Abstract
Dystrophic or ectopic mineral deposition occurs in many pathologic conditions, including atherosclerosis. Calcium mineral deposits that frequently accompany atherosclerosis are readily quantifiable radiographically, serve as a surrogate marker for the disease, and predict a higher risk of myocardial infarction and death. Accelerating research interest has been propelled by a clear need to understand how plaque structure, composition, and stability lead to devastating cardiovascular events. In atherosclerotic plaque, accumulating evidence is consistent with the notion that calcification involves the participation of arterial osteoblasts and osteoclasts. Here we summarize current models of intimal arterial plaque calcification and highlight intriguing questions that require further investigation. Because atherosclerosis is a chronic vascular inflammation, we propose that arterial plaque calcification is best conceptualized as a convergence of bone biology with vascular inflammatory pathobiology.
Plaque structure and composition importantly impact clinical expression of atherosclerosis. Molecular medicine in the 21st century has turned toward a comprehensive understanding of the dynamic processes that influence the composition and stability of atheroma and of how structural plaque components impact clinical outcomes. Recently, increasing interest has focused on understanding how atherosclerotic pathology is related to a common plaque constituent: calcium mineral deposits. Pathologists have long known that calcified atherosclerotic arteries can contain tissue that is histomorphologically indistinguishable from bone (1, 2). Important studies in the last decade have now spawned a model wherein calcification in atherosclerotic plaque is viewed as an active, complex, and therefore presumably regulated process that exhibits intriguing similarities to new bone formation, or remodeling. Ectopic and dystrophic mineral deposition and extracellular matrix calcification can occur in numerous pathologic conditions by passive precipitation. Here we focus on one specific type of mineral deposition with high relevance to cardiac pathology: intimal arterial calcification in the context of atherosclerotic plaque. The emerging view is that plaque calcification represents a meeting of bone biology with chronic plaque inflammation. Remarkable cellular ontogenetic versatility in atherosclerosis appears to effect profound structural alterations, with significant ramifications for plaque stability and clinical outcomes.
Clinical Significance
Atherosclerotic lesions frequently become calcified. The process can begin early and accelerates as the disease progresses and more complex lesions develop. Calcium deposits in coronary arteries indicate the presence of plaque, but the converse statement that an absence of coronary calcium indicates an absence of atheromatous plaque is not true (1). Because calcification is a surrogate measure of coronary atherosclerosis, clinical interest has focused on the usefulness of noninvasive detection of calcium as a coronary risk-stratification tool (Fig. 1). At present, the clinical usefulness of coronary artery calcium scanning is controversial. There is a high histologic correlation between total “plaque burden” and the extent of coronary calcium deposition (3). Coronary calcification detected independently by computed tomography predicts an increased likelihood of adverse coronary events (4), but relative risks have varied widely, specificity is disappointingly low, and there are other methodological concerns (4, 5). Furthermore, patients with little or no coronary artery calcium can and do suffer cardiac events (6). Calcium scanning may offer no advantage over standard risk factor assessment (7) and has not been shown to affect outcomes favorably (8).
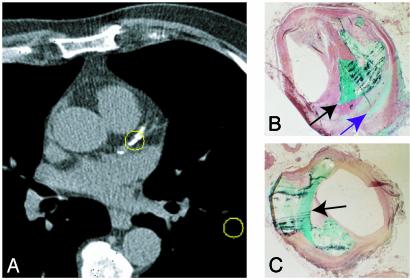
Fig. 1.
(A) Calcification (center circled region) in the proximal left anterior descending coronary artery shown on an electron beam computed tomography transverse section. Consecutive high-resolution non-contrast-enhanced computed tomography images are acquired from the top to the bottom of the heart to visualize arterial calcification. Regions of high computed tomography density representing calcium deposits are summed, and a calcium score is derived. This score is then used to ascertain the risk of future cardiac events. (B and C) Human coronary artery sections prepared by using undecalcified methylmethacrylate embedding and sectioning procedures and stained with Goldner–Masson trichrome stain. Two distinct patterns of calcification are evident: large focal mineral deposition (turquoise, black arrow in B and C) and a fine speckled pattern (purple arrow in B).
Both a comprehensive understanding of plaque structural dynamics and better prognostic indicators are needed. This conclusion is driven home by the devastating morbidity and mortality attributable to atherosclerosis. Although atherosclerosis is the pathobiologic substrate, coronary events are most often precipitated directly by rupture or erosion of structurally unstable plaque, and plaque vulnerability has little relationship to the extent of atherosclerosis (ref. 9 and Fig. 2). These considerations underscore the urgency of attaining a more complete knowledge of how the structural components of plaque are formed, regulated, and altered.
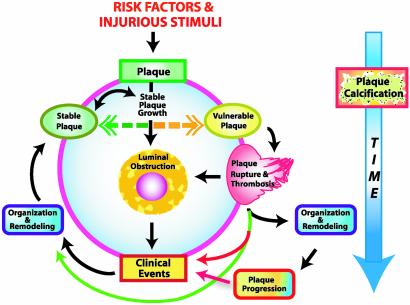
Fig. 2.
Interrelationships between development and stability of plaque and calcium deposition. There are significant uncertainties among three key plaque-related variables (presence, progression, and stability of plaque) and the variables that radiographic calcium scans measure (presence, quantity, and change in quantity of calcium deposits). The circle in the center represents possible sequences of events in the natural history of an individual plaque. Known risk factors and other injurious stimuli, such as infectious agents, initiate plaque formation. Plaque begins to grow and might proceed toward luminal obstruction and eventually cause a clinical event. The period of growth occurs at highly variable rates and tends to be episodic, with long periods of latency during which growth of the plaque may be minimal. At any point during the period of stable plaque growth, an individual lesion can become vulnerable, after which plaque rupture and thrombosis can result. Plaque rupture can lead directly to a clinical event but can also be silent and may itself be innocuous. Reorganization and remodeling may ensue with or without a supervening clinical event. Reorganization of ruptured plaque may proceed to a period of relatively rapid plaque progression but can also stabilize and remain stabilized indefinitely. From there, a period of dormancy may ensue, or, alternatively, further development of plaque can again take any of the pathways described above and result in a clinical event or remain clinically silent. This cycle repeats itself at each lesion site somewhat independently of what is occurring even at closely adjacent plaques. Calcification can begin at any point in this process. The sequence of events in plaque development does not seem to directly determine calcium deposition or progression of arterial calcification, in that there is no evidence that calcification develops episodically; instead, calcification appears to develop more linearly over time (large blue arrow on right). When an arterial tree such as the coronary arterial circulation is considered, overall plaque burden also proceeds roughly linearly and approximates the quantity of calcification. Time between and duration of individual stages of plaque development tend to be highly variable and more nonlinear than calcification.
Active Model Versus Passive Model of Arterial Calcification
In many pathologies where chronic inflammation occurs, concomitant soft-tissue calcification is thought to be a passive precipitation associated with areas of advanced tissue degeneration, or necrosis. Atherosclerosis involves both chronic inflammation and necrosis. Atherosclerotic arteries, however, may also contain bone-like tissues, including hematopoietic tissue (2) and hydroxyapatite (10). These features distinguish atherosclerotic arterial calcification from the dystrophic calcium deposition frequently observed in conditions involving chronic inflammation and necrosis and also from other types of vascular calcification, such as calcification of the medial layer of the artery. Histopathologically, some of the mineralization in plaque is diffuse or amorphous, but observations of bone-like regions within plaque and a series of remarkable studies in the last decade now support the idea that calcium deposition in plaque is an active and regulated process akin to bone formation. This concept is still somewhat controversial. An alternative “passive precipitation” model posits that it is only the presence of inhibitors under homeostatic conditions that prevents calcium precipitation from occurring (11). It is clear that inhibitory influences affect mineralization, but collectively, the available evidence would appear to be more consistent with an “active regulated” mechanism leading to arterial calcium deposition. Key cellular and molecular elements that participate in bone and cartilage formation have been characterized in calcific plaque and are summarized in Table 1 and Fig. 3. A brief review of the mechanism of normal bone formation and degradation will provide a relevant conceptual framework.
Table 1. Cell types and signaling-pathway components participating in bone metabolism that have been found to be associated with arteries, plaque, and / or arterial calcification.
| Name |
|---|
| Cell Type |
| Chondrocyte |
| Osteoblast |
| Osteoclast |
| Osteocyte |
| Proteins |
| BGP (osteocalcin) |
| MGP |
| OPN |
| ON |
| ALP |
| BSP |
| Signaling components |
| BMP-2 |
| BMP-4 |
| BMP-6 |
| BMP receptor |
| CSF-1 (M-CSF) |
| CSF-1R (c-fms) |
| RANKL |
| RANK |
| OPG |
| TNF-α |
| TNF-α receptor |
| Transcription factors |
| Cbfa 1 |
| Sox9 |
| Msx2 |
| Other |
| Matrix vesicles |
| Hydroxyapatite |
BGP, bone Gla (γ-carboxyglutamate) protein; MGP, matrix Gla protein; OPN, osteopontin; ON, osteonectin; ALP, alkaline phosphatase; BSP, bone sialoprotein; BMP, bone morphogenetic protein; CSF-1, colony-stimulating factor 1; TNF-α, tumor necrosis factor α; RANK, receptor activator of NF-κB; RANKL, RANK ligand. OPG is also known as osteoclastogenesis inhibitory factor (OCIF), TNF receptor-related molecule 1 (TR-1), follicular dendritic cell receptor 1 (FDCR-1), and TNF superfamily receptor 11B (TNFRSF-11B). Alternative names for RANKL include OPG-ligand (OPG-L), osteoclast differentiation factor (ODF), TNF-related activation-induced cytokine (TRANCE), stromal osteoclast-forming activity (SOFA), and TNF superfamily 11 (TNFSF-11). RANK has been termed osteoclast differentiation and activation receptor (ODAR), and TNF superfamily receptor 11A (TNFRSF-11A). Standardized nomenclature recommended by the American Society of Bone and Mineral Research is OPG, RANKL, and RANK.
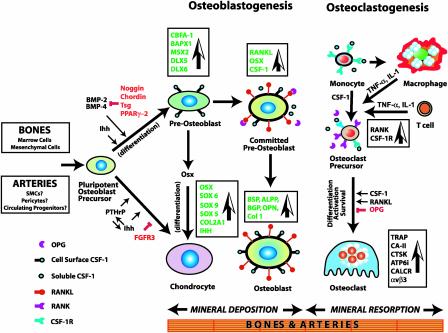
Fig. 3.
Mechanism of osteogenesis, showing the major genes, growth factors, and signaling pathways culminating in fully mature chondrocytes, osteoblasts, and osteoclasts. Inhibitory influences are shown in red. Considerable ontogenetic plasticity is retained at each step but appears to diminish as terminally differentiated cellular phenotypes are approached. The proposed mechanism of arterial calcification appears to involve many of the same components.
Bone Formation and Degradation
Osteoblasts derived from mesenchymal precursors are responsible for bone formation, and osteoclasts mediate the opposing process of mineral resorption (12, 13). Osteoclasts arise specifically from hematopoietic mononuclear phagocyte progenitors by the combined actions of CSF-1 (also known as macrophage CSF) and RANKL (see footnote to Table 1 concerning nomenclature). Chondrocytes, the third major cell type involved in bone formation, are derived from mesenchymal cells and function to generate an initial cartilage template on which mineral deposition may occur. Chondrocytes and osteoblasts thus differentiate from a common progenitor lineage (Fig. 3). Chondrocytes deposit a cartilage-specific extracellular matrix, proliferate, hypertrophy, and undergo apoptosis. Surrounding mesenchymal cells differentiate into osteoblasts, invade the zones occupied by hypertrophic and dying chondrocytes, and begin synthesizing a bone-specific matrix on the cartilage template, which osteoclasts begin to degrade. Simultaneously, neovascularization and osteoclastogenesis occur in the same region. The coordinated development of osteoblasts and osteoclasts involves cell–cell contact between precursors of both lineages and soluble cytokines (Fig. 3). The entire process is elegantly orchestrated by genetic programs that regulate the expression of a large number of specific molecules (Table 2) in an ordered spatiotemporal manner (14).
Table 2. List of molecules known to participate in the development and function of osteoblasts and osteoclasts, and their chromosomal locations in human and mouse.
| Chromosome/genetic locus, cM
|
|||
|---|---|---|---|
| Name | Gene | Human | Mouse |
| Matrix proteins | |||
| Aggrecan | AGCI | 15q26.1 | 7/39.0 |
| Alkaline phosphatase | ALPL | 1p36.1-p34 | 4/70.2 |
| Bone Gla protein (osteocalcin) | BGP | 1q25-q31 | 3/42.6 |
| Bone sialoprotein II | BSP | 4q21-q25 | 5/56.0 |
| Calcitonin receptor | CALCR | 7q21.3 | 6/3.0 |
| Carbonic anhydrase II | CA2 | 8q22 | 3/10.5 |
| Cartilage oligomeric matrix protein | COMP | 19p13.1 | 8/33.0 |
| Cathepsin K | CTSK | 1q21 | 3/47.9 |
| Chordin | CHRD | 3q27 | 16/14.0 |
| Collagen, type I, α1 | COL1A1 | 17q21.31-22 | 11/56.0 |
| Collagen, type I, α2 | COL1A2 | 7q22.1 | 6/068 |
| Collagen, type II, α1 | COL2A1 | 12q13.11-q13.2 | 1/15.0 |
| Collagen, type IX, α1 | COL9A1 | 6q13 | 15/54.5 |
| Collagen, type IX, α2 | COL9A2 | 1p33-p32.2 | 4/53.0 |
| Collagen, type XI, α1 | COL11A1 | 1p21 | 3/53.1 |
| Collagen, type XI, α2 | COL11A2 | 6p21.3 | 17/18.51 |
| Fibrillin 1 | FBN1 | 15q21.1 | 2/71.0 |
| Klotho | KL | 13q12 | 5/63 |
| MGP | MGP | 12p13.1-p12.3 | 6 |
| Monocyte chemotatic protein 1 | MCP1 (SCYA2) | 17q11.2-q12 | 11/46.5 |
| Noggin | NOG | 17q22 | 11/50.5 |
| Osteonectin | ON | 5q31.3-q32 | 11/29.9 |
| OPN (bone sialoprotein I) | OPN | 4q21-q25 | 5/56.0 |
| T cell, immune regulator I | ATP6i | 11q13.4-q13.5 | 19/6.0 |
| Tartrate-resistant acid phosphatase | ACPS | 19p13.3-p13.1 | 9/6.0 |
| Thrombospondin 1 | TSP-1 | 15q15 | 2/65.0 |
| Transcription factor | |||
| Osterix (mouse) | OSX | 12q12 | 15 |
| Runt-related transcription factor 2 | RUNX2 (CBFA-1) | 6p21 | 17/28.07 |
| SRY (sex-determining region Y)-box 9 | SOX9 | 17q24.3-q25.1 | 11/69.5 |
| SRY-box 5 | SOX5 | 12p12.1 | 6/69.5 |
| Signaling pathway components | |||
| BMP-2 | BMP-2 | 20p12 | 2/76.1 |
| BMP-4 | BMP-4 | 14q22-q23 | NA |
| CSF-1 receptor | CSF-1R (C-FMS) | 5p33.2-33.3 | 18/30.0 |
| Mothers against decapentaplegic homolog 6 | SMAD6 | 15q21-q22 | 9 |
| Osteoprotegerin | OPG | 8q24 | NA |
| RANK | RANK | 18q22.1 | NA |
| RANKL | RANKL | 13q14 | 14/45.0 |
| TRAF-6 | TRAF6 | 11p11.2 | NA |
| TNF-α | TNF | 6p21.3 | 17/19.06 |
| TNF receptor 1 | TNFR1 | 12p13.2 | 6/60.55 |
| Low density lipoprotein receptor-related protein 5 | LRP5 | 11q13.4 | 19.B |
| Homeobox genes | |||
| Bagpipe homeobox | BAPX1 | 4p16.1 | 5/23.0 |
| Distal-less homeobox 5 | DLX5 | 7q22 | 6/2.0 |
| Distal-less homeobox 6 | DLX6 | 7q22 | 6/2.0 |
| Muscle segment homeobox | MSX2 | 5q34-q35 | 13/32.0 |
| Growth factors | |||
| CSF-1 (macrophage CSF) | CSF-1 (M-CSF) | 1p21-p13 | 3/51.0 |
| Fibroblast growth factor 2 | FGF2 | 4q25-q27 | 3/19.3 |
| Fibroblast growth factor receptor 3 | FGFR3 | 4p16.3 | 5/20.0 |
| Insulin-like growth factor-binding protein 4 | IGFBP4 | 17q12-q21 | NA |
| Insulin-like growth factor-binding protein 5 | IGFBP5 | 2q33-q36 | 1/36.1 |
| Indian hedgehog | IHH | 2q33-q35 | 1/40.8 |
| Insulin-like growth factor 1 (somatomedin C) | IGF1 | 12q22-q24.1 | 10/48.0 |
| Insulin-like growth factor 2 (somatomedin A) | IGF2 | 11p15.5 | 7/69.09 |
| Interferon β | IFNB | 9p21 | 4/42.6 |
| Parathyroid hormone-related peptide (PTHrP) | PTHrP | 12p12.1-p11.2 | 6/74.0 |
| Platelet-derived growth factor, α polypeptide | PDGFA | 7p22 | 5/82.0 |
| Platelet-derived growth factor, β polypeptide | PDGFB | 22q12.3-q13.1 | 15/46.8 |
| Transforming growth factor β | TGFB1 | 19p13.1 | 7/6.5 |
| Hormones | |||
| Leptin | LEP | 7q31.3 | 5/10.5 |
| Leptin receptor | LEPR | 1p31 | 4/46.7 |
| Parathyroid hormone | PIH | 11p15.3-p15.1 | 7/52.5 |
cM, centimorgans; NA, not applicable; TRAF-6, TNF receptor-associated factor 6.
Passive Model of Arterial Calcification
Pathologic mineralization can also occur by passive, cell-autonomous mechanisms, notably in patients with metabolic disorders. Apoptotic cell fragments and cholesterol crystals can serve as a crystallization nidus, and mineralization may occur if local ionic concentrations exceed the salt solubility product. The passive model of arterial calcification postulates that arterial mineral deposition similarly occurs when inhibitors are no longer able to prevent calcium precipitation (11). Pathologic studies of medial arterial calcification (Mönckeberg's sclerosis), data on human genetic disorders, and analysis of phenotypes of genetically altered mice have produced evidence consistent with this model. Arterial calcification can also be produced in animals by certain manipulations, such as administration of 1α,25-(OH)2-vitamin D3 and nicotine (15). Price et al. (16) have produced calcification in the medial layer of rat arteries with injections of warfarin and vitamin K. Although this treatment causes extensive calcification in the medial arterial layer, calcification in atheroma is found typically in the intima near the base of the plaque (Fig. 1) and rarely in the media itself (1, 17). Because rats have little or no intima and are free of atherosclerosis, the relevance of the observed phenotype to calcification occurring in the context of atherosclerotic plaque remains uncertain.
MGP is expressed in chondrocytes (18) and in normal and atherosclerotic arteries (19). Deletion of the gene encoding MGP in mice results in extensive calcification of cartilage and the medial layer of arteries (20). These findings suggest that MGP might have a general function in tissues as diverse as cartilage and arteries: inhibition of mineral precipitation in the extracellular fluid (11), where calcium and phosphate concentrations approach the salt solubility product. On a molecular level, it is not yet known how MGP participates in calcification in bone and artery. MGP might have direct calcium-chelating effects or might operate indirectly, for example, by inhibiting BMPs (21). Microenvironments in atherosclerotic plaques might foster conditions that favor precipitation of calcium mineral. In particular, necrotic debris from foam cells that have undergone apoptosis could serve as a nidus for precipitation of mineral because they may release high concentrations of mitochondrial phosphate and phosphatidylserine-containing molecules. This series of events could, in principle, tip local ionic equilibria sufficiently to instigate precipitation.
In mice with targeted deletion of bone Gla protein (osteocalcin), increased bone formation occurs. This increased bone formation is expected because an abundantly expressed bone protein with affinity for mineral ions has been removed. No arterial calcification occurs, however, despite the fact that bone Gla protein is expressed in both normal and atherosclerotic arteries (19). OPN is another bone-matrix protein expressed in arteries. OPN inhibits mineral formation in vitro, but no arterial calcification occurs in OPN-knockout mice (22). However, this finding does not rule out a role for OPN in preventing mineral precipitation in atherosclerotic arteries. OPN is not found in normal arteries but is indeed expressed in plaque and colocalizes with calcified plaque regions (23). It is therefore possible that OPN might play a protective role where necessary and be expressed in plaque only in response to local conditions that might tend to favor mineralization.
Active Osteoblast-Like Arterial Cell Model
Almost a decade ago, a cell type that was similar to microvascular pericytes and could be induced to differentiate along an osteoblast-like lineage under certain in vitro conditions was discovered in both normal and diseased arteries. These cells, termed calcifying vascular cells (CVCs) colocalized with areas where expression of the potent osteogenic factor BMP-2 was also observed (24). Subsequent studies characterized similarities of CVCs and vascular smooth muscle cell (SMC)-derived osteoblast-like cells with osteoblast-specific traits and functions (1). Nevertheless, it is not yet clear what specific cell type(s) mediate arterial calcification. Under mineralizing cell-culture conditions, a number of cell types, including aortic SMCs, can acquire osteoblast-like characteristics. In normal human arteries, MGP is expressed in the medial layer; in plaque, MGP is associated with endothelial cells and SMCs but not macrophages (25). The transcription core binding factor α1 (Cbfa1, also called Runx2) is essential for the development of osteoblasts (26), colocalizes with calcified areas, and is expressed by macrophages in plaque microenvironments where MGP is absent (25). These observations are consistent with a model wherein MGP inhibits calcium deposition and Cbfa1 promotes differentiation of pluripotent arterial cells into an osteoblast-like phenotype in plaque microenvironments where focal calcification occurs.
There appears to be considerable versatility in the origin and ontogenetic fate of arterial cells in atherosclerotic plaque (27). Recent data in animal models of vascular disease (27) and in humans who have undergone bone marrow transplantation (28) indicate that SMCs and endothelial cells in atherosclerotic plaques can originate from bone marrow. Arteries may contain cells that are phenotypically similar to the major cell types involved in endochondral bone formation: chondrocytes, osteoblasts, and osteoclasts (2, 19, 20, 24, 29). These cells possess signaling pathways and express proteins normally associated with bone. Normal human aortae express a number of proteins associated with bone, albeit generally at low levels (19). However, fibrous-cap atheromatous lesions and fibrocalcific plaques express high levels of many of these proteins, particularly in regions of plaque where calcification is seen. Lamellar bone-like structures containing osteoblast-like cells may be found in association with matrix vesicles, and high expression of bone-matrix proteins in regions adjacent to mineralized plaque has been reported (19, 30). Collectively, these findings support the concept that arterial calcification occurs in plaque microenvironments in a manner that recapitulates osteogenesis. Most of the genetic and molecular mechanisms that mediate bone formation and degradation, however, have not yet been tested in any in vivo model of atherosclerotic calcification.
Genetically Altered Mice and Arterial Calcification
A valuable tool to unravel the genetic and molecular mechanisms of atherosclerotic calcification is the utilization of the numerous animal models that harbor gene mutations relevant to bone biology and mineral homeostasis. Studies reported thus far, however, have yielded conflicting results. Some genetically altered mouse models appear the most consistent with a passive mechanism of arterial calcification. For example, genetic deficiency of MGP (20) in mice results in extensive medial arterial calcium deposition, suggesting that MGP inhibits mineral deposition. The expression pattern of MGP in normal arteries and fibrocalcific plaque is also consistent with an inhibitory role of MGP in calcium deposition (19) but does not address the mechanism directly.
Other genetically altered murine models are consistent with an active model of arterial calcification. BMP-2 expression occurs in calcified arterial lesions (19, 24), and it is suspected that this potent osteogenic factor may be involved in calcium deposition in plaque. Smad6 is an intracellular inhibitor of BMP signaling. Mice lacking the gene encoding Smad6 exhibit extensive medial arterial calcification (31). Other mutations that can produce arterial calcification include deletions of the genes encoding the structural microfibrillar protein fibrillin-1 (32) and the aging-related gene klotho (33). Although some of these genetically altered animals exhibit impressive calcification and sometimes extramedullary hematopoiesis of the blood vessels, it should be emphasized that these data are not within the context of atherosclerotic disease. In these knockout mutations, calcification takes place predominantly within the media. Animals that do not have atherosclerosis develop little or no neointima, and it may be possible that the medial calcification is qualitatively and mechanistically different from that seen in atherosclerotic plaque.
Studies of inherited disorders in humans suggest that animal models may sometimes be misleading. Keutel syndrome is an inherited disorder that results in a nonfunctional MGP gene (34), but this syndrome is not characterized by the massive arterial calcification that is observed in MGP-knockout mice (20). Similarly, arterial calcification is observed in murine fibrillin-1 deficiency, but this deficiency has not been convincingly linked to arterial calcification in humans. Discordances between results of cell-culture studies and whole animal genetic models of calcification have also been reported. MGP-knockout mice exhibit extensive vascular calcification, but calcifying vascular cell-associated mineralization is accompanied by high levels of MGP expression in vitro (35). The extrapolation of cell-culture results to in vivo conditions is hazardous, and the discordances noted above only underscore the need for more direct mechanistic testing.
Osteoclast-Like Arterial Cells
A model of calcification that postulates bone-forming arterial cells raises the question how arterial mineralization might be opposed or limited. Unchecked rapidly progressing mineralization is neither biologically tolerable nor clinically observed. The process could be self-limiting, but other intriguing possibilities exist as well. As is the case in bone, might there be a counterbalancing mechanism involving osteoclast-like arterial cells? We have proposed that calcium deposition might be importantly determined by the activity of osteoclast-like cells (OLCs), arterial cells with the capacity to inhibit calcium deposition and/or resorb mineral (ref. 36 and Fig. 3).
If OLCs develop similarly in arteries and bone, then it would be expected that genetic alterations that interfere with osteoclast development or function would result in greater arterial calcium deposition. Specifically, mutations resulting in an osteopetrotic phenotype would be anticipated also to exhibit increased arterial calcification; in addition, osteoporotic phenotypes should show lesser amounts of calcium deposition in the arteries. Studies in osteopetrotic murine models are consistent with this idea (37, 38). Paradoxically, however, osteoprotegerin (OPG)-deficient mice develop both osteoporosis and arterial calcification (39), and administration of soluble OPG or transgenic OPG expression reverses the phenotype (40). The explanation for this finding is unclear, but in any case, most of these data have been obtained in mice that do not develop atherosclerosis; again, the relevance of the findings to plaque calcification remains uncertain. Furthermore, increased arterial calcification results from genetic alterations causing osteopetrosis but can also be seen with osteoporotic and osteopenic phenotypes (20, 38, 39). Further studies are needed to elucidate these issues.
A major breakthrough in osteoclast biology occurred with the characterization of key components of a signaling pathway required for osteoclast formation and function: RANKL, its cell surface receptor (RANK), and its decoy receptor OPG (ref. 41; see Table 1 legend). Deletion of the gene encoding RANKL or RANK results in nearly a complete lack of functional osteoclasts, and RANKL and CSF-1 signaling are necessary and sufficient for osteoclast survival and function. Several lines of evidence support a role for CSF-1 and RANKL signaling in the development of arterial OLCs from plaque mononuclear phagocytes (36). RANKL and its receptors are expressed in a number of vascular tissues, including arteries, and their expression patterns are altered as plaque forms and mineral deposits appear. OPG, RANK, and RANKL are normally expressed by osteoblastic stromal cells and osteoclast precursors but are also found in cells associated with calcified arterial lesions of OPG-deficient mice (39). RANKL signaling is associated with mononuclear phagocytic cells in plaque (42). RANKL, RANK, and OPG colocalize with multinucleated cells that are tartrate-resistant acid phosphatase-positive and cathepsin K-positive but negative for the macrophage antigen F4/80. Cathepsin K is an osteoclast-associated protease that is also expressed in atherosclerotic plaque (43). Collectively, these findings are consistent with a model in which development of multinucleated cells (OLCs) from hematopoietic precursors is facilitated in the microenvironment surrounding arterial calcium deposits, providing a possible mechanism to limit mineralization. Steitz et al. (44) recently used OPN–/– mice to show that OPN inhibits ectopic calcification and also promotes mineral resorption by inducing expression of carbonic anhydrase II by mononuclear phagocytic cells. The most intriguing finding within the present context was that OPN did not just inhibit calcium mineral growth but appeared to induce multinucleated giant cells and macrophage-like cells to dissolve mineral.
But what is the origin of OLCs? Atherosclerosis is conceptualized as a chronic inflammatory response to injury in which multiple cell types are known to participate (45). Tissue repair processes were thought previously to be largely the purview of resident parenchymal and/or mesenchymal cells; however, increasing evidence is consistent with a general model wherein somatic nonresident stem cells are mobilized and recruited to remote sites of injury where differentiation into the necessary lineages and tissue repair occur (46). Circulating bone-marrow-derived hematopoietic stem cells are thought to be recruited to developing atheromatous lesion sites, appear to possess the capacity to differentiate into either endothelial cells or vascular SMCs, and may actually account for the majority of cells participating in both atherogenesis and neointimal proliferation after arterial injury (27, 28, 47).
Bone Biology, the Immune System, and Vascular Inflammation
Osteoclast formation and function are influenced by numerous cytokines besides CSF-1 and RANKL, and some of these, particularly proinflammatory cytokines, have also been implicated in atherogenesis. TNF-α activates osteoclasts directly (Fig. 3) and also appears to potentiate the effects of RANKL signaling (48). Therefore, three key cytokines that are involved in osteoclast development and function and have proinflammatory properties (RANKL, CSF-1, and TNF-α) are present in atherosclerotic plaque and expressed in patterns that are consistent with the notion that arterial OLCs develop from mononuclear phagocytic-cell precursors.
Immune modulation plays an important role in both plaque development and bone growth and activity. RANKL is expressed by T cells and participates in immune functions distinct from its role in bone (41, 49). T cells participate directly in bone formation and metabolism. Recent reports indicate that there is considerable cross-talk between signaling pathways in bone and the immune system. Binding of RANKL to RANK results in recruitment of several adaptor proteins. These include TRAF-6, which is required to transmit RANKL signals to downstream effectors via the NF-κB and c-Jun N-terminal kinase pathways (41). TRAF-6 also participates in other inflammatory signaling pathways involving TNF-α, IL-1, IL-8, IL-17, and CD-40 ligand. TRAF-6-knockout mice exhibit defective osteoclast formation and are osteopetrotic (50). IFNs can directly affect osteoclast development and activity. For example, IFN-γ suppresses osteoclastogenesis by inhibiting activation of NF-κB and c-Jun N-terminal kinase by the RANKL–RANK signaling pathway (51). There is also cross-talk between RANKL and IFN-β signaling (52). Like mice with TRAF-6 deficiency, mice lacking c-Fos, a critical downstream target of RANKL signaling (13, 41), demonstrate defective osteoclast development and osteopetrosis (53). However, RANKL–RANK signaling-dependent increases in c-Fos expression result in upregulation of IFN-β in osteoclast precursors (52). IFN-β, in turn, inhibits expression of c-Fos. Thus, the effects of RANKL signaling on c-Fos expression cause inhibitory autoregulation by enhanced IFN-β expression and signaling.
The central inflammatory character of atherosclerosis (45), together with the role of inflammatory cytokines in bone metabolism, suggests a conceptual framework: inflammation and bone resorption share common signaling-pathway components that meet at crossroads in plaque microenvironments. The result is differentiation of vascular cells, recruitment of pluripotent precursors, and development of bone-like tissues and hydroxyapatite deposits. Aided and abetted by the immune system, vascular pathology may thus beget both bone formation and bone resorption.
Mechanism(s) in Need of Testing
By analogy with known bone biology, we propose a homeostatic mechanism wherein the two main cellular mediators are osteoblast-like cells and OLCs. Arterial mineral metabolism is normally in balance, but in proinflammatory plaque microenvironments, immune-modulating cytokines facilitate recruitment and development of osteoblast-like cells and OLCs, uncoupling of their activities, and net mineral deposition. But myriad questions remain unexplored. How do atherogenesis and the immune system interweave with bone-remodeling signaling pathways to determine differentiation of bone-like cells in the arterial wall? Which cells evolve from resident pluripotent cells, which cells are recruited, and how do these processes occur? Why do only some plaques calcify? How does plaque calcification influence disease progression and clinical outcomes? And importantly, where might therapeutic intervention be effectively targeted? The working out of the interrelationships among diverse tissues and bone and immune signaling pathways that ultimately meet at the arterial crossroads and drive plaque calcification should prove to be a fascinating journey in the years ahead.
Acknowledgments
We thank the Mayo Foundation, the Mirisch Foundation, United Hostesses Charities, the Eisner Foundation, the Grand Foundation, the Ornest Family Foundation, the Milken Family Foundation, and the Heart Fund at Cedars–Sinai Medical Center for their generous support. This work was supported by grants from Philip Morris USA, Inc., through the External Research Program (T.B.R.); grants from the National Heart, Lung, and Blood Institute to T.B.R. (HL51980 and HL58555), L.A.F. (HL51736), and R.C.D. (7RO1-HL43277-02); and National Institutes of Health National Center for Research Resources Grant K24RR017593-01 (to L.A.F.).
Abbreviations: BMP, bone morphogenetic protein; CSF-1, colony-stimulating factor 1; Gla, γ-carboxyglutamate; MGP, matrix Gla protein; OLCs, osteoclast-like cells; OPG, osteoprotegerin; OPN, osteopontin; RANK, receptor activator of NF-κB; RANKL, RANK ligand; SMC, smooth muscle cell; TNF-α, tumor necrosis factor α; TRAF-6, TNF receptor-associated factor 6.
References
- 1.Detrano, R. C., Doherty, T. M., Davies, M. J. & Stary, H. C. (2000) Curr. Probl. Cardiol. 25, 374–402. [DOI] [PubMed] [Google Scholar]
- 2.Jeziorska, M., McCollum, C. & Wooley, D. E. (1998) Virchows Arch. 433, 559–565. [DOI] [PubMed] [Google Scholar]
- 3.Sangiorgi, G., Rumberger, J. A., Severson, A., Edwards, W. D., Gregoire, J., Fitzpatrick, L. A. & Schwartz, R. S. (1998) J. Am. Coll. Cardiol. 31, 126–133. [DOI] [PubMed] [Google Scholar]
- 4.O'Malley, P. G., Taylor, A. J., Jackson, J. L., Doherty, T. M. & Detrano, R. C. (2000) Am. J. Cardiol. 85, 945–948. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke, R. A., Brundage, B. H., Froelicher, V. F., Greenland, P., Grundy, S. M., Hachamovitch, R., Pohost, G. M., Shaw, L. J., Weintraub, W. S. & Winters, W. L., Jr. (2000) J. Am. Coll. Cardiol. 36, 326–340. [DOI] [PubMed] [Google Scholar]
- 6.Doherty, T. M., Wong, N. D., Shavelle, R. M., Tang, W. & Detrano, R. C. (1999) Lancet 353, 41–42. [DOI] [PubMed] [Google Scholar]
- 7.Detrano, R. C., Wong, N. D., Doherty, T. M., Shavelle, R. M., Tang, W., Ginzton, L. E., Budoff, M. J. & Narahara, K. A. (1999) Circulation 99, 2633–2638. [DOI] [PubMed] [Google Scholar]
- 8.Pearson, T. A. (2002) Circulation 105, 886–892. [DOI] [PubMed] [Google Scholar]
- 9.Libby, P. (2001) Circulation 104, 365–372. [DOI] [PubMed] [Google Scholar]
- 10.Schmid, K., McSharry, W. O., Pameijer, C. H. & Binette, J. P. (1980) Atherosclerosis (Shannon, Irel.) 37, 199–210. [DOI] [PubMed] [Google Scholar]
- 11.Schinke, T., McKee, M. D. & Karsenty, G. (1999) Nat. Genet. 21, 150–151. [DOI] [PubMed] [Google Scholar]
- 12.Harada, S. & Rodan, G. A. (2003) Nature 423, 349–355. [DOI] [PubMed] [Google Scholar]
- 13.Boyle, W. J., Simonet, W. S. & Lacey, D. L. (2003) Nature 423, 337–342. [DOI] [PubMed] [Google Scholar]
- 14.Qi, H., Aguiar, D. J., Williams, S. M., La Pean, A., Pan, W. & Verfaillie, C. M. (2003) Proc. Natl. Acad. Sci. USA 100, 3305–3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niederhoffer, N., Bobryshev, Y. V., Lartaud-Idjouadiene, I., Giummelly, P. & Atkinson, J. (1997) J. Vasc. Res. 34, 386–398. [DOI] [PubMed] [Google Scholar]
- 16.Price, P. A., Faus, S. A. & Williamson, M. K. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 317–327. [DOI] [PubMed] [Google Scholar]
- 17.Stary, H. C. (2000) Z. Kardiol. 89, Suppl. 2, 28–35. [DOI] [PubMed] [Google Scholar]
- 18.Luo, G., D'Souza, R., Hogue, D. & Karsenty, G. (1995) J. Bone Miner. Res. 10, 325–334. [DOI] [PubMed] [Google Scholar]
- 19.Dhore, C. R., Cleutjens, J. P., Lutgens, E., Cleutjens, K. B., Geusens, P. P., Kitslaar, P. J., Tordoir, J. H., Spronk, H. M., Vermeer, C. & Daemen, M. J. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1998–2003. [DOI] [PubMed] [Google Scholar]
- 20.Luo, G., Ducy, P., McKee, M. D., Pinero, G. J., Loyer, E., Behringer, R. R. & Karsenty, G. (1997) Nature 386, 78–81. [DOI] [PubMed] [Google Scholar]
- 21.Zebboudj, A. F., Imura, M. & Bostrom, K. (2002) J. Biol. Chem. 277, 4388–4394. [DOI] [PubMed] [Google Scholar]
- 22.Liaw, L., Birk, D. E., Ballas, C. B., Whitsitt, J. S., Davidson, J. M. & Hogan, B. L. (1998) J. Clin. Invest. 101, 1468–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzpatrick, L. A., Severson, A., Edwards, W. D. & Ingram, R. T. (1994) J. Clin. Invest. 94, 1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bostrom, K., Watson, K. E., Horn, S., Wortham, C., Herman, I. M. & Demer, L. L. (1993) J. Clin. Invest. 91, 1800–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engelse, M. A., Neele, J. M., Bronckers, A. L., Pannekoek, H. & de Vries, C. J. (2001) Cardiovasc. Res. 52, 281–289. [DOI] [PubMed] [Google Scholar]
- 26.Komori, T., Yagi, H., Nomura, S., Yamaguchi, A., Sasaki, K., Deguchi, K., Shimizu, Y., Bronson, R. T., Gao, Y. H., Inada, M., et al. (1997) Cell 89, 755–764. [DOI] [PubMed] [Google Scholar]
- 27.Doherty, T. M., Shah, P. K. & Rajavashisth, T. B. (2003) FASEB J. 17, 592–597. [DOI] [PubMed] [Google Scholar]
- 28.Caplice, N. M., Bunch, T. J., Stalboerger, P. G., Wang, S., Simper, D., Miller, D. V., Russell, S. J., Litzow, M. R. & Edwards, W. D. (2003) Proc. Natl. Acad. Sci. USA 100, 4754–4759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt, J. L., Fairman, R., Mitchell, M. E., Carpenter, J. P., Golden, M., Khalapyan, T., Wolfe, M., Neschis, D., Milner, R., Scoll, B., et al. (2002) Stroke (Dallas) 33, 1214–1219. [DOI] [PubMed] [Google Scholar]
- 30.Canfield, A. E., Farrington, C., Dziobon, M. D., Boot-Handford, R. P., Heagerty, A. M., Kumar, S. N. & Roberts, I. S. (2002) J. Pathol. 196, 228–234. [DOI] [PubMed] [Google Scholar]
- 31.Galvin, K. M., Donovan, M. J., Lynch, C. A., Meyer, R. I., Paul, R. J., Lorenz, J. N., Fairchild-Huntress, V., Dixon, K. L., Dunmore, J. H., Gimbrone, M. A., Jr., et al. (2000) Nat. Genet. 24, 171–174. [DOI] [PubMed] [Google Scholar]
- 32.Pereira, L., Lee, S. Y., Gayraud, B., Andrikopoulos, K., Shapiro, S. D., Bunton, T., Biery, N. J., Dietz, H. C., Sakai, L. Y. & Ramirez, F. (1999) Proc. Natl. Acad. Sci. USA 96, 3819–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kuro-o, M., Matsumura, Y., Aizawa, H., Kawaguchi, H., Suga, T., Utsugi, T., Ohyama, Y., Kurabayashi, M., Kaname, T. & Kume, E., et al. (1997) Nature 390, 45–51. [DOI] [PubMed] [Google Scholar]
- 34.Munroe, P. B., Olgunturk, R. O., Fryns, J. P., Van Maldergem, L., Ziereisen, F., Yuksel, B., Gardiner, R. M. & Chung, E. (1999) Nat. Genet. 21, 142–144. [DOI] [PubMed] [Google Scholar]
- 35.Proudfoot, D., Skepper, J. N., Shanahan, C. M. & Weissberg, P. L. (1998) Arterioscler. Thromb. Vasc. Biol. 18, 379–388. [DOI] [PubMed] [Google Scholar]
- 36.Doherty, T. M., Uzui, H., Fitzpatrick, L. A., Tripathi, P. V., Dunstan, C. R., Asotra, K. & Rajavashisth, T. B. (2002) FASEB J. 16, 577–582. [DOI] [PubMed] [Google Scholar]
- 37.Spicer, S. S., Lewis, S. E., Tashian, R. E. & Schulte, B. A. (1989) Am. J. Pathol. 134, 947–954. [PMC free article] [PubMed] [Google Scholar]
- 38.Qiao, J.-H., Tripathi, J., Mishra, N. K., Cai, Y., Tripathi, S., Wang, X. P., Imes, S., Fishbein, M. C., Clinton, S. K., Libby, P., et al. (1997) Am. J. Pathol. 150, 1687–1699. [PMC free article] [PubMed] [Google Scholar]
- 39.Bucay, N., Sarosi, I., Dunstan, C. R., Morony, S., Tarpley, J., Capparelli, C., Scully, S., Tan, H. L., Xu, W., Lacey, D. L., et al. (1998) Genes Dev. 12, 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Min, H., Morony, S., Sarosi, I., Dunstan, C. R., Capparelli, C., Scully, S., Van, G., Kaufman, S., Kostenuik, P. J. & Lacey, D. L., et al. (2000) J. Exp. Med. 192, 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khosla, S. (2001) Endocrinology 142, 5050–5055. [DOI] [PubMed] [Google Scholar]
- 42.Tsurukai, T., Udagawa, N., Matsuzaki, K., Takahashi, N. & Suda, T. (2000) J. Bone Miner. Metab. 18, 177–184. [DOI] [PubMed] [Google Scholar]
- 43.Sukhova, G. K., Shi, G. P., Simon, D. I., Chapman, H. A. & Libby, P. (1998) J. Clin. Invest. 102, 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steitz, S. A., Speer, M. Y., McKee, M. D., Liaw, L., Almeida, M., Yang, H. & Giachelli, C. M. (2002) Am. J. Pathol. 161, 2035–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libby, P. (2002) Nature 420, 868–874. [DOI] [PubMed] [Google Scholar]
- 46.Orlic, D., Kajstura, J., Chimenti, S., Limana, F., Jakoniuk, I., Quaini, F., Nadal-Ginard, B., Bodine, D. M., Leri, A. & Anversa, P. (2001) Proc. Natl. Acad. Sci. USA 98, 10344–10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sata, M., Saiura, A., Kunisato, A., Tojo, A., Okada, S., Tokuhisa, T., Hirai, H., Makuuchi, M., Hirata, Y. & Nagai, R. (2002) Nat. Med. 8, 403–409. [DOI] [PubMed] [Google Scholar]
- 48.Fuller, K., Murphy, C., Kirstein, B., Fox, S. W. & Chambers, T. J. (2002) Endocrinology 143, 1108–1118. [DOI] [PubMed] [Google Scholar]
- 49.Anderson, D. M., Maraskovsky, E., Billingsley, W. L., Dougall, W. C., Tometsko, M. E., Roux, E. R., Teepe, M. C., DuBose, R. F., Cosman, D. & Galibert, L. (1997) Nature 390, 175–179. [DOI] [PubMed] [Google Scholar]
- 50.Lomaga, M. A., Yeh, W. C., Sarosi, I., Duncan, G. S., Furlonger, C., Ho, A., Morony, S., Capparelli, C., Van, G., Kaufman, S., et al. (1999) Genes Dev. 13, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takayanagi, H., Ogasawara, K., Hida, S., Chiba, T., Murata, S., Sato, K., Takaoka, A., Yokochi, T., Oda, H., Tanaka, K., et al. (2000) Nature 408, 600–605. [DOI] [PubMed] [Google Scholar]
- 52.Takayanagi, H., Kim, S., Matsuo, K., Suzuki, H., Suzuki, T., Sato, K., Yokochi, T., Oda, H., Nakamura, K., Ida, N., et al. (2002) 416, 744–749. [DOI] [PubMed]
- 53.Wang, Z. Q., Ovitt, C., Grigoriadis, A. E., Mohle-Steinlein, U., Ruther, U. & Wagner, E. F. (1992) Nature 360, 741–745. [DOI] [PubMed] [Google Scholar]