Abstract
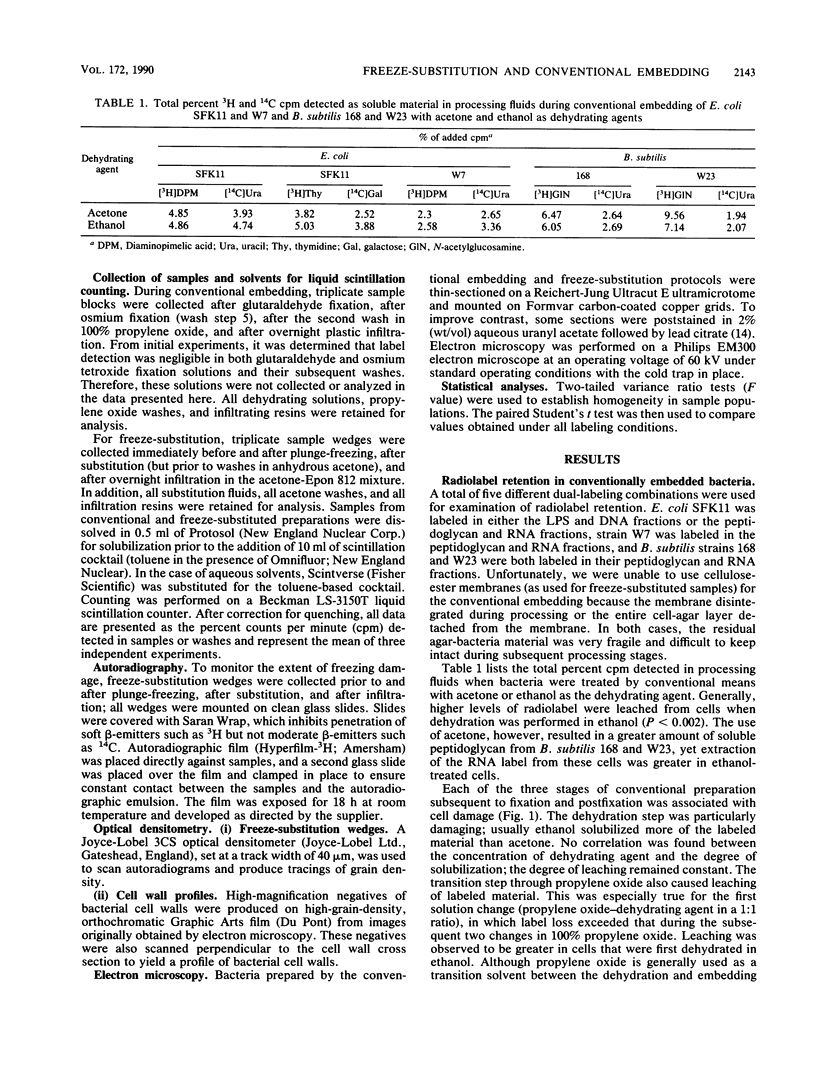
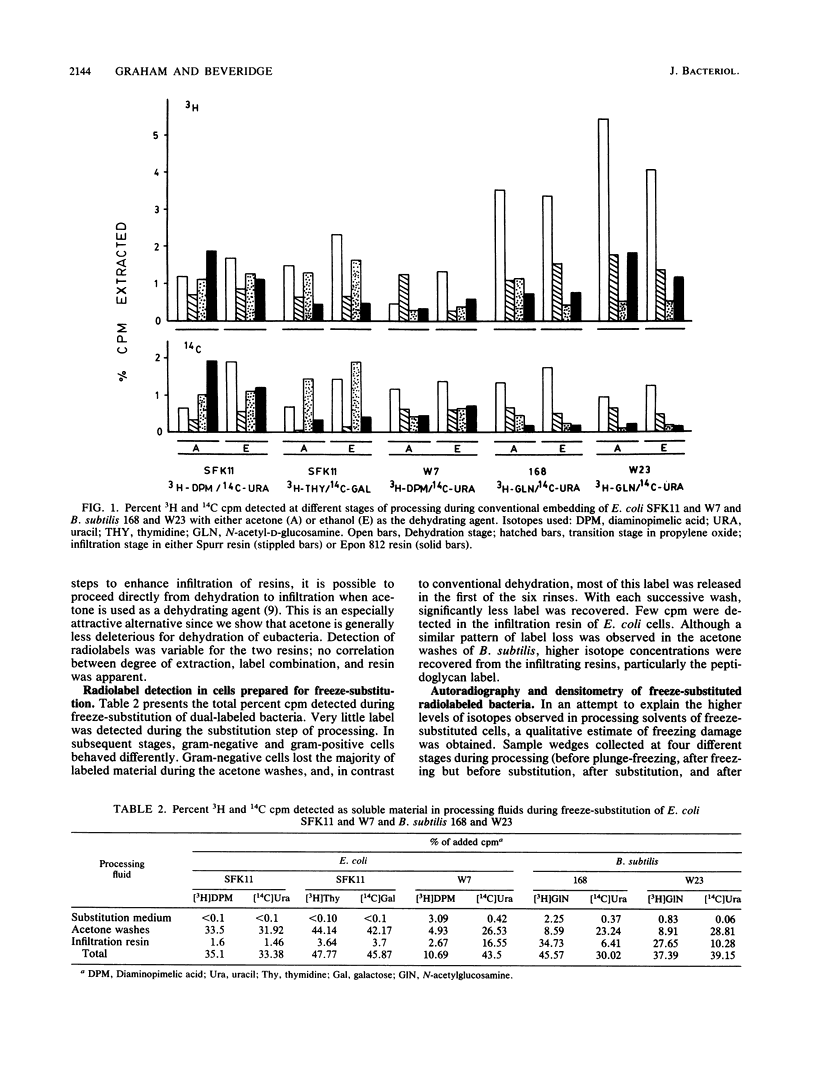
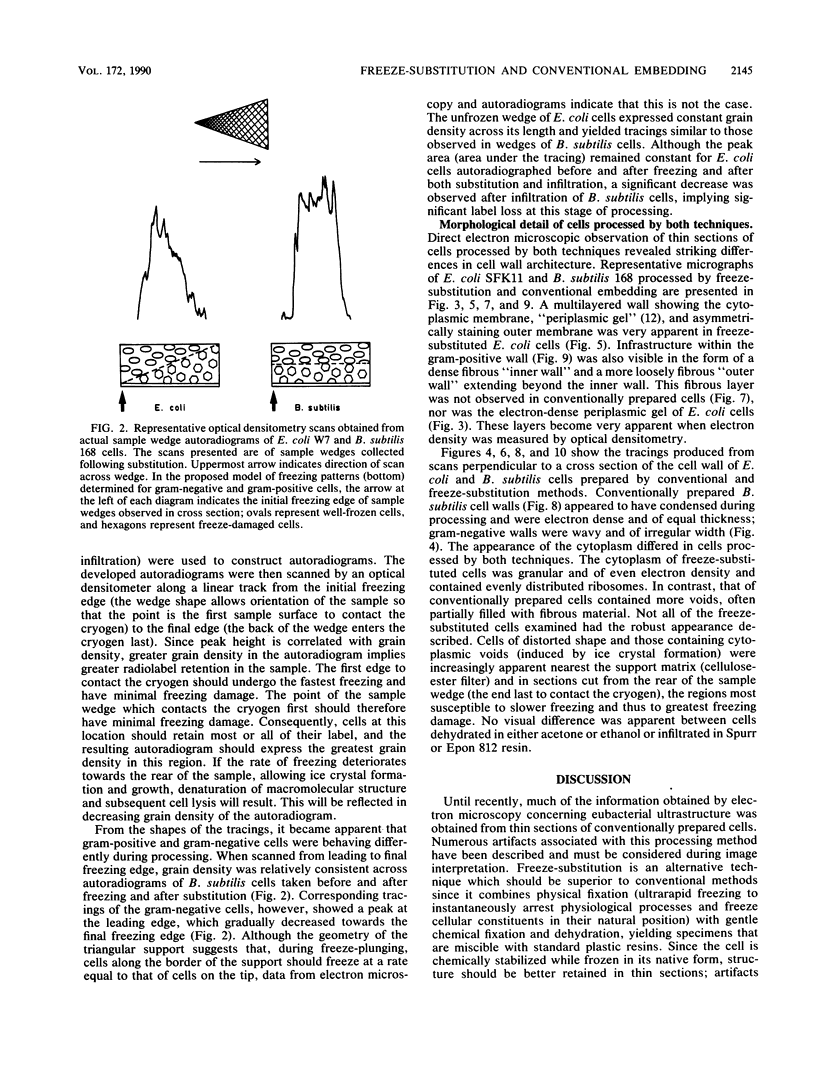
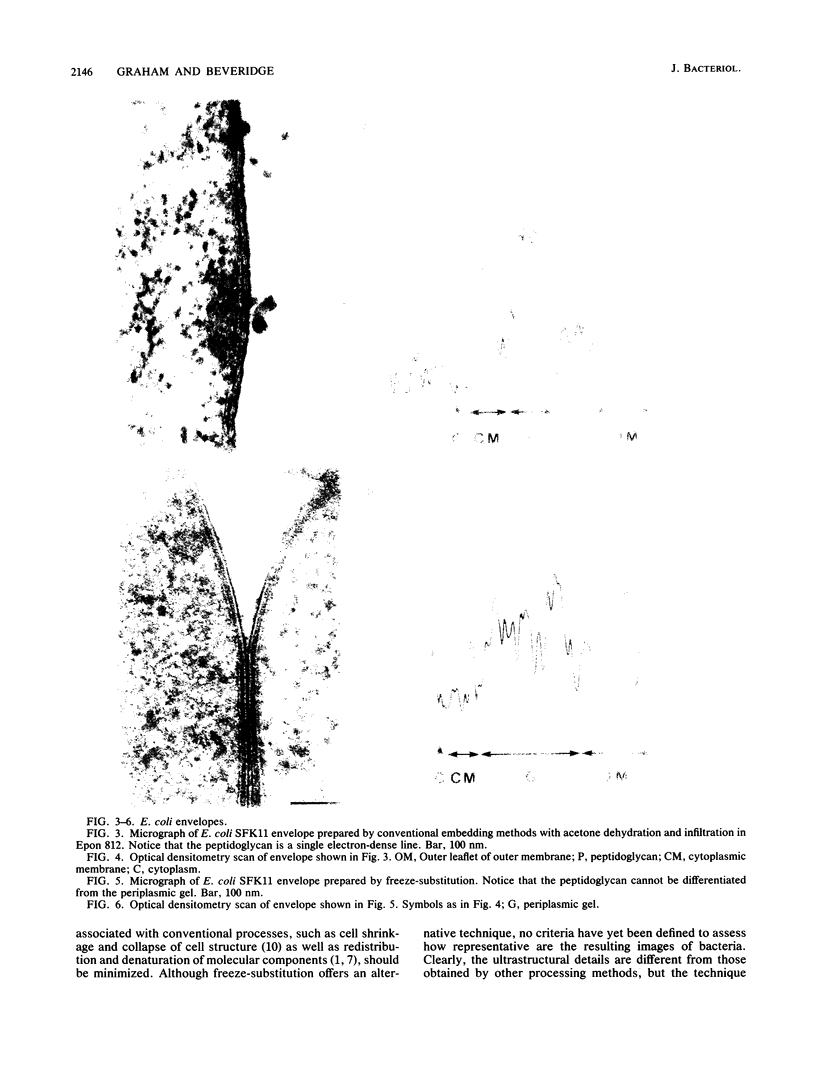
Freeze-substitution and more conventional embedding protocols were evaluated for their accurate preservation of eubacterial ultrastructure. Radioisotopes were specifically incorporated into the RNA, DNA, peptidoglycan, and lipopolysaccharide of two isogenic derivatives of Escherichia coli K-12 as representative gram-negative eubacteria and into the RNA and peptidoglycan of Bacillus subtilis strains 168 and W23 as representative gram-positive eubacteria. Radiolabeled bacteria were processed for electron microscopy by conventional methods with glutaraldehyde fixation, osmium tetroxide postfixation, dehydration in either a graded acetone or ethanol series, and infiltration in either Spurr or Epon 812 resin. A second set of cells were simultaneously freeze-substituted by plunge-freezing in liquid propane, substituting in anhydrous acetone containing 2% (wt/vol) osmium tetroxide, and 2% (wt/vol) uranyl acetate, and infiltrating in Epon 812. Extraction of radiolabeled cell components was monitored by liquid scintillation counting at all stages of processing to indicate retention of cell labels. Electron microscopy was also used to visually confirm ultrastructural integrity. Radiolabeled nucleic acid and wall components were extracted by both methods. In conventionally embedded specimens, dehydration was particularly damaging, with ethanol-dehydrated cells losing significantly more radiolabeled material during dehydration and subsequent infiltration than acetone-treated cells. For freeze-substituted specimens, postsubstitution washes in acetone were the most deleterious step for gram-negative cells, while infiltration was more damaging for gram-positive cells. Autoradiographs of specimens collected during freeze-substitution were scanned with an optical densitometer to provide an indication of freezing damage; the majority of label lost from freeze-substituted cells was a result of poor freezing to approximately one-half of the cell population, thus accounting for the relatively high levels of radiolabel detected in the processing fluids. These experiments revealed that gram-positive and gram-negative cells respond differently to freezing; these differences are discussed with reference to wall structure. It was apparent that the cells frozen first (ie., the first to contact the cryogen) retained the highest percentage of all radioisotopes, and the highest level of cellular infrastructure, indicative of better preservation. The preservation of these select cells was far superior to that obtained by more conventional techniques.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beveridge T. J., Davies J. A. Cellular responses of Bacillus subtilis and Escherichia coli to the Gram stain. J Bacteriol. 1983 Nov;156(2):846–858. doi: 10.1128/jb.156.2.846-858.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge T. J., Koval S. F. Binding of metals to cell envelopes of Escherichia coli K-12. Appl Environ Microbiol. 1981 Aug;42(2):325–335. doi: 10.1128/aem.42.2.325-335.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J., van der Merwe C. F. Extraction of carbon 14-labeled compounds from plant tissue during processing for electron microscopy. J Electron Microsc Tech. 1989 Feb;11(2):155–160. doi: 10.1002/jemt.1060110210. [DOI] [PubMed] [Google Scholar]
- Cope G. H., Williams M. A. Quantitative studies on the preservation of choline and ethanolamine phosphatides during tissue preparation for electron microscopy. I. Glutaraldehyde, osmium tetroxide, Araldite methods. J Microsc. 1969;90(1):31–46. doi: 10.1111/j.1365-2818.1969.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Cope G. H., Williams M. A. Quantitative studies on the preservation of choline and ethanolamine phosphatides during tissue preparation for electron microscopy. II. Other preparative methods. J Microsc. 1969;90(1):47–60. doi: 10.1111/j.1365-2818.1969.tb00693.x. [DOI] [PubMed] [Google Scholar]
- Ebersold H. R., Cordier J. L., Lüthy P. Bacterial mesosomes: method dependent artifacts. Arch Microbiol. 1981 Sep;130(1):19–22. doi: 10.1007/BF00527066. [DOI] [PubMed] [Google Scholar]
- Graham L. L., Beveridge T. J. Effect of chemical fixatives on accurate preservation of Escherichia coli and Bacillus subtilis structure in cells prepared by freeze-substitution. J Bacteriol. 1990 Apr;172(4):2150–2159. doi: 10.1128/jb.172.4.2150-2159.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Carlemalm E., Villiger W., Kellenberger E. Periplasmic gel: new concept resulting from the reinvestigation of bacterial cell envelope ultrastructure by new methods. J Bacteriol. 1984 Oct;160(1):143–152. doi: 10.1128/jb.160.1.143-152.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobot J. A., Villiger W., Escaig J., Maeder M., Ryter A., Kellenberger E. Shape and fine structure of nucleoids observed on sections of ultrarapidly frozen and cryosubstituted bacteria. J Bacteriol. 1985 Jun;162(3):960–971. doi: 10.1128/jb.162.3.960-971.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobley H. L., Doyle R. J., Streips U. N., Langemeier S. O. Transport and incorporation of N-acetyl-D-glucosamine in Bacillus subtilis. J Bacteriol. 1982 Apr;150(1):8–15. doi: 10.1128/jb.150.1.8-15.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Sousa J. C. Ultrastructure of the cell wall and cytoplasmic membrane of gram-negative bacteria with different fixation techniques. J Bacteriol. 1973 Feb;113(2):953–962. doi: 10.1128/jb.113.2.953-962.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibull C., Christiansson A., Carlemalm E. Extraction of membrane lipids during fixation, dehydration and embedding of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1983 Feb;129(Pt 2):201–207. doi: 10.1111/j.1365-2818.1983.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Weibull C., Christiansson A. Extraction of proteins and membrane lipids during low temperature embedding of biological material for electron microscopy. J Microsc. 1986 Apr;142(Pt 1):79–86. doi: 10.1111/j.1365-2818.1986.tb02739.x. [DOI] [PubMed] [Google Scholar]
- Weibull C., Villiger W., Carlemalm E. Extraction of lipids during freeze-substitution of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1984 May;134(Pt 2):213–216. doi: 10.1111/j.1365-2818.1984.tb02513.x. [DOI] [PubMed] [Google Scholar]










