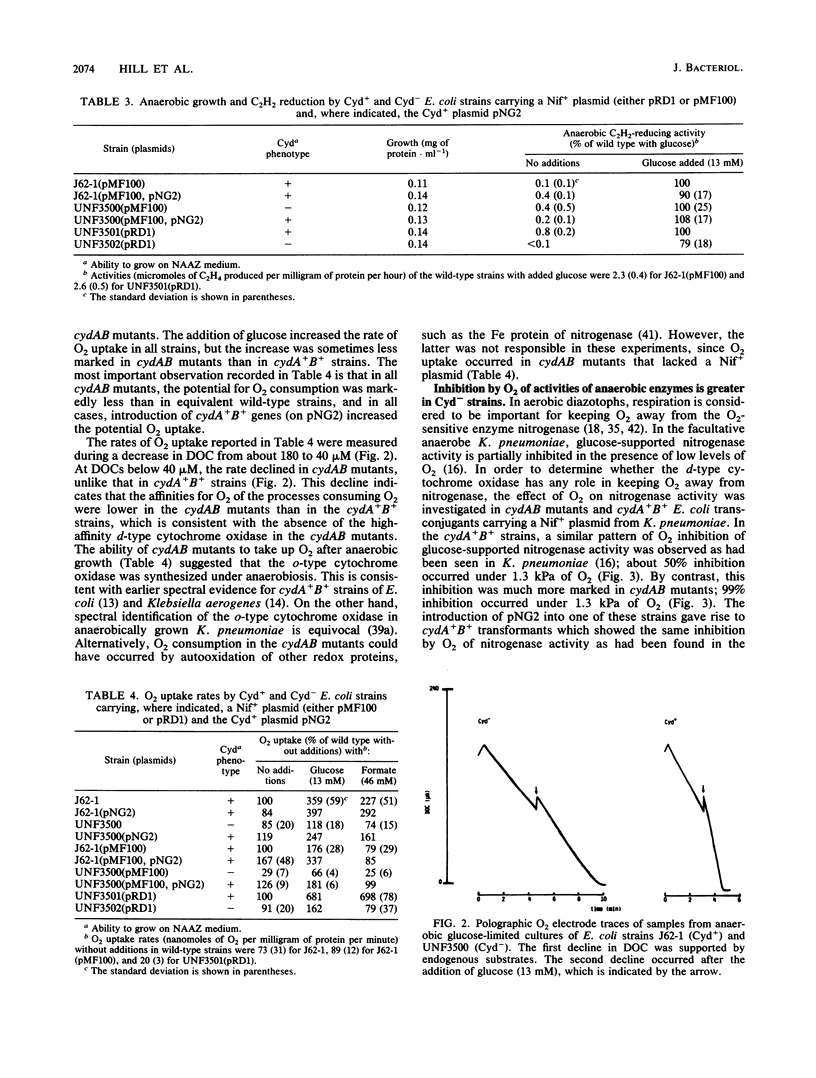
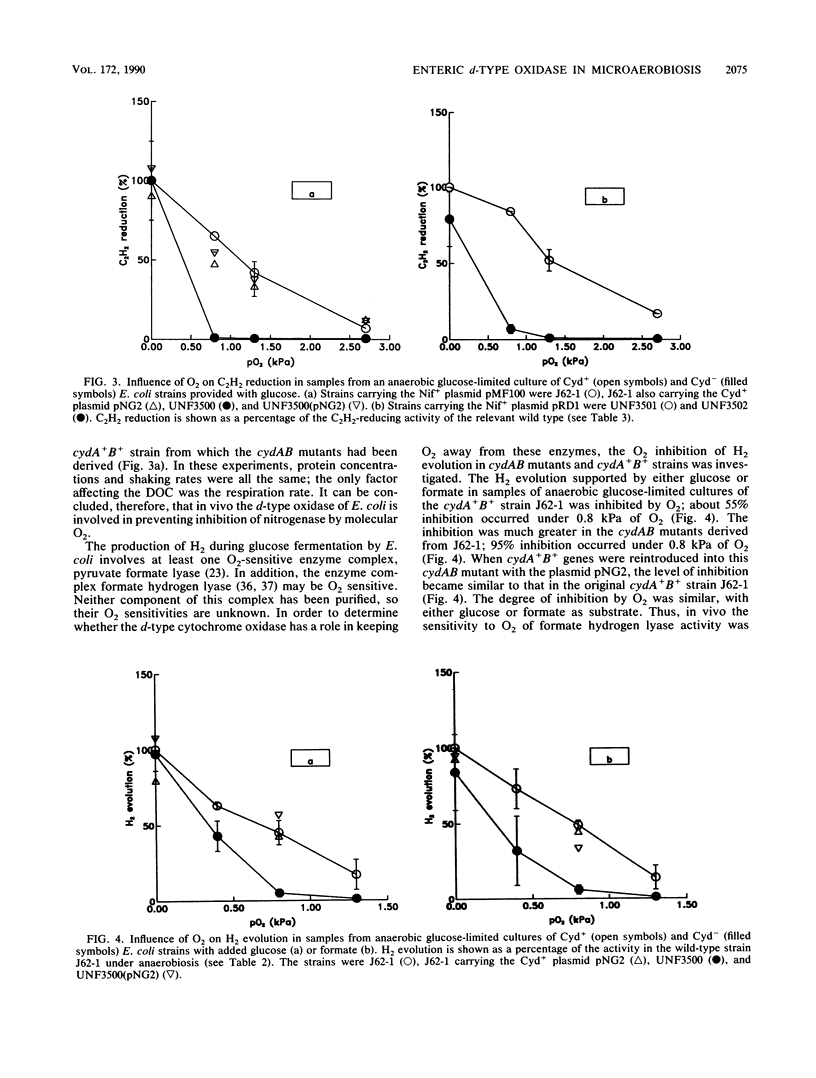
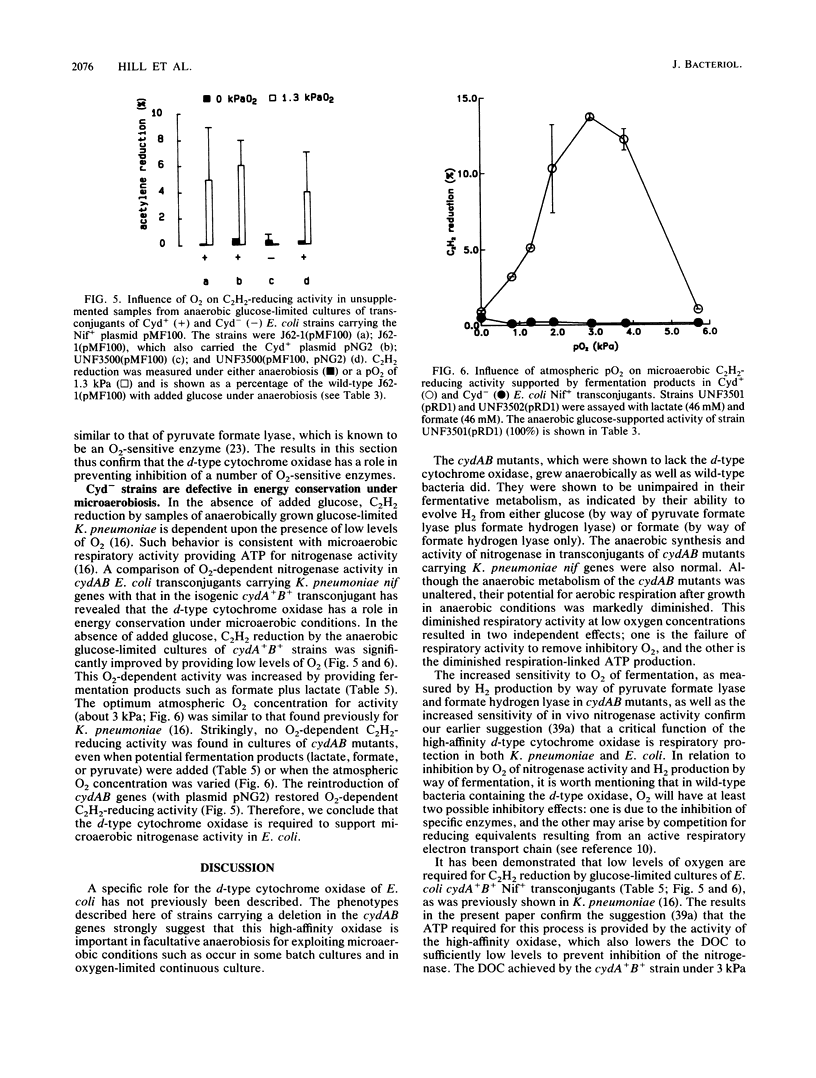
Escherichia coli strains that lacked the d-type cytochrome oxidase, the terminal oxidase with a high affinity for O2, grew anaerobically as well as the wild type did and were not impaired in the ability to evolve H2 from either glucose or formate. The anaerobic synthesis and activity of nitrogenase in transconjugants of these strains carrying Klebsiella pneumoniae nif genes were also normal. However, the behavior towards O2 of anaerobically grown bacteria lacking the d-type oxidase differed from that of the wild type in the following ways: the potential O2 uptake was lower, H2 evolution and nitrogenase activity supported by fermentation were more strongly inhibited by O2, and microaerobic O2-dependent nitrogenase activity in the absence of a fermentable carbon source did not occur. These results show that the d-type oxidase serves two functions in enteric bacteria--to conserve energy under microaerobic conditions and to protect anaerobic processes from inhibition by O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Au D. C., Lorence R. M., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking the cytochrome o terminal oxidase. J Bacteriol. 1985 Jan;161(1):123–127. doi: 10.1128/jb.161.1.123-127.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R., Cannon F., Kondorosi A. Construction of a P plasmid carrying nitrogen fixation genes from Klebsiella pneumoniae. Nature. 1976 Mar 18;260(5548):268–271. doi: 10.1038/260268a0. [DOI] [PubMed] [Google Scholar]
- Filser M., Merrick M., Cannon F. Cloning and characterisation of nifLA regulatory mutations from Klebsiella pneumoniae. Mol Gen Genet. 1983;191(3):485–491. doi: 10.1007/BF00425767. [DOI] [PubMed] [Google Scholar]
- Georgiou C. D., Dueweke T. J., Gennis R. B. Regulation of expression of the cytochrome d terminal oxidase in Escherichia coli is transcriptional. J Bacteriol. 1988 Feb;170(2):961–966. doi: 10.1128/jb.170.2.961-966.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg I., Nadler V., Hochman A. Mechanism of nitrogenase switch-off by oxygen. J Bacteriol. 1987 Feb;169(2):874–879. doi: 10.1128/jb.169.2.874-879.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Gennis R. B. Isolation and characterization of an Escherichia coli mutant lacking cytochrome d terminal oxidase. J Bacteriol. 1983 Jun;154(3):1269–1275. doi: 10.1128/jb.154.3.1269-1275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. N., Kranz J. E., Gennis R. B. Cloning the cyd gene locus coding for the cytochrome d complex of Escherichia coli. Gene. 1984 Dec;32(1-2):99–106. doi: 10.1016/0378-1119(84)90037-4. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Downie J. A., Garland P. B. Kinetic characterization of the membrane-bound cytochromes of Escherichia coli grown under a variety of conditions by using a stopped-flow dual-wavelength spectrophotometer. Biochem J. 1976 Feb 15;154(2):285–294. doi: 10.1042/bj1540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. E. A study of the effect of growth conditions on chemostat-grown Klebsiella aerogenes and kinetic changes of A 500-nm absorption band. Biochim Biophys Acta. 1972 Jul 12;275(1):83–92. doi: 10.1016/0005-2728(72)90026-6. [DOI] [PubMed] [Google Scholar]
- Harrison D. E., Loveless J. E. The effect of growth conditions on respiratory activity and growth efficiency in facultative anaerobes grown in chemostat culture. J Gen Microbiol. 1971 Sep;68(1):35–43. doi: 10.1099/00221287-68-1-35. [DOI] [PubMed] [Google Scholar]
- Hill S. How is nitrogenase regulated by oxygen? FEMS Microbiol Rev. 1988 Apr-Jun;4(2):111–129. doi: 10.1111/j.1574-6968.1988.tb02738.x. [DOI] [PubMed] [Google Scholar]
- Hill S. Influence of atmospheric oxygen concentration on acetylene reduction and efficiency of nitrogen fixation in intact Klebsiella pneumoniae. J Gen Microbiol. 1976 Apr;93(2):335–345. doi: 10.1099/00221287-93-2-335. [DOI] [PubMed] [Google Scholar]
- Hill S., Kavanagh E. P. Roles of nifF and nifJ gene products in electron transport to nitrogenase in Klebsiella pneumoniae. J Bacteriol. 1980 Feb;141(2):470–475. doi: 10.1128/jb.141.2.470-475.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill S. The apparent ATP requirement for nitrogen fixation in growing Klebsiella pneumoniae. J Gen Microbiol. 1976 Aug;96(2):297–312. doi: 10.1099/00221287-95-2-297. [DOI] [PubMed] [Google Scholar]
- Hill S., Turner G. L., Bergersen F. J. Synthesis and activity of nitrogenase in Klebsiella pneumoniae exposed to low concentrations of oxygen. J Gen Microbiol. 1984 May;130(5):1061–1067. doi: 10.1099/00221287-130-5-1061. [DOI] [PubMed] [Google Scholar]
- Ingledew W. J., Poole R. K. The respiratory chains of Escherichia coli. Microbiol Rev. 1984 Sep;48(3):222–271. doi: 10.1128/mr.48.3.222-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita K., Konishi K., Anraku Y. Terminal oxidases of Escherichia coli aerobic respiratory chain. II. Purification and properties of cytochrome b558-d complex from cells grown with limited oxygen and evidence of branched electron-carrying systems. J Biol Chem. 1984 Mar 10;259(5):3375–3381. [PubMed] [Google Scholar]
- McInerney M. J., Holmes K. S., DerVartanian D. V. Effect of O2 limitation on growth and respiration of the wild type and an ascorbate-tetramethyl-p-phenylenediamine-oxidase-negative mutant strain of Azotobacter vinelandii. J Bioenerg Biomembr. 1982 Dec;14(5-6):451–456. doi: 10.1007/BF00743070. [DOI] [PubMed] [Google Scholar]
- Merrick M. J., Gibbins J. R., Postgate J. R. A rapid and efficient method for plasmid transformation of Klebsiella pneumoniae and Escherichia coli. J Gen Microbiol. 1987 Aug;133(8):2053–2057. doi: 10.1099/00221287-133-8-2053. [DOI] [PubMed] [Google Scholar]
- Miller M. J., Gennis R. B. The purification and characterization of the cytochrome d terminal oxidase complex of the Escherichia coli aerobic respiratory chain. J Biol Chem. 1983 Aug 10;258(15):9159–9165. [PubMed] [Google Scholar]
- Poole R. K., Waring A. J., Chance B. The reaction of cytochrome omicron in Escherichia coli with oxygen. Low-temperature kinetic and spectral studies. Biochem J. 1979 Nov 15;184(2):379–389. doi: 10.1042/bj1840379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos J. L., Robson R. L. Lesions in citrate synthase that affect aerobic nitrogen fixation by Azotobacter chroococcum. J Bacteriol. 1985 May;162(2):746–751. doi: 10.1128/jb.162.2.746-751.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice C. W., Hempfling W. P. Oxygen-limited continuous culture and respiratory energy conservation in Escherichia coli. J Bacteriol. 1978 Apr;134(1):115–124. doi: 10.1128/jb.134.1.115-124.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson R. L., Postgate J. R. Oxygen and hydrogen in biological nitrogen fixation. Annu Rev Microbiol. 1980;34:183–207. doi: 10.1146/annurev.mi.34.100180.001151. [DOI] [PubMed] [Google Scholar]
- Sankar P., Lee J. H., Shanmugam K. T. Gene-product relationships of fhlA and fdv genes of Escherichia coli. J Bacteriol. 1988 Dec;170(12):5440–5445. doi: 10.1128/jb.170.12.5440-5445.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers R. G., Ballantine S. P., Boxer D. H. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: evidence for a third isoenzyme. J Bacteriol. 1985 Dec;164(3):1324–1331. doi: 10.1128/jb.164.3.1324-1331.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Stacey G., Brill W. J. Electron transport to nitrogenase. Purification and characterization of pyruvate:flavodoxin oxidoreductase. The nifJ gene product. J Biol Chem. 1983 Oct 10;258(19):12064–12068. [PubMed] [Google Scholar]
- Smith A., Hill S., Anthony C. A haemoprotein is not involved in the control by oxygen of enteric nitrogenase synthesis. J Gen Microbiol. 1988 Jun;134(6):1499–1507. doi: 10.1099/00221287-134-6-1499. [DOI] [PubMed] [Google Scholar]
- Smith A., Hill S., Anthony C. The purification, characterization and role of the d-type cytochrome oxidase of Klebsiella pneumoniae during nitrogen fixation. J Gen Microbiol. 1990 Jan;136(1):171–180. doi: 10.1099/00221287-136-1-171. [DOI] [PubMed] [Google Scholar]
- Thomas A. D., Doelle H. W., Westwood A. W., Gordon G. L. Effect of oxygen on several enzymes involved in the aerobic and anaerobic utilization of glucose in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1099–1105. doi: 10.1128/jb.112.3.1099-1105.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Ashby G. A. Oxidation of nitrogenase iron protein by dioxygen without inactivation could contribute to high respiration rates of Azotobacter species and facilitate nitrogen fixation in other aerobic environments. Biochem J. 1989 Jul 1;261(1):181–187. doi: 10.1042/bj2610181. [DOI] [PMC free article] [PubMed] [Google Scholar]



