Abstract
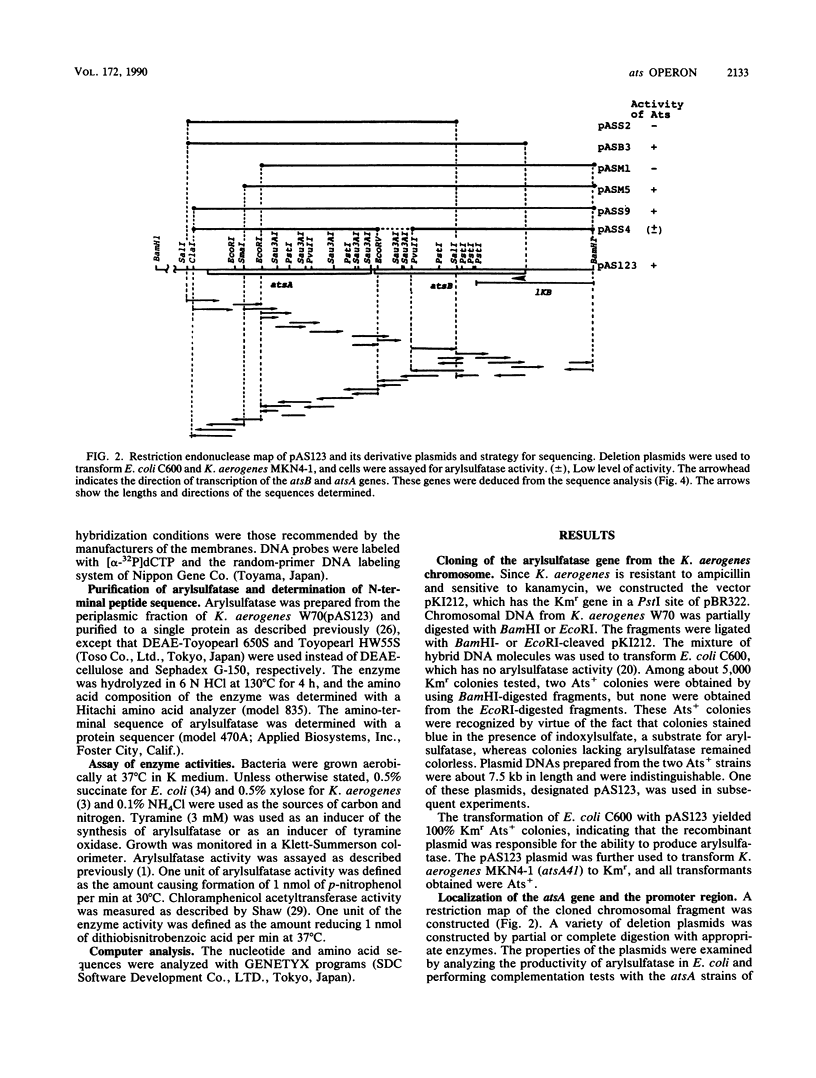
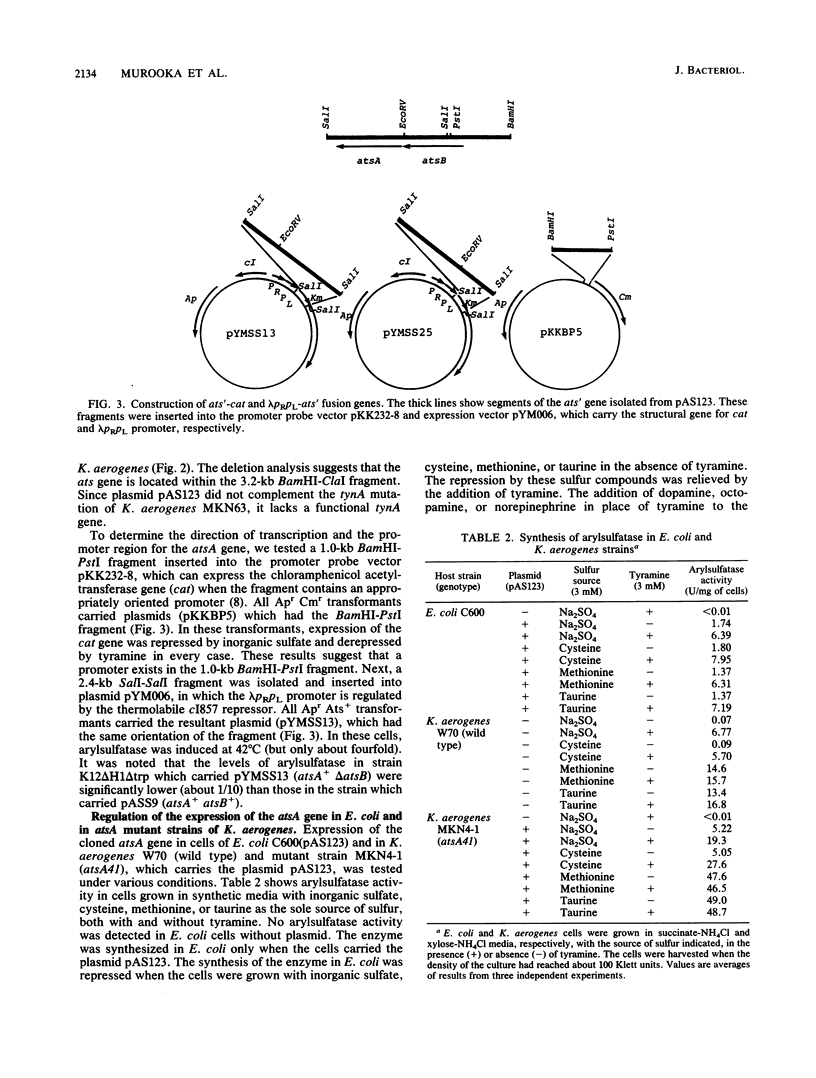
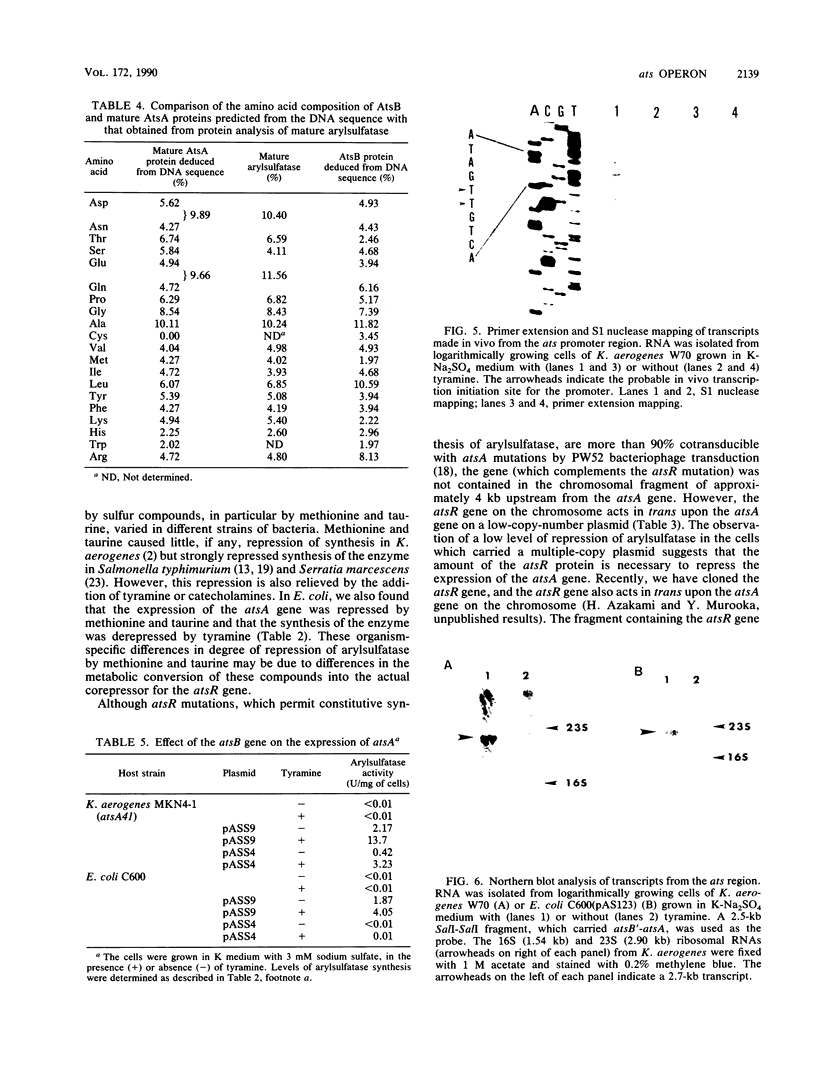
The structural gene for arylsulfatase (atsA) of Klebsiella aerogenes was cloned into a pKI212 vector in Escherichia coli. Deletion analysis showed that the atsA gene with the promoter region was located within a 3.2-kilobase cloned segment. In E. coli cells which carried the plasmid, the synthesis of arylsulfatase was repressed by various sources of sulfur; the repression was relieved, in each case, by tyramine. Transfer of the plasmid into atsA or constitutive atsR mutant strains of K. aerogenes resulted in complementation of atsA but not of atsR. The nucleotide sequence of the 3.2-kilobase fragment was determined. Two open reading frames, the atsA gene and an unknown gene (atsB), were found. These are located between a potential promoter and a transcriptional terminator sequence. Deletion analysis suggests that atsB is a potential positive factor for the regulation of arylsulfatase. Analysis of the amino acid sequences of the first 13 amino acids from the N terminus of the purified secreted arysulfatase agrees with that of the nucleotide sequence of atsA. The leader peptide extends over 20 amino acids and has the characteristics of a signal sequence. Primer extension mapping of transcripts generated in vivo suggests that the synthesis of mRNA starts at a site 31 or 32 bases upstream from the ATG initiation codon of the atsB gene. By Northern (RNA) blot analysis of the transcripts induced by tyramine, we found a 2.7-kilobase transcript which is identical in size to the total sequence of the atsB and atsA genes. Thus, the ats operon is composed of two cistrons and is regulated by sulfur and tyramine.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi T., Murooka Y., Harada T. Derepression of arylsulfatase synthesis in Aerobacter aerogenes by tyramine. J Bacteriol. 1973 Oct;116(1):19–24. doi: 10.1128/jb.116.1.19-24.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Murooka Y., Harada T. Regulation of arylsulfatase synthesis by sulfur compounds in Klebsiella aerogenes. J Bacteriol. 1975 Jan;121(1):29–35. doi: 10.1128/jb.121.1.29-35.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adachi T., Okamura H., Murooka Y., Harada T. Catabolite repression and derepression of arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1974 Nov;120(2):880–885. doi: 10.1128/jb.120.2.880-885.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia coli cells. J Biol Chem. 1981 Nov 25;256(22):11905–11910. [PubMed] [Google Scholar]
- Berkner K. L., Folk W. R. Polynucleotide kinase exchange reaction: quantitave assay for restriction endonuclease-generated 5'-phosphoroyl termini in DNA. J Biol Chem. 1977 May 25;252(10):3176–3184. [PubMed] [Google Scholar]
- Bernard H. U., Remaut E., Hershfield M. V., Das H. K., Helinski D. R., Yanofsky C., Franklin N. Construction of plasmid cloning vehicles that promote gene expression from the bacteriophage lambda pL promoter. Gene. 1979 Jan;5(1):59–76. doi: 10.1016/0378-1119(79)90092-1. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Close T. J., Rodriguez R. L. Construction and characterization of the chloramphenicol-resistance gene cartridge: a new approach to the transcriptional mapping of extrachromosomal elements. Gene. 1982 Dec;20(2):305–316. doi: 10.1016/0378-1119(82)90048-8. [DOI] [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Chamberlin M. J. Developmental and genetic regulation of Bacillus subtilis genes transcribed by sigma 28-RNA polymerase. Cell. 1983 Nov;35(1):285–293. doi: 10.1016/0092-8674(83)90231-3. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson M. J., Milazzo F. H. Arylsulfatase in Salmonella typhimurium: detection and influence of carbon source and tyramine on its synthesis. J Bacteriol. 1979 Jul;139(1):80–87. doi: 10.1128/jb.139.1.80-87.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D. G., Sutherland I. W., Wilkinson J. F. Transduction in Klebsiella. Nature. 1969 Feb 1;221(5179):475–476. doi: 10.1038/221475a0. [DOI] [PubMed] [Google Scholar]
- Mizusawa S., Nishimura S., Seela F. Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res. 1986 Feb 11;14(3):1319–1324. doi: 10.1093/nar/14.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Adachi T., Okamura H., Harada T. Genetic control of arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Apr;130(1):74–81. doi: 10.1128/jb.130.1.74-81.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Harada T. Regulation of derepressed synthesis of arylsulfatase by tyramine oxidase in Salmonella typhimurium. J Bacteriol. 1981 Feb;145(2):796–802. doi: 10.1128/jb.145.2.796-802.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Higashiura T., Harada T. Genetic mapping of tyramine oxidase and arylsulfatase genes and their regulation in intergeneric hybrids of enteric bacteria. J Bacteriol. 1978 Nov;136(2):714–722. doi: 10.1128/jb.136.2.714-722.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Yamada T., Tanabe S., Harada T. Immunological study of the regulation of cellular arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Oct;132(1):247–253. doi: 10.1128/jb.132.1.247-253.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murooka Y., Yim M. H., Harada T. Formation and Purification of Serratia marcescens Arylsulfatase. Appl Environ Microbiol. 1980 Apr;39(4):812–817. doi: 10.1128/aem.39.4.812-817.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka M., Murooka Y., Harada T. Genetic control of tyramine oxidase, which is involved in derepressed synthesis of arylsulfatase in Klebsiella aerogenes. J Bacteriol. 1980 Jul;143(1):321–327. doi: 10.1128/jb.143.1.321-327.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura H., Murooka Y., Harada T. Tyramine oxidase and regulation of arylsulfatase synthesis in Klebsiella aerogenes. J Bacteriol. 1977 Jan;129(1):59–65. doi: 10.1128/jb.129.1.59-65.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. Chloramphenicol acetyltransferase from chloramphenicol-resistant bacteria. Methods Enzymol. 1975;43:737–755. doi: 10.1016/0076-6879(75)43141-x. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Streicher S. L., Bender R. A., Magasanik B. Genetic control of glutamine synthetase in Klebiella aerogenes. J Bacteriol. 1975 Jan;121(1):320–331. doi: 10.1128/jb.121.1.320-331.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Murooka Y., Harada T. Comparative immunological studies on arylsulfatase in bacteria of the family Enterobacteriaceae: occurrence of latent arylsulfatase protein regulated by sulfur compounds and tyramine. J Bacteriol. 1978 Feb;133(2):536–541. doi: 10.1128/jb.133.2.536-541.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]